Summary
Background
Ionotropic glutamate receptors (iGluRs) are glutamate-gated ion channels that mediate excitatory neurotransmission in the central nervous system. Based on both molecular and pharmacological criteria, iGluRs have been divided into two major classes, the non-NMDA-class, which includes both AMPA and kainate subtypes of receptors, and the NMDA-class. One evolutionarily conserved feature of iGluRs is their desensitization in the continued presence of glutamate. Thus, when in a desensitized state, iGluRs can be bound to glutamate, yet the channel remains closed. However, the relevance of desensitization to nervous system function has remained enigmatic.
Results
Here, we report the identification and characterization of a novel polypeptide (con-ikot-ikot) from the venom of a predatory marine snail Conus striatus that specifically disrupts the desensitization of AMPA receptors (AMPARs). The stoichiometry of con-ikot-ikot appears reminiscent of the proposed subunit organization of AMPARs, i.e., a dimer of dimers, suggesting that it acts as a molecular four-legged clamp that holds the AMPAR channel open. Application of con-ikot-ikot to hippocampal slices caused a large and rapid increase in resting AMPAR-mediated current leading to neuronal death.
Conclusions
Our findings provide insight into the mechanisms that regulate receptor desensitization, and demonstrate that in the arms race between prey and predators, evolution has selected for a toxin that blocks AMPAR desensitization, thus revealing the fundamental importance of desensitization for regulating neural function.
Introduction
Fast, excitatory neurotransmission in the vertebrate nervous system is primarily mediated by the neurotransmitter glutamate, which activates two major classes of pore-forming ionotropic glutamate receptors (iGluRs) that are distinguished on the basis of molecular and pharmacological criteria. Of these, the receptors activated by α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPARs) have a primary role in the transient depolarization of the postsynaptic membrane caused by synaptic release of glutamate [1]. The time course of AMPAR-mediated changes of the synaptic potential is rapid, on the order of ms, and is influenced by both the rate at which glutamate is removed from the synaptic cleft and by the processes of receptor deactivation and desensitization. AMPARs are tetrameric arrangements of 4 subunits assembled as a dimer of dimers [2]. Binding of glutamate to the S1-S2 extracellular regions of an individual subunit causes a conformational change of the receptor leading to pore opening [3, 4]. In the continued presence of glutamate an additional conformational change closes the pore – this process is called desensitization [5].
Recent studies indicate that at least three classes of auxiliary proteins modulate the kinetics of AMPAR desensitization [6–9]. Members of the Transmembrane AMPAR Regulatory Protein (TARP) family have been identified in both vertebrates [10] and invertebrates [8]. Vertebrate TARPs act as obligate chaperones for AMPAR export from the endoplasmic reticulum, localize AMPARs to synapses and contribute to AMPAR desensitization and deactivation kinetics [6]. More recently, in invertebrates a second class of AMPAR auxiliary proteins has been described [7, 9, 11]. The founding member of these CUB-domain containing proteins, SOL-1, was identified in C. elegans and shown to slow the rate of AMPAR desensitization. sol-1 mutants have disrupted glutamatergic neurotransmission and AMPAR-dependent behaviors, thus demonstrating the biological importance of regulating iGluR desensitization. Related CUB-domain proteins have now been identified in vertebrates and modify the kinetics of NMDA and kainate receptors [12, 13]. Finally, a recent study showed that cornichon, a three-pass transmembrane protein, associates with AMPARs and modulates the kinetics of deactivation and desensitization [14].
The cell body and dendrites of mammalian hippocampal CA1 neurons express extrasynaptic and synaptic AMPARs that can exchange locations [15–18], and that associate with auxiliary proteins that are known to strongly affect the biophysical properties of the receptors [6, 11, 19–21]. Studying how desensitization contributes to the function of these classes of AMPARs has been difficult because existing modulators of AMPAR desensitization are nonspecific. For example, the widely used drug cyclothiazide (CTZ), which blocks AMPAR desensitization, also blocks ionotropic GABA receptors [22], facilitates presynaptic release by suppressing potassium currents [23] and also increases the AMPAR affinity for glutamate [24]. Therefore, new pharmacological agents are required for a rigorous test of the contribution of desensitization to neuronal AMPARs. We hypothesized that if desensitization of AMPARs was of critical importance for neuronal function and survival, evolution may have selected for mechanisms that disrupt desensitization. We therefore screened venoms from various species of Conus snails [25] for toxins that specifically disrupted the desensitization of AMPAR-mediated glutamate-gated currents.
Here, we report the discovery and characterization of a toxin (con-ikot-ikot) from the fish-hunting snail Conus striatus [26]. We show that con-ikot-ikot has a profound effect on AMPAR-mediated currents by inhibiting channel desensitization. The effect was specific for AMPARs and con-ikot-ikot binds to a site distinct to that of CTZ. Con-ikot-ikot is a much larger peptide than previous toxins isolated from cone snails, and the active protein appears to be formed from a dimer of dimers thus mimicking the proposed stoichiometry of iGluRs. The unprecedented biochemical properties of this toxin suggest that it might act as a molecular four-legged clamp which, when bound to the iGluR channel, locks it in an open conformation. In addition to providing a mechanistic insight into receptor desensitization, the discovery of this novel Conus snail toxin may present an opportunity for the development of therapeutic drugs that modulate this important aspect of nervous system function.
