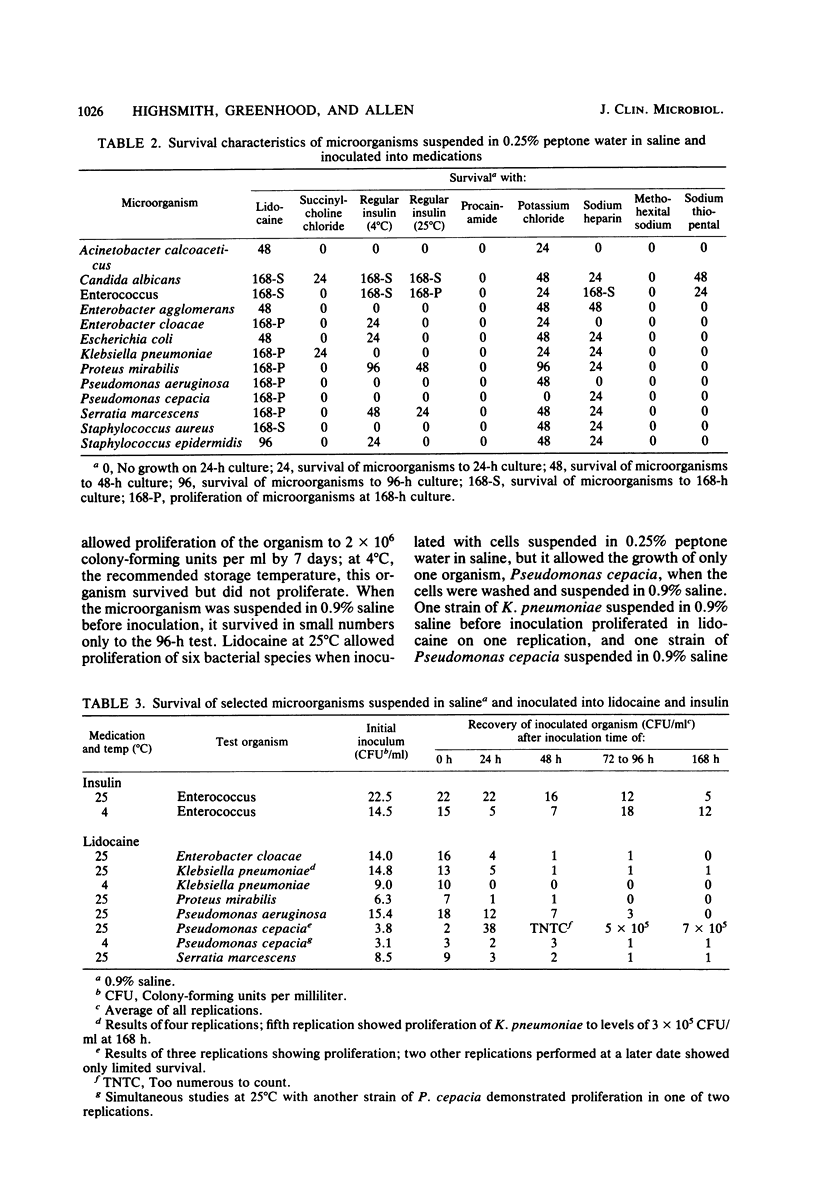
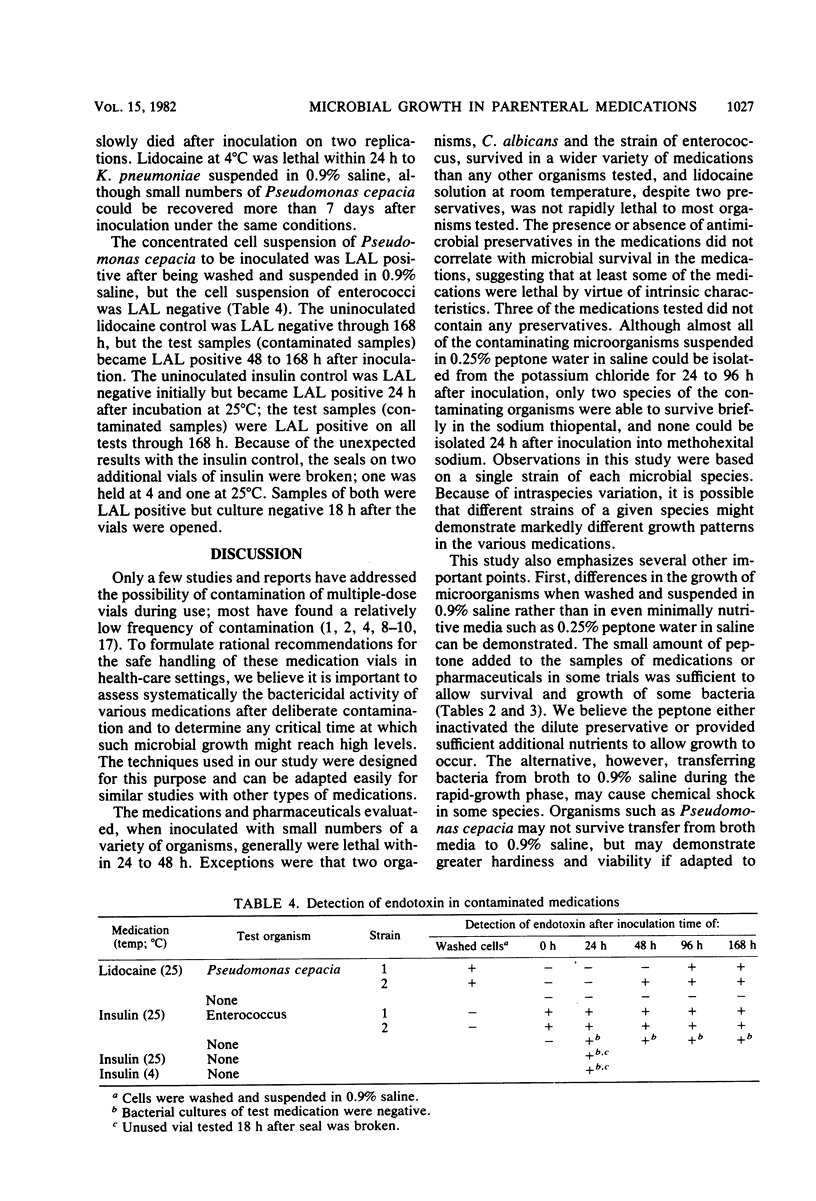
Abstract
The extent to which microbial contamination of medications dispensed in multiple-dose vials might serve as a source of infection to patients has not been fully investigated. To characterize the effects of microbial contamination, we studied the growth-supporting properties of eight medications dispensed in multiple-dose vials. Two medications, procainamide and methohexital, demonstrated no survival of any microbes 24 h after inoculation. Succinylcholine chloride, regular insulin, potassium chloride, heparin, and thiopental slowly killed or allowed limited survival of several of the microorganisms used as contaminants. Lidocaine allowed survival or proliferation of several microbial strains suspended in 0.25% peptone water in saline, but slowly killed all strains except Pseudomonas cepacia suspended in 0.9% saline. Endotoxin, measured by the Limulus amebocyte lysate assay, was found in the two medications tested, lidocaine contaminated with Pseudomonas cepacia and insulin contaminated with enterococci. Inadvertent microbial contamination of at least some parenteral medications in multiple-dose vials may result in the exposure of patients to viable organisms. The potential, however, for medications such as lidocaine to support growth of organisms under selected circumstances should be noted by those responsible for preparing and administering these drugs. The potential hazard to patients from endotoxin in contaminated medications under these circumstances has not been assessed. Additional studies of this type should be pursued to provide more complete information about the risk of microbial contamination of products for parenteral use.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bothe J. Study shows contamination in multiple-dose vials. AORN J. 1973 May;17(5):111–114. doi: 10.1016/s0001-2092(07)60791-1. [DOI] [PubMed] [Google Scholar]
- Corley C. E., Manos J. P., Thomas J. D. Multiple dose vials: a source of contamination? J S C Med Assoc. 1968 Nov;64(11):461–464. [PubMed] [Google Scholar]
- Highsmith A. K., Anderson R. L., West C. M., Dixon R. E. Characterization of an endotoxin-like substance recovered from an intravenous antibiotic solution involving pyrogenic reactions. Prog Clin Biol Res. 1979;29:465–471. [PubMed] [Google Scholar]
