Abstract
BACKGROUND:
The REduction of Atherothrombosis for Continued Health (REACH) Registry is an international, prospective cohort of 68,236 patients with established coronary artery, cerebrovascular or peripheral arterial disease, or three or more atherothrombotic risk factors. Baseline data from the 1976 Canadian patients in the REACH Registry provide opportunities to assess atherothrombotic risk and treatment in a real-world Canadian setting.
OBJECTIVES:
To present baseline characteristics of Canadian REACH Registry patients, and to compare cardiovascular risk and treatment among Canadian, United States (USA) and global patients.
METHODS:
Patients 45 years of age or older with established atherosclerotic vascular disease or three or more cardiovascular risk factors were enrolled during 2004. Baseline data were used in analyses of risk factor prevalence and control and medication use. Comparisons between the Canadian and USA populations, Canadian and global populations, and the Canadian regions were conducted.
RESULTS:
Of the 1976 Canadian REACH patients, 82.5% had documented vascular disease, 12.6% of whom had manifestations in more than one vascular bed (polyvascular disease). A high prevalence of hypercholesterolemia (84.4%), hypertension (76.6%) and diabetes mellitus (43.7%) were noted, and 75.1% of patients were overweight or obese. Of the 1976 Canadian REACH patients, 75.1% were at target cholesterol levels, 67.4% were at target fasting blood glucose levels and 60.6% were at target blood pressure levels. Significant differences existed in the prevalence of risk factors and their management among Canadian, USA and global REACH populations, as well as within Canada.
CONCLUSIONS:
Canada compared favourably with USA and global REACH populations in the use of proven risk-reducing medications.
Keywords: Atherothrombosis, Cardiovascular risk reduction, Coronary artery disease, Ischemic stroke, Peripheral arterial disease
Abstract
HISTORIQUE :
Le registre REACH sur la réduction de la thrombose athéroscléreuse pour une santé continue est une cohorte prospective internationale de 68 236 patients atteints d’une coronaropathie, d’une maladie vasculaire cérébrale ou d’une maladie des artères périphériques ou présentant au moins trois facteurs de risque de thrombose athéroscléreuse. Les données de départ des 1 976 patients canadiens figurant dans le registre REACH permettent d’évaluer le risque de thrombose athéroscléreuse et le traitement en situation réelle au Canada.
OBJECTIFS :
Présenter les caractéristiques de départ des patients canadiens du registre REACH et comparer le risque cardiovasculaire et le traitement entre les patients canadiens, ceux des États-Unis (ÉU) et sur la scène mondiale.
MÉTHODOLOGIE :
En 2004, on a recruté des patients de 45 ans ou plus atteints d’une maladie vasculaire athéroscléreuse établie ou présentant au moins trois facteurs de risque cardiovasculaire. Les données de bases ont permis d’analyser la prévalence et le contrôle des facteurs de risque ainsi que l’utilisation des médicaments. On a comparé le Canada et les ÉU, le Canada et les populations mondiales et les diverses régions canadiennes.
RÉSULTATS :
Des 1 976 patients canadiens du registre REACH, 82,5 % présentaient une maladie vasculaire documentée, dont 12,6 %, des manifestations dans plus d’un foyer vasculaire (maladie polyvasculaire). Les chercheurs ont constaté une forte prévalence d’hypercholestérolémie (84,4 %), d’hypertension (76,6 %) et de diabète (43,7 %), et 75,1 % des patients faisaient de l’embonpoint ou étaient obèses. Des 1 976 patients canadiens du registre REACH, 75,1 % avaient un taux de cholestérol cible, 67,4 %, une glycémie à jeun cible et 60,6 %, une tension artérielle cible. On remarquait une différence significative entre la prévalence des facteurs de risque et leur prise en charge entre les populations canadiennes, américaines et mondiales du registre REACH, ainsi qu’au sein même du Canada.
CONCLUSIONS :
Le Canada se comparait favorablement aux populations américaines et mondiales du registre REACH pour ce qui est de l’utilisation de médicaments assurant une réduction du risque démontrée.
Manifestations of atherothrombosis, such as ischemic heart disease and cerebrovascular disease (CVD), are the leading causes of death and disability in developed countries, and are projected to remain so through the year 2030 (1). To better define the real-world profile of patients at risk for atherothrombosis and identify predictors of atherothrombotic events in an outpatient setting, the international, observational REduction of Atherothrombosis for Continued Health (REACH) Registry was initiated (2). The study was originally designed to prospectively collect data at baseline, one year and two years, but was extended to collect annual follow-up data for a total of four years. International baseline and one-year data have been published for 67,888 at-risk patients from 44 countries and include patient profiles and their use of drug therapies associated with cardiovascular risk reduction, including antihypertensive, antiplatelet and lipid-lowering therapies (3,4).