Results
Conus striatus venom contains a peptide that blocks AMPAR desensitization
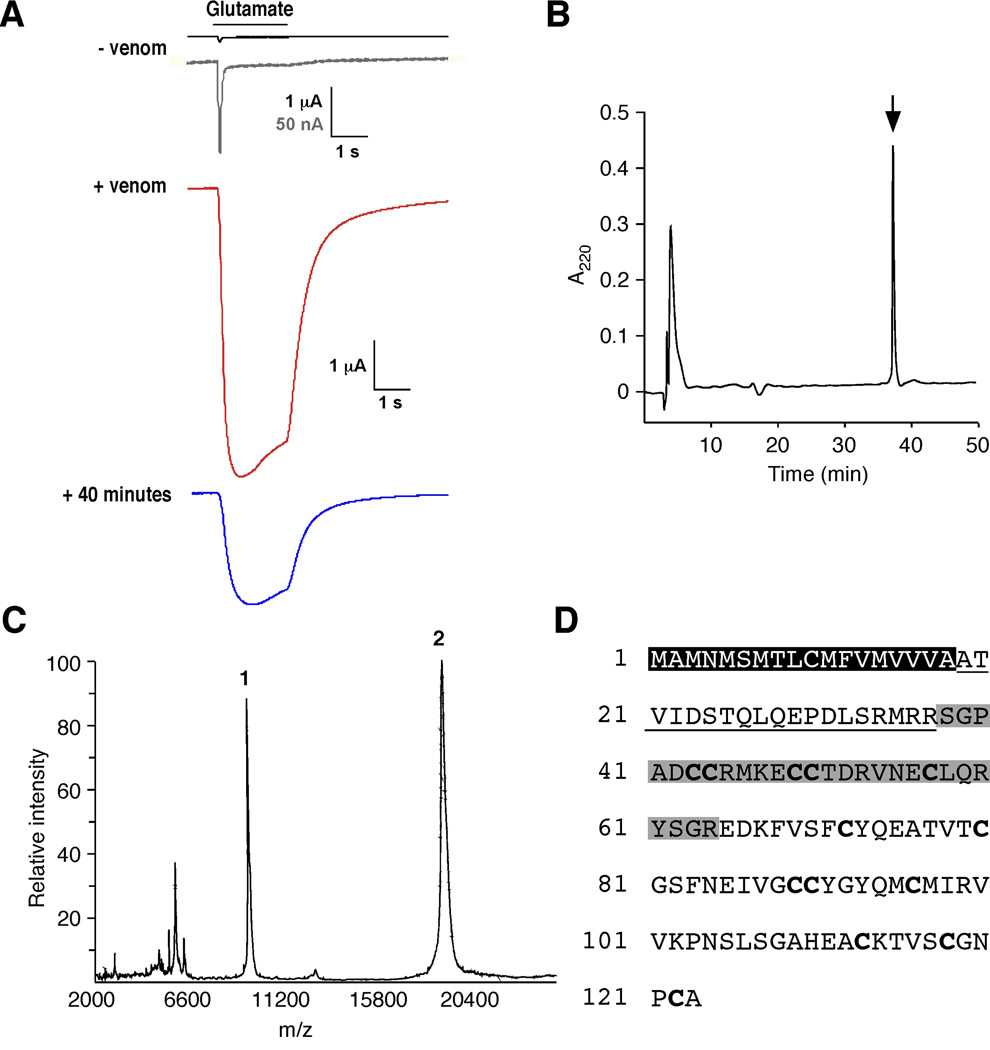
To identify molecules that modulate iGluR desensitization, we screened a number of Conus venoms for activities that modified glutamate-gated currents in Xenopus oocytes that expressed the GluA1 (GluR1) AMPAR subunit [27]. In the absence of venom, we recorded small, rapidly desensitizing currents in response to bath application of 1 mM glutamate (Figure 1A). In contrast, preincubation with venom from Conus striatus increased the size of the peak current by over an order of magnitude; however, the before and after currents do not scale, presumably a consequence of a toxin-mediated decrease in the rate of receptor desensitization (Figure 1A). The effect was still evident after a 40-minute wash (Figure 1A). Following serial HPLC-fractionation of the venom, we identified a single peak (Figure 1B) with biological activity identical to that of the original fraction (data not shown). MALDI mass spectrometry showed two major peaks with average molecular weights of 9, 443 and 18, 868 kD (Figure 1C). These results were confirmed by electrospray mass spectrometry analysis, which also revealed two peaks centered at 18, 864 kD (primary) and 9, 433 (secondary) (data not shown). Peptide sequencing produced a 27 amino acid (a.a.) N-terminal sequence on which we based degenerate oligonucleotides used to identify a single cDNA from Conus striatus. The cDNA encodes a predicted 123 a.a. peptide with an 18 a.a. signal sequence, a 19 a.a. propeptide region and an 86 a.a. mature peptide with 13 cysteines and a calculated molecular weight of 9, 432 kD (Figure 1D). This weight is in close agreement with the mass spectrometry estimates. We named this toxin con-ikot-ikot (ikot-ikot is a Filipino word that translates to “spinning around” or “turning around”, a reference to the swimming phenotype observed in fish injected with the toxin (see below).
Figure 1.
Isolation and identification of con-ikot-ikot. (A) Glutamate-gated currents recorded from Xenopus oocytes that expressed GluA1(flop) before (−venom), and 1 minute (+venom) or 40 minutes after (+ 40 minutes) the application of 0.18 mg venom from Conus striatus. Gray record is a scaled version of −venom trace. Application of 1 mM glutamate indicated by bar. (B) Final reverse phase HPLC separation of the active component (arrow). (C) MALDI spectra of the active component. Peaks 1 and 2 correspond to the predicted molecular weights of the monomeric and dimeric peptides. (D) Con-ikot-ikot amino acid sequence. Black box, predicted signal sequence; underline, propeptide; and gray box, sequence determined by Edman degradation.
Active con-ikot-ikot is formed from a dimer of dimers
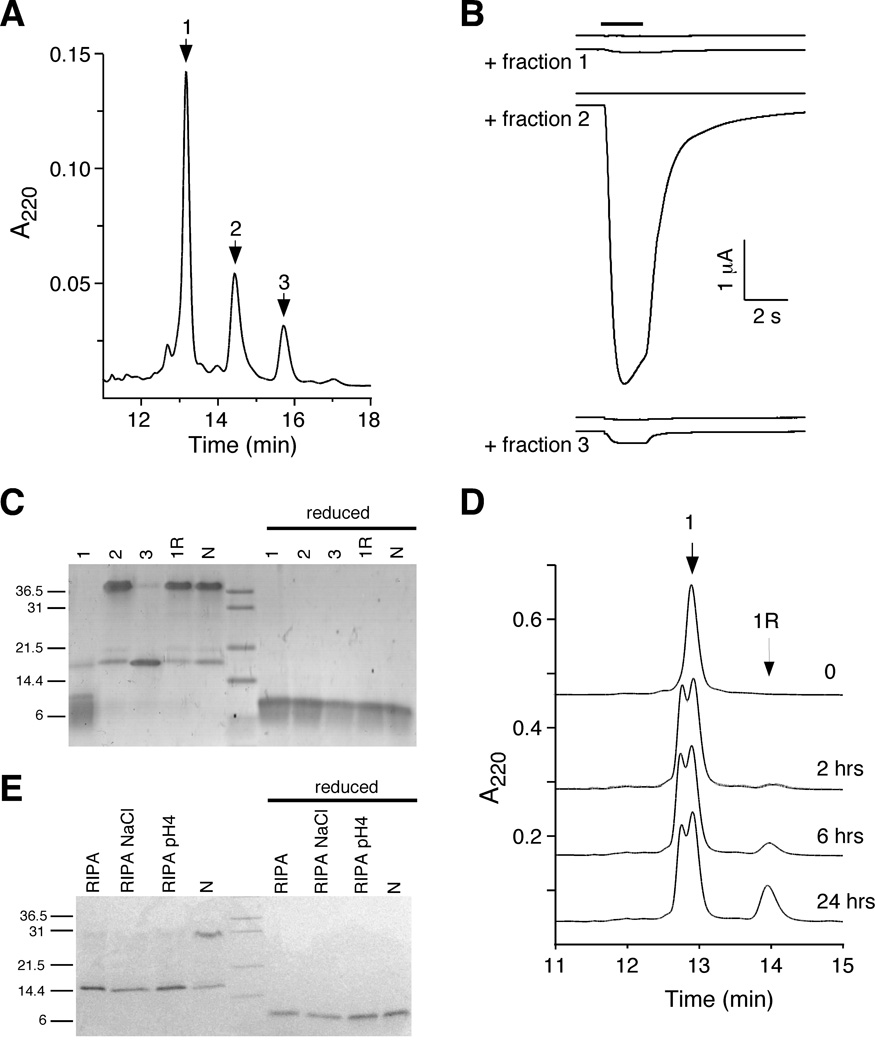
To establish that con-ikot-ikot was the active component of the venom, we expressed a 6xHis-tagged thioredoxin-toxin fusion protein in bacteria. The recombinant protein was concentrated by Ni2+ column chromatography, treated with protease to release the toxin, and further fractionated by HPLC, which revealed a chromatogram consisting of 3 major peaks. Peak 1 was the major component and first to elute, followed by minor peaks 2 and 3 (Figure 2A). When tested for the ability to block AMPAR desensitization in oocytes, peaks 1 and 3 had little activity, whereas peak 2 slowed the kinetics of desensitization thus greatly increasing the current magnitude (Figure 2B). Analysis of the peaks and native toxin by gel electrophoreseis revealed 3 major bands (Figure 2C). In the inactive peak 1 fraction, we observed a dominant band at approximately 9.5 kD (monomer), a minor band at roughly twice this size (19 kD), and no band at a position corresponding to four times the size (38 kD). In the inactive peak 3 fraction, we observed only a 19 kD band (dimer). In contrast, in the active peak 2 fraction, we observed a predominant 38 kD band (tetramer) as well as the minor 19 kD band. The bands observed in peak 2 were identical to that of native toxin. Protein refolding experiments showed that the inactive monomeric species could be converted to an active tetramer under conditions that promoted oxidation/reduction (1R) (Figure 2C, D). All bands collapsed to the monomeric form under reducing conditions (Figure 2C). Prolonged incubation of native toxin in RIPA buffer changed the ratio of bands from predominantly tetrameric to dimeric (Figure 2E). These data, together with the mass spectrometry results (Figure 1C), suggest that con-ikot-ikot forms a covalently linked dimer, presumably through an unpaired cysteine in the monomer, and that the mature toxin is a non-covalently linked dimer of dimers (Figure 2C, E).