- KOHAN S., CARLIN H., WHITEHEAD R. A study of contamination of multiple-dose medication vials. Hospitals. 1962 Jul 16;36:78–78. [PubMed] [Google Scholar]
- Lehmann C. R. Effect of refrigeration on bactericidal activity of four preserved multiple-dose injectable drug products. Am J Hosp Pharm. 1977 Nov;34(11):1196–1200. [PubMed] [Google Scholar]
- Maki D. G., Rhame F. S., Mackel D. C., Bennett J. V. Nationwide epidemic of septicemia caused by contaminated intravenous products. I. Epidemiologic and clinical features. Am J Med. 1976 Apr;60(4):471–485. doi: 10.1016/0002-9343(76)90713-0. [DOI] [PubMed] [Google Scholar]
- Meers P. D., Calder M. W., Mazhar M. M., Lawrie G. M. Intravenous infusion of contaminated dextrose solution: The Deveonport incident. Lancet. 1973 Nov 24;2(7839):1189–1192. doi: 10.1016/s0140-6736(73)92950-4. [DOI] [PubMed] [Google Scholar]
- Petty W. C., Heggers J. P., Shelton D. F., Mendenhall M. K. Viral and bacterial contamination of multiple-dose drug vials kept in anesthesia machines. Anesthesiology. 1969 Apr;30(4):465–470. doi: 10.1097/00000542-196904000-00019. [DOI] [PubMed] [Google Scholar]
- ROSENZWEIG A. L. POTENTIAL HEALTH HAZARDS IN THE MULTIPLE-DOSE VIAL. Hospitals. 1964 Jun 16;38:71–74. [PubMed] [Google Scholar]
- Ridgway J. F., McAuliff W. K. The sterility of multiple-dose vials in challenged. Hosp Manage. 1967 Jul;104(1):67–72. [PubMed] [Google Scholar]
- Spalter J., JACKSON M., Matz R. Bacterial in medications. Growth during emergency resuscitation. N Y State J Med. 1976 May;76(5):693–694. [PubMed] [Google Scholar]
- Steere A. C., Tenney J. H., Mackel D. C., Snyder M. J., Polakavetz S., Dunne M. E., Dixon R. Pseudomonas species bacteremia caused by contaminated normal human serum albumin. J Infect Dis. 1977 May;135(5):729–735. doi: 10.1093/infdis/135.5.729. [DOI] [PubMed] [Google Scholar]
- Walton J. R., Shapiro B. A., Harrison R. A., Davidson R., Reisberg B. E. Serratia bacteremia from mean arterial pressure monitors. Anesthesiology. 1975 Jul;43(1):113–114. doi: 10.1097/00000542-197507000-00027. [DOI] [PubMed] [Google Scholar]
- YOUNG J. A., COLLETTE T. S., BREHM W. F. Sterility of multiple dose vials after repeated use. Am Surg. 1958 Nov;24(11):811–814. [PubMed] [Google Scholar]