Because of Canada’s expansive geography, diverse population, decentralized provincial jurisdiction of health care and primary care-focused patient management, both in-country and international differences in patient risk factor profiles and management strategies would be expected. Canada’s contribution to the international REACH Registry provides a valuable opportunity to compare atherothrombotic risk factor profiles and their management among Canadian, United States (USA) and global populations, as well as within Canada. The present article provides baseline characteristics of Canadian patients enrolled in the international REACH Registry and relevant comparisons of risk factor prevalence and medication use between Canada and the USA, Canada and the rest of the world, and among Canadian regions.
METHODS
Details of the international REACH study design were published previously (2). Briefly, consecutive Canadian outpatients 45 years of age or older with at least three atherothrombotic risk factors or documented coronary artery disease (CAD), CVD or peripheral arterial disease (PAD) were enrolled in the study between January 2004 and October 2004 by physicians in the Maritimes (Newfoundland and Labrador, Prince Edward Island, Nova Scotia and New Brunswick), Quebec, Ontario, the Prairies (Alberta, Manitoba and Saskatchewan) and British Columbia. A physician recruitment grid was developed using Canadian epidemiological data, as well as physician distribution and practice specialty data, to ensure a representative selection of physicians, and thus patients, from all regions of the country. To reflect the Canadian health care system and the emphasis on primary care, a target of 75% general practitioners/family physicians was predefined, and no physician was to recruit more than 15 patients. Physician participation was based on their willingness and ability to recruit patients into the study. Of the 237 physicians approached using the physician recruitment grid, 154 (65%) actively enrolled patients. Importantly, the distribution of physicians, both with regard to practice specialty and Canadian province/region, was approximately the same as the targets proposed in the original study design. A comparison of the percentage of initiated REACH sites per region with Canadian population data from 2005 (5) showed that British Columbia was slightly under-represented (9.6% of initiated sites versus 13.1% of the population), while the Prairies were slightly over-represented (19.1% of initiated sites versus 16.8% of the population). All other regions were represented to within 1.5% of their respective populations.
Atherothrombotic risk factors included type 1 or 2 diabetes mellitus currently treated with hypoglycemic agents, evidence of diabetic nephropathy (microalbuminuria 30 μg/mL or more), ankle-brachial index (ABI) of 0.9 or lower, presence of at least one carotid plaque as evidenced by intima-media thickness twice that of neighbouring sites, asymptomatic carotid stenosis of 70% or greater, systolic blood pressure (BP) of 150 mmHg or greater despite therapy for three months or longer, hypercholesterolemia currently treated with medication, current smoking (mean of 15 cigarettes/day or more within the month before entry), and age of 65 years or older for men and 70 years or older for women. Documented CAD included stable angina with known CAD, history of unstable angina with known CAD, history of percutaneous coronary intervention, history of coronary artery bypass graft surgery or previous myocardial infarction. Documented CVD included previous transient ischemic attack or previous ischemic stroke. Documented PAD consisted of current intermittent claudication with an ABI of less than 0.9 or a history of intermittent claudication together with a previous related intervention.
Exclusion criteria included current hospitalization, participation in a clinical trial or difficulty in returning for follow-up visits. The protocol was submitted to central and local review boards, and all patients provided signed informed consent before participation.
Data were collected centrally using a standardized subject data form. In addition to atherothrombotic risk factor data and previous history of ischemic heart disease, the baseline visit also included collection of data regarding demographics, physical examination (weight, height, waist circumference, and seated systolic and diastolic BPs), other medical history (including smoking status and/or current symptoms) and medications. If performed within the past 12 months, values for serum creatinine, fasting blood glucose, total cholesterol, triglycerides, carotid intima-media thickness, ABI and microalbuminuria were recorded. For assessment of risk factor control, targets for baseline serum measurements were less than 7.0 mmol/L for fasting serum glucose and less than 5.2 mmol/L for total serum cholesterol (6–8). Baseline BP targets were less than 140/90 mmHg for nondiabetic patients and less than 130/80 mmHg for diabetic patients (9). The Canadian Cardiovascular Society (CCS) criteria for the definition of the metabolic syndrome is based on the presence of three of the following: presence of abdominal obesity, elevated fasting glucose, elevated triglycerides, reduced high-density lipoprotein (HDL) cholesterol and elevated BP (10). The REACH study included all of these measures except HDL cholesterol, so a modified definition of the metabolic syndrome was the presence of three of the four available study criteria.
Site visits and data audits were conducted at 6% of randomly selected Canadian sites between baseline and the 12±3 months follow-up. All sites receiving quality control visits were monitored for source documentation and accuracy for all enrolled patients. For consistency, statistical analyses were similar to those performed for the international baseline data (4). Statistical analyses were performed using SAS software version 8 (SAS Institute Inc, USA). Age and sex adjustments were made using a multiple logistic regression model giving the least-squares mean of the logit for each percentage. All statistical analyses were performed at the 5% significance level using two-sided tests. Logistic regression with age and sex in the model, followed by country or region, was used to obtain an overall P-value for country or region. If the overall P-value was statistically significant, multiple logistic regression was used to examine pair-wise comparisons.