Figure 2.
Active con-ikot-ikot toxin is a dimer of dimers. (A) Reverse phase HPLC chromatogram of recombinant, column-purified con-ikot-ikot (see Experimental Procedures). Three major fractions were isolated. (B) Glutamate-gated currents recorded from Xenopus oocytes expressing GluA1(flop) and treated with fractions 1, 2 or 3 (6 µM). (C) SDS-PAGE of native and recombinant toxin. Indicated are the fractions described above, the refolded fraction 1 (1R), and the native toxin (N). Reducing conditions included 1 mM DTT. (D) HPLC chromatograms showing fraction 1 and refolded fraction 1 (1R) of the recombinant toxin. Time of incubation (hrs) of fraction 1 in mixture of oxidized and reduced glutathione indicated next to trace. (E) SDS-PAGE following RIPA buffer incubation of native and recombinant toxin. Reducing conditions included 1 mM DTT.
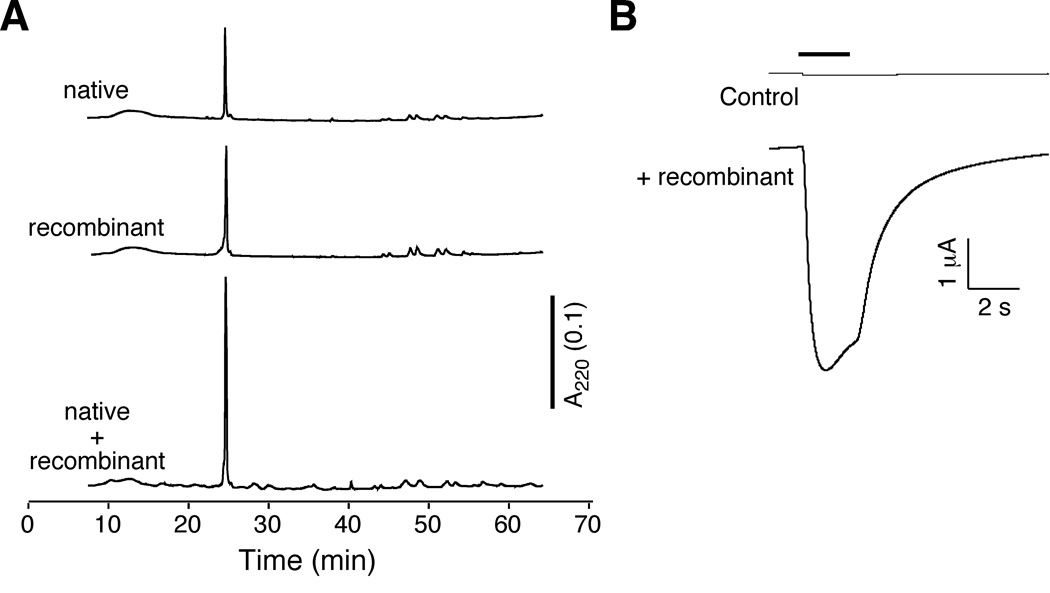
Many Conus toxins are covalently modified post-translationally [28]. To verify that recombinant con-ikot-ikot from the peak 2 fraction was equivalent to the native toxin, we showed that these peptides had identical co-elution profiles (Figure 3A), molecular weight by MALDI (9, 433 and 18, 865; data not shown), and biological activity (Figure 3B).
Figure 3.
Recombinant con-ikot-ikot is equivalent to the native toxin. (A) HPLC co-elution of native and recombinant con-ikot-ikot. (B) Glutamate-gated current traces from Xenopus oocytes that expressed GluA1(flop) before and after application of 6 µM recombinant con-ikot-ikot.
Con-ikot-ikot activity is specific for a subset of iGluRs
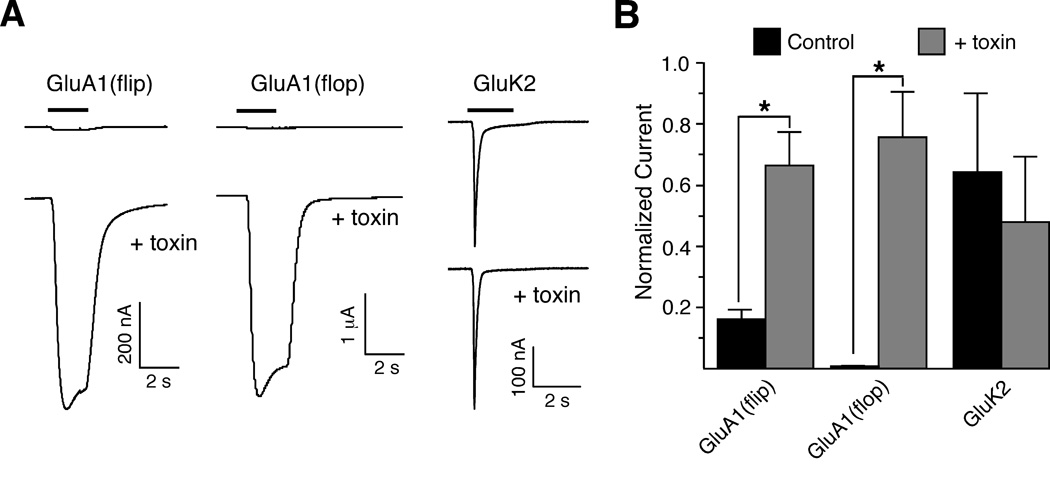
To assess the pharmacological specificity of con-ikot-ikot, we tested its ability to modify the desensitization of different classes of ligand-gated ion channels [1], including the iGluRs preferentially activated by either kainate or N-methyl-D-aspartic acid (NMDA), and GABA receptors. We found that whereas con-ikot-ikot enhanced either the flip or flop variants of the GluA1 AMPAR, it had no detectible activity on GluK2 (GluR6) kainate receptors (Figure 4A, B), GluN1/GluN2A (NR1/NR2A) NMDA receptors (data not shown), or GABA-A receptors (data not shown). The greater enhancement for GluA1 flop vs. flip is the opposite of that observed for CTZ [29]. We also observed enhancement of GluA2 (GluR2), GluA3 (GluR3) and GluA4 (GluR4) AMPAR-mediated currents (data not shown).
