Abstract
The present report describes two female patients aged 39 and 57 years who experienced loss of consciousness and chest pain due to high-grade atrioventricular block. Both patients demonstrated noncontraction centred on the cardiac apex and excessive contraction at the cardiac base on cardiac ultrasonography and left ventriculography, but neither of them demonstrated any significant stenotic lesions on coronary angiography. Furthermore, neither patient showed elevated serum biomarkers of cardiac injury or serum viral antibodies. In a repeat left ventriculogram two weeks later, the left ventricular wall motion disorder had improved in both patients. Based on these findings, the patients were diagnosed with takotsubo cardiomyopathy. Because the high-grade atrioventricular conduction disorder did not improve in spite of the improvement of left ventricular wall motion disorder, permanent pacemaker implantation was performed. It is extremely rare for takotsubo cardiomyopathy to be complicated by high-grade atrioventricular block. In the present study, both patients had takotsubo cardiomyopathy complicated by high-grade atrioventricular block and eventually underwent permanent pacemaker implantation.
Keywords: High-grade atrioventricular block, Permanent pacemaker implantation, Takotsubo cardiomyopathy
Takotsubo cardiomyopathy may be stress induced, and is characterized by noncontraction centred on the cardiac apex and compensatory excessive contraction at the cardiac base. Because the end-systolic shape of the heart on a left ventriculogram is similar to a takotsubo (Japanese for ‘octopus trap’), this condition was named takotsubo cardiomyopathy.
Severe cases in the acute stage of takotsubo cardiomyopathy have been complicated by torsades de pointes and accompanied by QT interval prolongation (1). However, in most cases, the myocardial damage is transient and improves dramatically in the short term, showing favourable prognosis. Furthermore, takotsubo cardiomyopathy mainly occurs with wall motion disorder and with no abnormality in the stimulus conduction pathway.
In the present study, two patients with takotsubo cardiomyopathy complicated by high-grade atrioventricular conduction disorder, and in whom the conduction disorder did not improve in spite of the dramatic improvement of myocardial damage, are described. Both patients eventually underwent permanent pacemaker implantation.
CASE PRESENTATION
Case 1
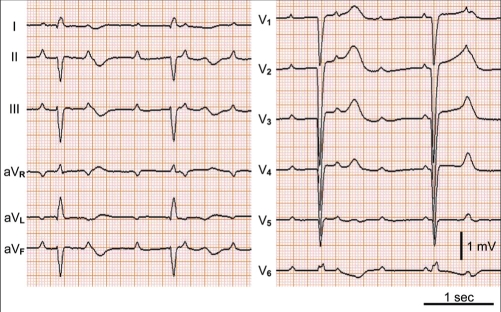
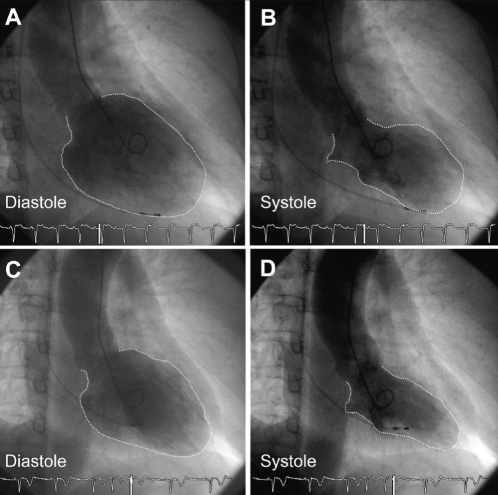
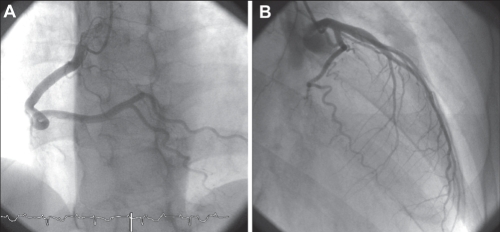
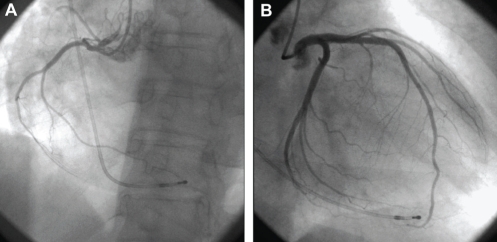
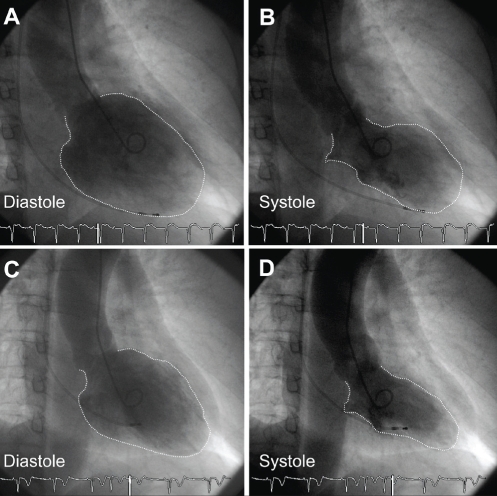
