The Canadian Association of Gastroenterology’s (CAG) Endoscopy Quality Initiative (EQI) was developed in 2007 to promote quality assurance and quality improvement, and to facilitate these activities in becoming standard of care for endoscopy services in Canada. To achieve these goals, the Steering Committee of the EQI has established a quality assurance program based on two essential tools: the Global Rating Scale (GRS) and the EQI practice audit.
The GRS is a scale designed for periodic, repeated use within the endoscopy unit to provide an assessment of the quality of endoscopy services and to guide quality improvement efforts. Its key construct of quality is that of a patient-centred service. Use of the GRS is supported by a dedicated Internet site for data entry, tracking of progress, action planning and access to a large electronic library of resources and case studies. Since its creation in 2004 by Dr Roland Valori and colleagues, the GRS has enjoyed rapid uptake and success in the United Kingdom (UK); this was paralleled by dramatic wait time reductions within the National Health Service. EQI participants are expected to complete the GRS biannually and to perform activities that will improve their GRS rating over time. To support participation in Canada, the CAG, with the help of the UK’s GRS leadership, has developed the Canadian GRS Web site. In addition, in May 2008, CAG sponsored a series of workshops at several Canadian centres led by Debbie Johnston, a member of the UK’s National Endoscopy Committee and co-author of the GRS.
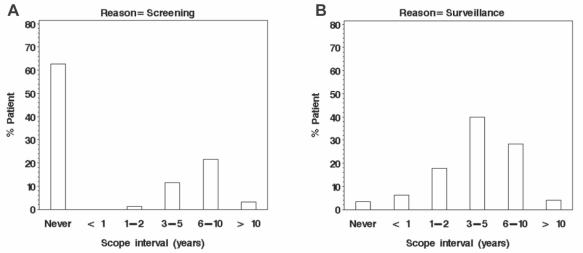
A pilot practice audit on colonoscopy was completed in 2007, with findings reported at the Canadian Digestive Diseases Week (CDDW) 2008 meeting (1). This pilot audit formed the basis for the current EQI colonoscopy practice audit, the preliminary results from which were presented at CDDW 2009 (Figure 1) (2–4). As of May 2009, more than 60 endoscopists from 21 sites across the country have provided data on 1260 colonoscopies.
Figure 1.
Screening (A) and surveillance (B) intervals from 822 colonoscopies recorded in the Endoscopy Quality Initiative practice audit. Data adapted from reference 4
This database, collected in real-time on smartphones, provides a comprehensive profile of the patient encounter including demographics, indication for the procedure, quality of the procedure, findings and any complications. The reporting site allows individual endoscopists to review their own data and compare with national data; individuals will also have the ability to longitudinally track improvements.
Members of the EQI Steering Committee attended the American Society for Gastrointestinal Endoscopy (ASGE) ‘Improving Quality and Safety in Your Endoscopy Unit’ Course, which is part of the ASGE Endoscopy Recognition Program, in October 2008, and future collaboration with the ASGE and World Organization of Digestive Endoscopy quality programs are in the planning stages.
Funding is a major hurdle for a national quality initiative. The CAG EQI has recently received support from The Canadian Partnership Against Cancer. Since most provinces are now planning or implementing a colorectal cancer screening program, it is anticipated that initiatives such as the EQI, together with screening programs, will have a synergistic effect on the quality of endoscopy services and the adoption of quality assurance activities.
The EQI was introduced at the gastroenterology fellow endoscopy course at McMaster University (Hamilton, Ontario) in July 2008, and was well received by the next generation of endoscopists.
It is abundantly clear that nationally and around the world, endoscopy stakeholders are catching the quality wave.
A national consensus meeting on quality and safety indicators for endoscopy in Canada is planned for 2010.
Previously, in this article we outlined the aims of the EQI:
-
Demonstrate a mechanism for continuous quality improvement in endoscopy.
The Canadian GRS Web site is up and running. More than 60 endoscopists from 21 centres have contributed data on 1260 colonoscopies.
-
Demonstrate that gastroenterologists provide quality care.
Following CDDW 2009, the University of Calgary (Calgary, Alberta) hosted a ‘Train-the-Colonoscopy-Trainers’ course led by Drs Roland Valori and John Anderson from the UK. Gastroenterologists from Alberta, Quebec and Ontario participated in this intensive two-day course assessing endoscopy, training, communication and evaluation skills.
-
Allow gastroenterologists a ‘quality endorsement’.
The EQI has proposed a quality ‘brand’ of recognition to units committed to participation in the EQI.
-
Communication among endoscopists, endoscopic nurses and administrators.
The EQI/GRS provides the template for collaboration and communication within and between units. A monthly EQI newsletter has helped participants with first steps and stumbles in the process. The CAG, with support from the Canadian Association of General Surgeons, is also extending the EQI program to general surgeons practicing endoscopy.
-
Demonstrate improvements in outcome measures.
The groundwork has been laid for a national strategy on outcome measures.
Much has been achieved in the past few years and there has been a groundswell of interest in quality endoscopy from all quarters. However, there is much still to be accomplished. The national consensus meeting on safety and quality indicators, formally recognizing units committed to quality in endoscopy, measuring patient satisfaction and improving outcomes, introducing quality assurance measures to trainees and developing ‘Train-the-Colonoscopy-Trainer’ programs for gastroenterologists across the country are all on the next wave.
Check out the EQI on the CAG Web site and catch the wave at <www.cag-acg.org/about/special-projectsendoscopyqualityinitiative.aspx>.
Footnotes
The CAG is proud to acknowledge its Benefactor Corporate Sponsors:
- Abbott Canada
- AstraZeneca Canada Inc
- Axcan Pharma Inc
- Olympus Canada Inc
- Pentax Canada Inc
- P&G GI Health
- Schering-Plough Canada Inc
- UCB Pharma Inc
REFERENCES
- 1.Armstrong D, Hollingworth R, Daniels S. Point-of-care data collection: Colonoscopy. Can J Gastroenterol. 2008;22(Suppl A):A194. [Google Scholar]
- 2.Armstrong D, MacIntosh D, Hollingworth R, et al. Prolonged wait times for colonoscopy in Canada: The Canadian Association of Gastroenterology (CAG) endoscopy quality initiative (EQI) pilot project. Can J Gastroenterol. 2009;23 doi: 10.1155/2009/130912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong D, Hollingworth R, MacIntosh D, et al. Quality indicators for colonoscopy in Canada: The Canadian Association of Gastroenterology (CAG) endoscopy quality initiative (EQI) practice audit project. Can J Gastroenterol. 2009;23 [Google Scholar]
- 4.Armstrong D, Hollingworth R, MacIntosh D, et al. Endoscopy unit quality indicators for colonoscopy: The Canadian Association of Gastroenterology (CAG) endoscopy quality initiative (EQI) pilot project. Can J Gastroenterol. 2009;23 [Google Scholar]