Figure 4.
Con-ikot-ikot specifically targets a subset of iGluRs. (A) Current traces from Xenopus oocytes that express GluA1(flip), GluA1(flop), and GluK2 before (control) and after toxin application. (B) Average relative increase of peak current in response to con-ikot-ikot application. *, p < 0.02 (Mann-Whitney; n = 8, GluA1-flip; n = 5, GluA1-flop; n = 5, GluK2).
Modulation by con-ikot-ikot does not require the N-terminal domain of GluA1
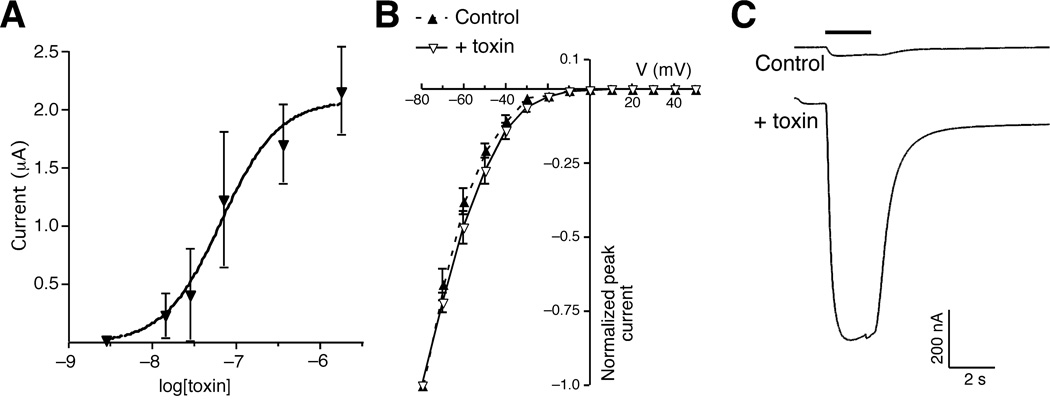
We characterized the potency of con-ikot-ikot by evaluating its dose-response relation. Con-ikot-ikot had an apparent EC50 of 67 nM (Figure 5A); however, this value is almost certainly an underestimate due to the relatively slow rate of perfusion in our oocyte recording chamber, which limits the detection of fast currents. In contrast to the marked effects on receptor desensitization, the toxin did not modify the current-voltage relation, indicating that ion permeation was not altered by toxin (Figure 5B). In addition, the estimated EC50 from the dose-response relation in the presence of toxin is 419 µM, which is consistent with published results [1], and suggests that the toxin did not increase the affinity for glutamate (Supplementary Figure 1). The extracellular portion of iGluRs consists of a large N-terminal domain and two smaller regions defined as S1 and S2, the sites of glutamate binding [2]. Previous work has shown that recombinant receptors lacking the N-terminal domain (NT) were functional and could be gated open by glutamate [30]. To assess the necessity of the GluA1 N-terminal domain for toxin-mediated modulation, we tested con-ikot-ikot on oocytes that expressed recombinant NT-GluA1(flip). We found that the toxin enhanced glutamate-gated currents, indicating that the N-terminal domain was not critical for con-ikot-ikot binding (Figure 5C).
Figure 5.
Con-ikot-ikot potently enhances AMPAR-mediated current. (A) Dose-response curve of glutamate-gated currents recorded from Xenopus oocytes that expressed GluA1(flip). (B) Current-voltage curves before (control) and after con-ikot-ikot application. (C) Glutamate-gated current in Xenopus oocytes that expressed an N-terminal deletion variant of GluA1(flip) (Δ60–471) both before (control) and after toxin application.
Con-ikot-ikot and cyclothiazide (CTZ) bind to distinct sites on GluA1
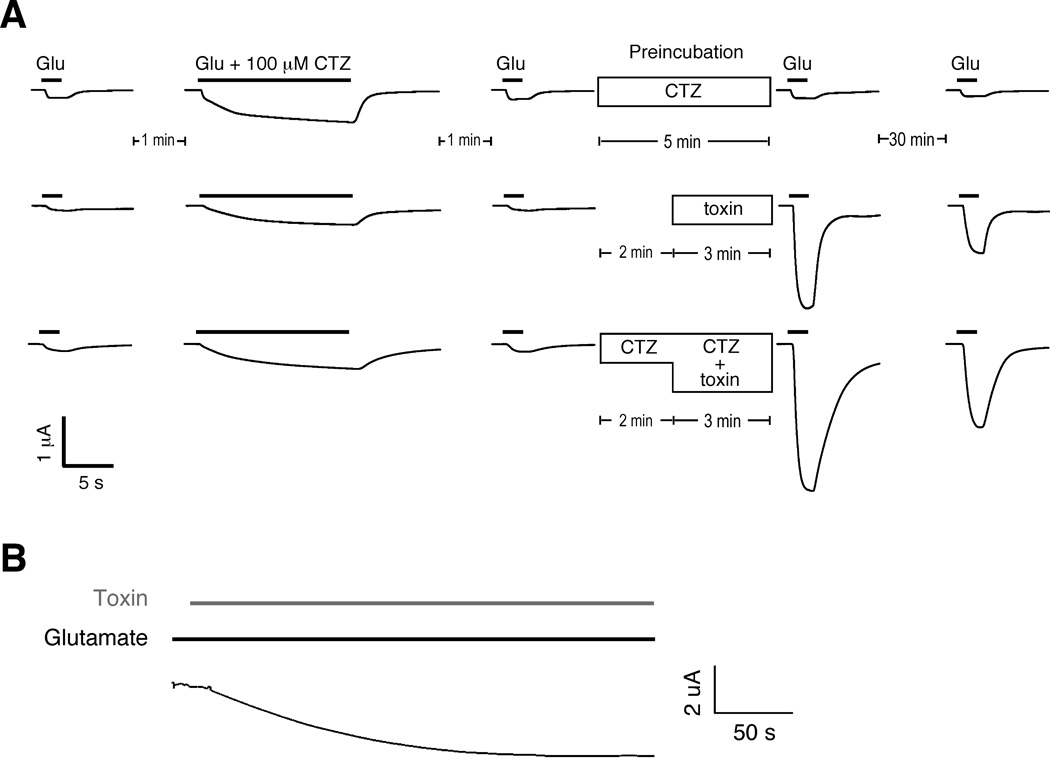
The non-specific drug CTZ is a well known modulator of desensitization of AMPARs [31], causing block of desensitization of GluA1(flip) receptors [29] and slowly potentiating AMPAR-mediated currents [24]. To address whether con-ikot-ikot and CTZ bound to a common site, we examined the effects of CTZ preincubation on con-ikot-ikot enhancement of GluA1(flip)-mediated current in Xenopus oocytes. We found that the effects of 6 µM con-ikot-ikot were long-lasting and that preincubation of toxin-treated oocytes with 100 µM CTZ did not block the effects of of con-ikot-ikot, although we did note an apparent decrease in the rate of deactivation when both drugs were applied (Figure 6A). These results indicate that the two drugs bind to different sites on the receptor. To determine whether con-ikot-ikot can open already desensitized GluA1 receptors, we first incubated oocytes in 1 mM glutamate to desensitize the receptors, and then added toxin in the continued presence of glutamate (Figure 6B). Toxin application was followed by a dramatic increase in current that reached maximal amplitude in approximately 3 min.