A 39-year-old woman was hospitalized for loss of consciousness. On admission to the Fukuoka University Chikushi Hospital (Fukuoka, Japan), the patient had a clear sensorium, a blood pressure of 97/52 mmHg and a pulse rate of 50 beats/min. Blood biochemical tests found normal levels of cardiac enzymes and a test for inflammatory markers was negative. Her serum viral antibodies were negative (Table 1), making myocardial infarction and viral myocarditis unlikely. A chest x-ray showed neither cardiac enlargement or pulmonary congestion. Figure 1 is the patient’s electrocardiogram at the time of admission showing complete atrioventricular block and a ventricular escape rhythm with a pulse rate of 35 beats/min. In cardiac ultrasonography, noncontraction at the left ventricular cardiac apex and excessive contraction at the cardiac base were recognized. A left ventriculogram showed similar findings (Figure 2). On the other hand, no significant stenotic lesions were recognized on coronary angiography (Figure 3). Based on these findings, the patient was diagnosed with takotsubo cardiomyopathy. For the treatment of atrioventricular block, a temporary pacemaker was inserted. In a left ventriculogram performed again two weeks later, the wall motion at the cardiac apex had become normal (Figure 2). However, the atrioventricular block was persistent even in the recovery stage of cardiomyopathy, making it necessary to perform permanent pacemaker implantation.
TABLE 1.
Cardiac enzymes, inflammatory markers and serum viral antibodies (on admission and after two weeks) in both case 1 and case 2
|
Case 1 |
Case 2 |
|||
|---|---|---|---|---|
| On admission | After 2 weeks | On admission | After 2 weeks | |
| WBC count (normal range 3.5×109/L to 9×109/L) | 12.9 | 6.2 | 6.6 | 4.6 |
| AST (normal range 13 U/L to 33 U/L) | 30 | 20 | 64 | 32 |
| ASL (normal range 6 U/L to 30 U/L) | 54 | 22 | 46 | 17 |
| LDH (normal range 119U/L to 229 U/L) | 168 | 204 | 236 | 186 |
| CK (normal range 45 U/L to 163 U/L) | 72 | 41 | 114 | 34 |
| C-reactive protein (mg/L) | 0.1 | 1.4 | 2.2 | 4.5 |
| Adenovirus | 4 | 4 | 4 | 4 |
| Measles virus | 4 | 4 | 4 | 4 |
| Cytomegalovirus | 128 | 128 | 128 | 128 |
| Coxsackie virus | 4 | 4 | 4 | 4 |
| Herpes virus | 4 | 4 | 4 | 8 |
ASL Argininosuccinate lyase; AST Aspartate aminotransferase; CK Creatine kinase; LDH Lactate dehydrogenase; WBC White blood cell
Figure 1).
Case 1. Electrocardiogram showing complete atrioventricular block and a ventricular escape rhythm with a heart rate of 35 beats/min
Figure 2).
Case 1. Left ventriculogram performed on admission (A,B) and two weeks after admission (C,D). B End-systolic phase ventriculogram demonstrating the typical apical and midventricular wall motion abnormalities characteristic of takotsubo cardiomyopathy. C,D Ventriculogram two weeks later demonstrating normalization of left ventricular wall motion
Figure 3).