Figure 6.
Con-ikot-ikot blocks desensitization of GluA1 and increases current. (A) Glutamate-gated currents in Xenopus oocytes that expressed GluA1(flip) both before and after application of either toxin alone or toxin and 100 µM cyclothiazide (CTZ). (B) Con-ikot-ikot-induced current in Xenopus oocytes that expressed chronically desensitized GluA1(flip).
Con-ikot-ikot is active on native mammalian AMPARs
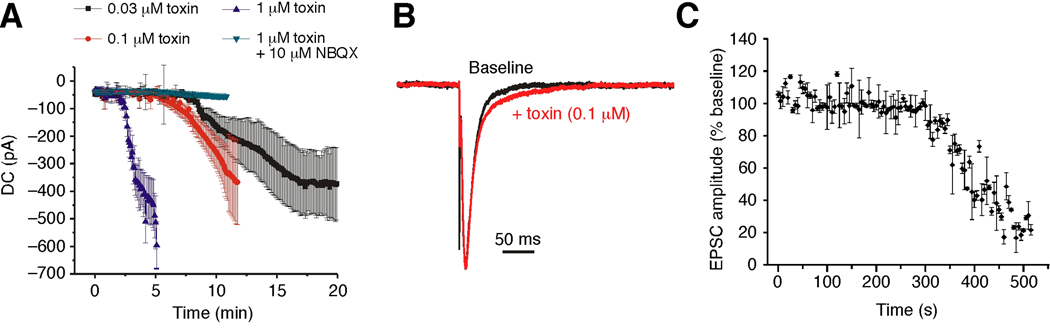
To examine whether the toxin had similar effects on native AMPARs in mammalian neurons, we used whole-cell patch clamp recordings to study CA1 pyramidal neurons in rat hippocampal slices. Bath application of 1 µM toxin resulted in a dramatic increase in direct current (DC) during voltage-clamp recordings that caused the loss of recordings within 5 minutes (Figure 7A). The timecourse of this increase was similar to that observed in ooctyes (Figure 6B). A less rapid increase in DC occurred with 0.1 µM toxin, but stable recordings were still lost after 10 −12 minutes. Lower toxin concentrations (0.03 µM) caused a smaller increase in DC that cells could tolerate. The effects of 1 µM toxin on DC were completely blocked by co-application of 10 µM NBQX demonstrating that the increase in DC caused by the toxin was mediated by AMPARs (Figure 7A). These findings demonstrate that the toxin caused massive AMPAR activation in CA1 pyramidal neurons. To address whether an increase in action potential frequency contributed to the change in DC, we repeated the experiment in the presence of tetrodotoxin (TTX), a blocker of Na+-dependent action potentials. We found that TTX did not block the toxin-induced change in DC (Supplementary Figure 2). We also monitored AMPAR-mediated excitatory postsynaptic currents (EPSCs) during toxin application to slices. We found that 0.1 µM toxin caused a depression of EPSC amplitude and a small prolongation of EPSC time course (weighted time constant from double exponential fits in the absence and presence of 0.1 µM toxin were 18.0 ± 0.9 ms and 24.5 ± 1.6 ms, respectively, P < 0.05, n = 4) (Figure 7B, C). The effect of toxin on EPSC time course could be due to the toxin blocking a component of AMPAR desensitization during EPSC decay and/or to a loss of voltage-clamp efficiency caused by the increase in DC.
Figure 7.
Con-ikot-ikot produces a standing AMPAR-mediated current in CA1 pyramidal neurons. (A) Direct current (DC) in whole-cell voltage-clamp recordings from CA1 pyramidal neurons in hippocampal slices during bath application of toxin (starting at 0 min) at concentrations indicated (n = 4 for 1 µM; n = 4 for 0.1 µM; n = 4 for 0.03 µM) and with the co-application of 1 µM toxin and 10 µM NBQX (n = 3). (B) EPSCs (scaled, averages of 12 traces) from an example experiment before and during toxin application. (C) AMPAR-mediated EPSC amplitude during application of 0.1 µM toxin (n = 4).
Con-ikot-ikot blocks desensitization of extrasynaptic AMPARs
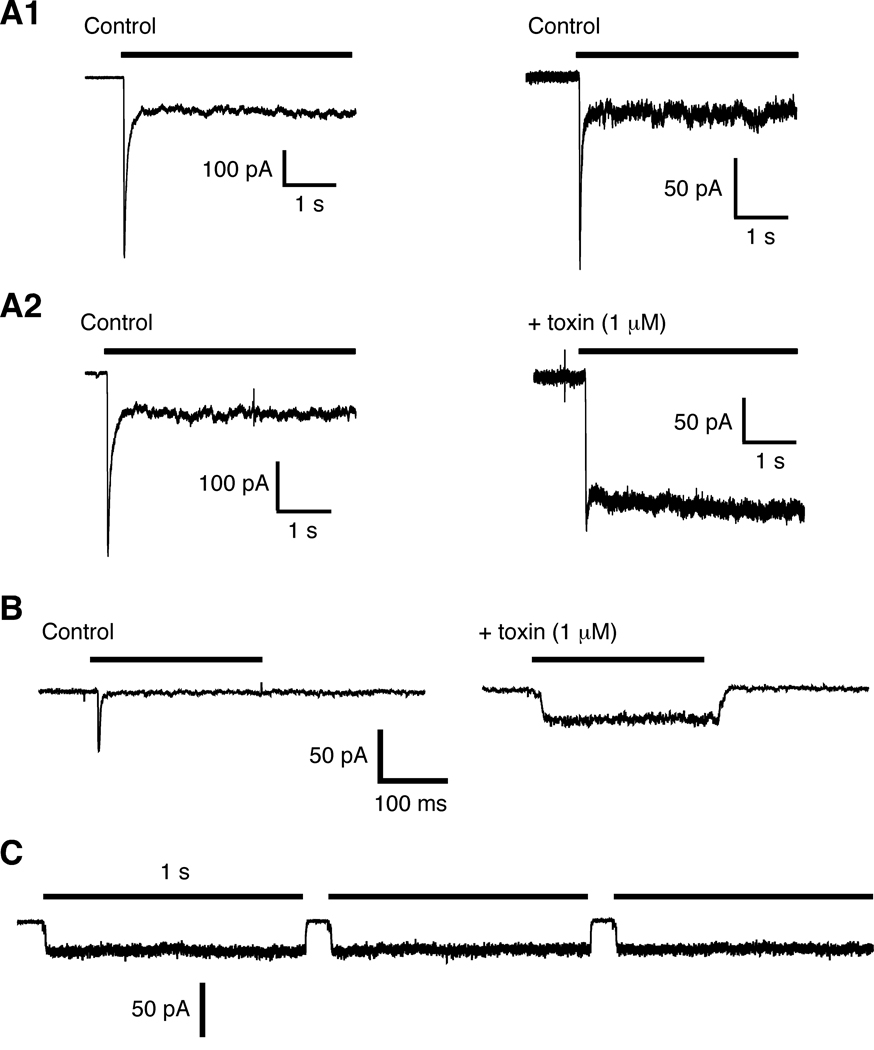
To directly examine the effect of con-ikot-ikot on AMPARs, we pulled outside-out patches from the soma of CA1 pyramidal cells and used rapid perfusion techniques to examine glutamate-gated currents. In the absence of con-ikot-ikot, the current desensitized within ms; however, following incubation with toxin desensitization was blocked (Figure 8A). Using the same techniques, we also studied GluA1 receptors expressed in HEK 293 cells. Again, we found that incubation with toxin blocked desensitization (Figure 8B), even during prolonged applications of glutamate (Figure 8C). The toxin also appeared to have a mild effect on the rate of receptor deactivation (Supplementary Figure 3).
Figure 8.
Con-ikot-ikot blocks desensitization of AMPARs. Rapid application of glutamate reveals rapid activation and subsequent desensitization in the continued presence of glutamate. (A) Currents in response to 10 mM glutamate from outside/out patches pulled from the soma of mouse hippocampal CA1 neurons. (A1) Control traces separated by 15 min (no toxin). (A2) Current before (left) and 15 minutes after (right) incubation with 1 µM con-ikot-ikot. (B and C) Currents in response to 3 mM glutamate from outside/out patches pulled from HEK 293 cells that expressed vertebrate GluA1 in the absence of toxin (control) and following incubation with toxin (B), and in response to repeated long duration applications of glutamate (C).
Discussion
Con-ikot-ikot is likely to play an important role in the prey capture strategy of Conus striatus given that it is a prominent component of the venom from this fish hunting cone snail. Conus striatus, one of the largest, most common species of piscivorous cone snails, is found from Hawaii to the Red Sea and East Africa. It aggressively stalks its fish prey, and can extend its proboscis at least 6 times the length of its shell. This allows large specimens of Conus striatus, which can reach up to 15 cm long, to strike their prey from a considerable distance. The immediate effect after the prey is struck is a rapid tetanic paralysis, resulting from a generalized excitotoxic shock [32]. These venom components acting together are called “the lightning strike cabal”. Our discovery of con-ikot-ikot reveals a previously unrecognized mechanistic aspect to the lightning-strike cabal. Con-ikot-ikot presumably acts on glutamatergic circuitry in the peripheral nervous system of fish with the likely targeted circuitry in the vestibular pathway. Depolarization of neurons by unblocking desensitization should cause hyperexcitability of the nervous system, leading to the rapid immobilization of the prey.
Our study of con-ikot-ikot’s action in the mammalian CNS demonstrates that the toxin produces a large sustained AMPAR-mediated current in neurons. This finding indicates that a population of AMPARs on CA1 pyramidal neurons that might be chronically desensitized becomes activated and passes current when toxin is present. However, we also show that the toxin does not activate receptors in the absence of glutamate, suggesting that either ambient levels of glutamate are higher than previously estimated [33], or that the toxin has additional effects, such as interfering with glutamate uptake, that lead to increased extracellular glutamate. The small effect of the toxin on AMPAR EPSC decay is consistent with other work that indicates that desensitization has only a minor role in determining the EPSC time course at CA1 synapses [34, 35]. Alternatively, the synapse may not be particularly accessible to the large peptide toxin. Other studies that used cyclothiazide to block AMPAR desensitization showed a prolongation of hippocampal AMPAR-mediated EPSCs [35–37]. However, this effect could also be attributed to the other known actions of cyclothiazide, e.g., enhancing and desynchronizing synaptic release [37], increasing AMPAR glutamate affinity [36, 38] or a slowing of deactivation [24].
Con-ikot-ikot also caused a large progressive depression of AMPA EPSC amplitude. This may be secondary to postsynaptic changes caused by the prolonged DC current. Alternatively, the toxin may cause activation of presynaptic AMPARs [39], which negatively regulate glutamate release and which are tonically desensitized by extracellular glutamate levels in the slice. To date, most modulators of AMPARs have been of limited therapeutic value. Con-ikot-ikot or soluble drugs modeled on con-ikot-ikot may provide powerful tools to vary the percentage of chronically desensitized receptors and alter synaptic transmission.
Experimental Procedures
Biochemistry
200 mg of crude venom isolated from C. striatus was purified by reverse phase HPLC using a binary buffer system (Buffer A: 0.1% TFA. Buffer B: 0.1% TFA, 90% acetonitrile). Gradients were 5–65% Buffer B, 30 minutes, followed by additional separation at 65–100% Buffer B, 10 minutes. All purifications were performed using Vydac C18 columns: preparative columns (22 mm x 25 cm, 15 µm particle size, 300 Å pore size), semi-preparative (10 mm x 25 cm, 5 µm particle size, 300 Å pore) or analytical columns (4.6 mm x 25 cm, 5 µm particle size, 300 Å pore size). Active fractions were further purified by two C18 separations from 23–45% B90 in 45 min and 25–40% B90 in 50 min. Purified peptide was sequenced by the University of Utah Core Facility using Edman N-terminal peptide sequencing. ESI/MS and MALDI TOF analysis was performed by the University of Utah Mass Spectrometry and Proteomics Core Facility. RNA was isolated from venom ducts by grinding with a mortar and pestle in ice cold RLT (RNeasy mini kit Qiagen). Primers designed to the con-ikot-ikot sequence were used to perform 5’ and 3’ RACE to identify the full cDNA sequence.
Recombinant con-ikot-ikot
cDNA encoding con-ikot-ikot was cloned in frame with thioredoxin pET32b+ (Novagen) (pCSW164). E. coli Rosetta-gami B (Novagen) containing pCSW164 were harvested 4 hrs after IPTG induction. Bacteria were lysed using an Avestin homoginizer in binding buffer (20 mM Phosphate 0.5 M NaCl, 20 mM imidazole pH7.4, and 1x Complete Protease Inhibitors (Roche)). FPLC purification of the protein fusion was performed using His-trap HP Nickel affinity column (GE healthcare). Recombinant protein was digested with enterokinase light chain (New England Biolabs: 2 mM Phosphate 50 mM NaCl 20 mM imidazole). The final purification was performed by reverse phase HPLC. Refolding experiments used a mixture of oxidized and reduced glutathione (1 mM GSSG, 2 mM GSH, 100 mM Tris, 10 mM EDTA pH7.5).