Case 1. Coronary angiography showing a normal coronary artery of the right coronary artery (A) and left coronary artery (B)
Case 2
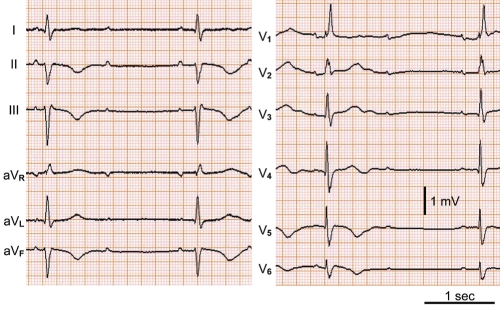
An originally healthy 57-year-old woman was admitted to the hospital because of dizziness and persistent chest pain followed by a 2 min to 3 min loss of consciousness. On admission, the patient had a clear sensorium, a blood pressure of 122/72 mmHg and a pulse rate of 35 beats/min. Similar to case 1, blood biochemical tests found no definitive abnormal findings, and tests for serum viral antibodies were negative (Table 1), making myocardial infarction and viral myocarditis unlikely. In a chest x-ray, cardiothoracic ratio was 53%, protrusion was seen at the right second arch, and left third and fourth arches, and a slight increase of hilar area shadow was recognized. On the other hand, the electrocardiogram showed atrioventricular block of 2:1, and T wave negative conversion (leads II, III, aVF, V5, V6) was recognized (Figure 4). Similar to case 1, no significant stenotic lesions were recognized on coronary angiography (Figure 5). However, on cardiac ultrasonography and left ventriculography, wide-range noncontraction at the cardiac apex and excessive contraction at the cardiac base were recognized (Figure 6). Based on these findings, takotsubo cardiomyopathy was diagnosed. For the treatment of atrioventricular block, a temporary pacemaker was inserted. In a left ventriculogram performed two weeks after admission, the decreased wall motion at the cardiac apex had showed clear improvement (Figure 6). However, the atrioventricular block was persistent in spite of the improvement of cardiomyopathy, making it necessary to perform permanent pacemaker implantation.
Figure 4).
Case 2. Electrocardiogram showing atrioventricular block of 2:1 and T wave negative conversion in leads of II, III, aVF, V5 and V6
Figure 5).
Case 2. Coronary angiography showing a normal coronary artery of the right coronary artery (A) and left coronary artery (B)
Figure 6).
Case 2. Left ventriculogram performed on admission (A,B) and two weeks after admission (C,D). B End-systolic phase ventriculogram demonstrating the typical apical and midventricular wall motion abnormalities characteristic of takotsubo cardiomyopathy. C,D Ventriculogram two weeks later demonstrating normalization of left ventricular wall motion
DISCUSSION
Takotsubo cardiomyopathy is characterized by noncontraction centred on the left ventricular cardiac apex and compensatory excessive contraction at the cardiac base in the acute phase. Its name was derived from the fact that the end-systolic shape of the heart on a left ventriculogram is similar to a takotsubo (Japanese for ‘octopus trap’). The onset of takotsubo cardiomyopathy is similar to acute myocardial infarction, but differs in the site of left ventricular wall motion disorder and prognosis. In other words, while myocardial infarction triggers myocardial wall motion disorder in the region supported by the obstructed coronary artery, takotsubo cardiomyopathy shows no significant stenosis in the coronary artery and the wall motion disorder range exceeds the region supported by the coronary artery branch (2). Also, noncontraction centred on the left ventricular cardiac apex and excessive contraction at the cardiac base are observed in takotsubo cardiomyopathy. The wall motion disorder improves dramatically within two weeks, similar to ‘stunned myocardium’ in acute ischemia, and the prognosis is favourable.
Takotsubo cardiomyopathy is often seen after or with subarachnoid hemorrhage (3), pheochromocytoma (4), noncardiac surgical operation in the elderly (5), Guillain-Barré syndrome (6), rapid administration of a large dose of catecholamine (7) and violent mental shock (8). The involvement of a catecholamine is suggested, but in most cases, they are not involved as precursor events.
Catecholamines enhance contractility and excitability of myocardial cells; however, the persistent exposure of the myocardium to high concentrations of noradrenaline induces cardiomyopathy (9). Possible causes include blood flow disturbance mediated by alpha-receptors (10,11) and ‘stunned myocardium’ due to multiple-branch coronary artery spasm (2). Animal experiments (12) have shown that the alpha-receptor-mediated action of noradrenaline induces coronary artery spasms and cardiomyopathy, and diffuse multiple-branch coronary artery spasms were induced in the acute-phase induction experiment. However, it is not clear whether the cardiomyopathy is caused by the direct action of the wall motion disorder on the alpha-receptors of myocardial cells or by vasoconstriction mediated by the alpha-receptors residing in the coronary artery wall. Pathologically, contraction band necrosis (7) and myocardial focal myocytolysis (3) were observed. This cardiomyopathy is manifested most conspicuously in patients with phenochromocytoma. Furthermore, the catecholamine cardiomyopathy accompanying phenochromocytoma may become a dilated myocardiopathy; thus, takotsubo cardiomyopathy is occasionally referred to as an acute and transient catecholamine cardiomyopathy.