Protein electrophoresis
Samples were prepared by adding purified native protein to RIPA buffer (1% Triton X-100, 0.5% DOC, 0.1% SDS, 0.15 M NaCl) with 50 mM Tris pH 7.5 or 50 mM Tris pH 7.5 and 1 M NaCl, or 0.01 M Na-acetate pH4. Samples were incubated at 37 °C 1 hour or 4 °C for pH 4 samples. Equal volume of sample buffer (6% SDS) with or without DTT (10 mM) were added to samples and then boiled 10 min before being separated on 15% precast SDS gel (Biorad). Alternatively, samples were directly added to an equal volume of SDS buffer with or without DTT.
Reconstitution studies studies
Xenopus oocytes were injected with approximately 10 ng cRNA prepared from p59/2, GluA1(flop); GluA1(flip) plasmid; pRB14, GluA2; pRB312, GluA3; pK46, GluA4; U08261, GluN1; pNR2A251, GluN2A; pDM1318, GluA1 flip – LIVBP D60–471. Oocyte electrophysiological recordings were performed as described previously [40]. Currents recorded in response to 1 mM glutamate. HEK 293 cells were transfected with GluA1 (Figure 8) or GluA1 and stargazin (Supplementary Figure 3). Glutamate-gated currents were recorded using rapid piezo-driven exchange of solutions as described previously (exchange time < 1 ms) [8].
Hippocampal slices and recordings
2 week old Wistar rats were anesthetized with isoflurane and then decapitated in accordance with NIH animal care and use guidelines. Transverse hippocampal slices (400 µm thick) were cut in ice cold artificial cerebrospinal fluid (ACSF) containing (mM): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 9 MgSO4, 1 NaH2PO4, 26.2 NaHCO3, 11 glucose equilibrated with 95% O2 and 5% CO2. Slices were then allowed to recover for at least one hour in ACSF at room temperature (composition as above except for 1.3 mM MgSO4). Whole cell patch clamp recordings were made from visually identified CA1 pyramidal neurons in the presence of 50 µM picrotoxin at room temperature. The whole-cell solution contained (mM) 115 CsMeSO4, 20 CsCl2, 10 HEPES, 2.5 MgCl2, 4 NaATP, 0.4 NaGTP, 10 NaCreatine, and 0.6 EGTA (pH 7.2).
EPSCs were evoked by electrical stimulation of Schaffer collateral/commissural axons using a bipolar stimulating electrode placed in stratum radiatum of CA1 (0.2 Hz stimulation frequency). Cells were voltage-clamped at −70 mV. Recordings were performed using a Multiclamp 700B patch-clamp amplifier (Axon Instruments, Foster City, CA); signals were filtered at 4 kHz, digitized at 10 Hz and displayed and analyzed on-line using pClamp 9.2 (Axon Instruments). For fast agonist application, out-side-out patches were pulled from the soma of CA1 pyramidal cells. Currents were elicited in patches using rapid local perfusion of 10 mM glutamate by means of a gravity fed solution system controlled by a solenoid valve. Agonist was applied typically for 2 seconds with an estimated exchange time for agonist application across the patch of < 10 ms. EPSC decay kinetics were fit with a double exponential using OriginLab software (Northampton, MA) and are expressed as a weighted decay time constant. Statistical significance for the effects of toxin on EPSC decay time constant was tested using a student’s t-test. NBQX was obtained from Tocris Cookson.
Supplementary Material
Supplementary Figure 1. Dose-response relation. Following 5 minutes incubation in 6 µM con-ikot-ikot, glutamate-gated currents were recorded from Xenopus oocytes that expressed vertebrate GluA1.
Supplementary Figure 2. Con-ikot-ikot produces a standing AMPAR-mediated current in CA1 pyramidal neurons in the presence of TTX. Direct current (DC) in whole-cell voltage-clamp recordings from CA1 pyramidal neurons in hippocampal slices during bath application of 0.1 µM toxin. Bath solution contained 1 µM TTX.
Supplementary Figure 3. Con-ikot-ikot appears to modify the deactivation of GluA1 AMPARs. (A and B) Rapid application of 3 mM glutamate evoked currents in outside/out patches pulled from the soma of mouse hippocampal CA1 neurons (A), or HEK 293 cells (B) before and after application of 1 µM con-ikot-ikot.
Acknowledgements
We thank R. Shackman and C. Nielsen of the University of Utah Peptide Synthesis and Mass Spectrometry Facilities. This research was made possible by support from NIH Grants MH067747 (AVM) and GM48677 (BO), the NINDS Intramural program (JAM, JTRI), and a PRAT Fellowship (JAM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 2.Armstrong N, Sun Y, Chen GQ, Gouaux E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature. 1998;395:913–917. doi: 10.1038/27692. [DOI] [PubMed] [Google Scholar]
- 3.Mayer ML. Glutamate receptors at atomic resolution. Nature. 2006;440:456–462. doi: 10.1038/nature04709. [DOI] [PubMed] [Google Scholar]
- 4.Hansen KB, Yuan H, Traynelis SF. Structural aspects of AMPA receptor activation, desensitization and deactivation. Curr Opin Neurobiol. 2007;17:281–288. doi: 10.1016/j.conb.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- 6.Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Y, Mellem JE, Brockie PJ, Madsen DM, Maricq AV. SOL-1 is a CUB-domain protein required for GLR-1 glutamate receptor function in C. elegans. Nature. 2004;427:451–457. doi: 10.1038/nature02244. [DOI] [PubMed] [Google Scholar]
- 8.Walker CS, Brockie PJ, Madsen DM, Francis MM, Zheng Y, Koduri S, Mellem JE, Strutz-Seebohm N, Maricq AV. Reconstitution of invertebrate glutamate receptor function depends on stargazin-like proteins. Proc Natl Acad Sci U S A. 2006;103:10781–10786. doi: 10.1073/pnas.0604482103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker CS, Francis MM, Brockie PJ, Madsen DM, Zheng Y, Maricq AV. Conserved SOL-1 proteins regulate ionotropic glutamate receptor desensitization. Proc Natl Acad Sci U S A. 2006;103:10787–10792. doi: 10.1073/pnas.0604520103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y, Brockie PJ, Mellem JE, Madsen DM, Walker CS, Francis MM, Maricq AV. SOL-1 is an auxiliary subunit that modulates the gating of GLR-1 glutamate receptors in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2006;103:1100–1105. doi: 10.1073/pnas.0504612103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng D, Pitcher GM, Szilard RK, Sertie A, Kanisek M, Clapcote SJ, Lipina T, Kalia LV, Joo D, McKerlie C, et al. Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLoS Biol. 2009;7:e41. doi: 10.1371/journal.pbio.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W, St-Gelais F, Grabner CP, Trinidad JC, Sumioka A, Morimoto-Tomita M, Kim KS, Straub C, Burlingame AL, Howe JR, et al. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron. 2009;61:385–396. doi: 10.1016/j.neuron.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwenk J, Harmel N, Zolles G, Bildl W, Kulik A, Heimrich B, Chisaka O, Jonas P, Schulte U, Fakler B, et al. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science. 2009;323:1313–1319. doi: 10.1126/science.1167852. [DOI] [PubMed] [Google Scholar]