As mentioned above, takotsubo cardiomyopathy is a disease status based mainly on the presence of ventricular wall motion disorder and is not usually associated with a conduction pathway disorder. However, our two patients experienced takotsubo cardiomyopathy complicated by atrioventricular conduction disorder. The mechanism of takotsubo cardiomyopathy is unclear. A possible mechanism is via the frequent occurrence of diffuse spasms of the multiple-branch coronary arteries, especially the right coronary arteries – the continual ischemic status may lead to atrioventricular conduction disorder, conduction pathway fibrosis, etc. Usually, the myocardial damage of takotsubo cardiomyopathy is transient and has a favourable prognosis. The atrioventricular conduction disorder seen concomitantly in these patients was expected to improve rapidly. However, even in the recovery stage in which the wall motion disorder normalized, the high-grade atrioventricular conduction disorder did not improve, making it necessary to perform permanent pacemaker implantation.
REFERENCES
- 1.Elkhateeb OE, Beydoun HK. Recurrent long QT syndrome and syncope in transient apical ballooning syndrome (takotsubo cardiomyopathy) Can J Cardiol. 2008;24:917–9. doi: 10.1016/s0828-282x(08)70700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dote K. Five patients with extensive myocardial stunning in diffuse coronary spasm. J Cardiol. 1991;21:213–4. [Google Scholar]
- 3.Sakamoto H, Nishimura H, Imataka K, Ieki K, Horie T, Fujii J. Abnormal Q wave, ST-segment elevation, T-wave inversion, and widespread focal myocytolysis associated with subarachnoid hemorrhage. Jpn Circ J. 1996;60:254–7. doi: 10.1253/jcj.60.254. [DOI] [PubMed] [Google Scholar]
- 4.Yamanaka O, Yasumasa F, Nakamura T, et al. “Myocardial stunning”-like phenomenon during a crisis of pheochromocytoma. Jpn Circ J. 1994;58:737–42. doi: 10.1253/jcj.58.737. [DOI] [PubMed] [Google Scholar]
- 5.Tokioka M, Miura H, Masaoka Y, et al. Transient appearance of asynergy on the echocardiogram and electrocardiographic changes simulating acute myocardial infarction following non-cardiac surgery. J Cardiogr. 1985;15:639–53. [PubMed] [Google Scholar]
- 6.Iga K, Himura Y, Izumi C, et al. Reversible left ventricular dysfunction associated with Guillain-Barre syndrome – an expression of catecholamine cardiotoxicity? Jpn Circ J. 1995;59:236–40. doi: 10.1253/jcj.59.236. [DOI] [PubMed] [Google Scholar]
- 7.Annzai H. One patient with stunned myocardium in catecholamine induced acute myocardial damage and confirmation this pathologic findings. Kokyu To Junkan. 1996;44:199–204. [Google Scholar]
- 8.Kadota K. Analysis of myocardial stunning similar to acute extensive anteroseptal myocardial infarction. Jpn Int Med J. 1996;85:102. [Google Scholar]
- 9.Reichenbach DD, Benditt EP. Catecholamines and cardiomyopathy: The pathogenesis and potential importance of myofibrillar degeneration. Hum Pathol. 1970;1:125–50. [Google Scholar]
- 10.Downing SE, Lee JC. Contribution of alpha-adrenoreceptor activity to the pathogenesis of norepinephrine cardiomyopathy. Circ Res. 1983;52:471–8. doi: 10.1161/01.res.52.4.471. [DOI] [PubMed] [Google Scholar]
- 11.Simons M, Downing SE. Coronary vasoconstriction and catecholamine cardiomyopathy. Am Heart J. 1985;109:297–304. doi: 10.1016/0002-8703(85)90597-6. [DOI] [PubMed] [Google Scholar]
- 12.Sato H. A stunned myocardium of “octopus pot type” with multi coronary spasm. Sci Rev. 1990:56–64. [Google Scholar]