- 15.Borgdorff AJ, Choquet D. Regulation of AMPA receptor lateral movements. Nature. 2002;417:649–653. doi: 10.1038/nature00780. [DOI] [PubMed] [Google Scholar]
- 16.Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- 17.Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron. 2005;48:977–985. doi: 10.1016/j.neuron.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 18.Heine M, Groc L, Frischknecht R, Beique JC, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–205. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang R, Walker CS, Brockie PJ, Francis MM, Mellem JE, Madsen DM, Maricq AV. Evolutionary conserved role for TARPs in the gating of glutamate receptors and tuning of synaptic function. Neuron. 2008;59:997–1008. doi: 10.1016/j.neuron.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rouach N, Byrd K, Petralia RS, Elias GM, Adesnik H, Tomita S, Karimzadegan S, Kealey C, Bredt DS, Nicoll RA. TARP gamma-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nat Neurosci. 2005;8:1525–1533. doi: 10.1038/nn1551. [DOI] [PubMed] [Google Scholar]
- 21.Kato AS, Siuda ER, Nisenbaum ES, Bredt DS. AMPA receptor subunit-specific regulation by a distinct family of type II TARPs. Neuron. 2008;59:986–996. doi: 10.1016/j.neuron.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 22.Deng L, Chen G. Cyclothiazide potently inhibits gamma-aminobutyric acid type A receptors in addition to enhancing glutamate responses. Proc Natl Acad Sci U S A. 2003;100:13025–13029. doi: 10.1073/pnas.2133370100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishikawa T, Takahashi T. Mechanisms underlying presynaptic facilitatory effect of cyclothiazide at the calyx of Held of juvenile rats. J Physiol. 2001;533:423–431. doi: 10.1111/j.1469-7793.2001.0423a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fucile S, Miledi R, Eusebi F. Effects of cyclothiazide on GluR1/AMPA receptors. Proc Natl Acad Sci U S A. 2006;103:2943–2947. doi: 10.1073/pnas.0511063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olivera BM. E.E. Just Lecture, 1996. Conus venom peptides, receptor and ion channel targets, and drug design: 50 million years of neuropharmacology. Mol Biol Cell. 1997;8:2101–2109. doi: 10.1091/mbc.8.11.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terlau H, Olivera BM. Conus venoms: a rich source of novel ion channel-targeted peptides. Physiol Rev. 2004;84:41–68. doi: 10.1152/physrev.00020.2003. [DOI] [PubMed] [Google Scholar]
- 27.Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craig AG, Bandyopadhyay P, Olivera BM. Post-translationally modified neuropeptides from Conus venoms. Eur J Biochem. 1999;264:271–275. doi: 10.1046/j.1432-1327.1999.00624.x. [DOI] [PubMed] [Google Scholar]
- 29.Partin KM, Fleck MW, Mayer ML. AMPA receptor flip/flop mutants affecting deactivation, desensitization, and modulation by cyclothiazide, aniracetam, and thiocyanate. J Neurosci. 1996;16:6634–6647. doi: 10.1523/JNEUROSCI.16-21-06634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasternack A, Coleman SK, Jouppila A, Mottershead DG, Lindfors M, Pasternack M, Keinanen K. Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor channels lacking the N-terminal domain. J Biol Chem. 2002;277:49662–49667. doi: 10.1074/jbc.M208349200. [DOI] [PubMed] [Google Scholar]
- 31.Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets. 2007;8:583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- 32.Terlau H, Shon KJ, Grilley M, Stocker M, Stuhmer W, Olivera BM. Strategy for rapid immobilization of prey by a fish-hunting marine snail. Nature. 1996;381:148–151. doi: 10.1038/381148a0. [DOI] [PubMed] [Google Scholar]
- 33.Herman MA, Jahr CE. Extracellular glutamate concentration in hippocampal slice. J Neurosci. 2007;27:9736–9741. doi: 10.1523/JNEUROSCI.3009-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jonas P, Spruston N. Mechanisms shaping glutamate-mediated excitatory postsynaptic currents in the CNS. Curr Opin Neurobiol. 1994;4:366–372. doi: 10.1016/0959-4388(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 35.Hjelmstad GO, Isaac JT, Nicoll RA, Malenka RC. Lack of AMPA receptor desensitization during basal synaptic transmission in the hippocampal slice. J Neurophysiol. 1999;81:3096–3099. doi: 10.1152/jn.1999.81.6.3096. [DOI] [PubMed] [Google Scholar]
- 36.Yamada KA, Tang CM. Benzothiadiazides inhibit rapid glutamate receptor desensitization and enhance glutamatergic synaptic currents. J Neurosci. 1993;13:3904–3915. doi: 10.1523/JNEUROSCI.13-09-03904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diamond JS, Jahr CE. Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC. Neuron. 1995;15:1097–1107. doi: 10.1016/0896-6273(95)90098-5. [DOI] [PubMed] [Google Scholar]
- 38.Patneau DK, Vyklicky L, Jr, Mayer ML. Hippocampal neurons exhibit cyclothiazide-sensitive rapidly desensitizing responses to kainate. J Neurosci. 1993;13:3496–3509. doi: 10.1523/JNEUROSCI.13-08-03496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schenk U, Matteoli M. Presynaptic AMPA receptors: more than just ion channels? Biol Cell. 2004;96:257–260. doi: 10.1016/j.biolcel.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Strutz-Seebohm N, Werner M, Madsen DM, Seebohm G, Zheng Y, Walker CS, Maricq AV, Hollmann M. Functional analysis of Caenorhabditis elegans glutamate receptor subunits by domain transplantation. J Biol Chem. 2003;278:44691–44701. doi: 10.1074/jbc.M305497200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Dose-response relation. Following 5 minutes incubation in 6 µM con-ikot-ikot, glutamate-gated currents were recorded from Xenopus oocytes that expressed vertebrate GluA1.
Supplementary Figure 2. Con-ikot-ikot produces a standing AMPAR-mediated current in CA1 pyramidal neurons in the presence of TTX. Direct current (DC) in whole-cell voltage-clamp recordings from CA1 pyramidal neurons in hippocampal slices during bath application of 0.1 µM toxin. Bath solution contained 1 µM TTX.
Supplementary Figure 3. Con-ikot-ikot appears to modify the deactivation of GluA1 AMPARs. (A and B) Rapid application of 3 mM glutamate evoked currents in outside/out patches pulled from the soma of mouse hippocampal CA1 neurons (A), or HEK 293 cells (B) before and after application of 1 µM con-ikot-ikot.