RESULTS
Baseline demographics
A total of 1998 patients were enrolled by 154 physicians across Canada. Baseline data were available for 1976 of these patients. Of the 22 patients with no baseline data, 20 did not meet inclusion criteria and two withdrew their consent. Internationally, baseline data were available for 68,236 patients, including 25,763 in the USA. Three-quarters of the participating Canadian physicians were in general or family practice, compared with 44% of those in the global registry.
The baseline characteristics of the Canadian REACH patients are summarized in Table 1. The mean (± SD) age was 68.4±9.9 years, compared with 70.0±10.4 years in the USA cohort. More male patients were were documented among the Canadian population (69.0%) than among the global (63.7%; P<0.001) and USA populations (57.0%; P<0.001). Of the Canadian patients, 1631 (82.5%) had documented symptomatic vascular disease, while the remaining 345 (17.5%) were asymptomatic for vascular disease but had three or more atherothrombotic risk factors. The percentage of Canadian patients enrolled with risk factors only was similar to the global population (18.2%), but significantly less than the USA population (24.5%; P<0.001). Compared with both the symptomatic global and USA populations, significantly more symptomatic Canadians were enrolled with single arterial bed disease and significantly fewer symptomatic Canadians were enrolled with polyvascular disease (P<0.001 for all comparisons; Figure 1). CAD was the most prevalent vascular morbidity and was present in approximately two-thirds of all patients, followed by CVD and PAD. Compared with USA and global patients, there were more Canadian patients with CAD and fewer with CVD and PAD (Figure 1).
TABLE 1.
Baseline characteristics of Canadian participants in the international REduction of Atherothrombosis for Continued Health (REACH) Registry
| Total (n=1976) | Asymptomatic* (n=345) | Symptomatic (n=1631)† | CAD (n=1362) | CVD (394) | PAD (n=148) | |
|---|---|---|---|---|---|---|
| Age, years, mean ± SD | 68.4±9.9 | 67.8±9.9 | 68.6±9.8 | 68.3±9.8 | 71.1±9.8 | 69.3±9.6 |
| Men, % | 69.0 | 56.8 | 71.5 | 73.4 | 65.5 | 68.9 |
| Diabetes‡, % | 43.7 | 84.1 | 35.2 | 36.2 | 35.0 | 46.6 |
| Hypertension§, % | 76.6 | 88.4 | 74.1 | 73.2 | 82.5 | 81.8 |
| Hypercholesterolemia, % | 84.4 | 86.4 | 84.0 | 86.9 | 72.8 | 81.8 |
| Abdominal obesity¶, % | 51.7 | 68.5 | 48.2 | 48.1 | 48.5 | 46.9 |
| Overweight/obese (BMI ≥25 kg/m2), % | 75.1 | 80.5 | 73.9 | 75.3 | 69.3 | 67.3 |
| Smokers, % | ||||||
| Former** | 46.5 | 32.2 | 49.6 | 50.3 | 46.1 | 51.7 |
| Current†† | 15.7 | 21.2 | 14.5 | 13.9 | 13.8 | 31.0 |
| Modified metabolic syndrome‡‡, % | 29.1 | 51.0 | 24.5 | 24.3 | 25.6 | 31.1 |
| Impaired fasting glucose§§, % | 22.8 | 22.3 | 22.9 | 24.2 | 18.0 | 20.3 |
Asymptomatic refers to patients enrolled with three or more atherothrombotic risk factors but no overt ischemic event;
Patients may present with polyvascular disease; therefore, this value does not equal the total of coronary artery disease (CAD) + cerebrovascular disease (CVD) + peripheral arterial disease (PAD);
Patients with type 1 or 2 diabetes currently treated with hypoglycemic agents or a history of diabetes;
Patients with systolic blood pressure ≥150 mmHg despite therapy for ≥3 months or a history of hypertension;
Men: waist circumference ≥102 cm; women: waist circumference ≥88 cm;
≥5 cigarettes/day (mean) >1 month before enrolment;
≥5 cigarettes/day (mean) within 1 month of enrolment;
Patients with ≥3 of the following: abdominal obesity, triglycerides ≥1.7 mmol/L, blood pressure ≥130/85 mmHg, fasting glucose >6.1 mmol/L;
Patients with fasting glucose >5.6 mmol/L and <7.0 mmol/L. BMI Body mass index
Figure 1).
Prevalence of atherothrombotic disease among symptomatic Canadian, global and United States (USA) patients enrolled in the REduction of Atherothrombosis for Continued Health (REACH) Registry. All values are adjusted for age and sex. *0.001<P<0.05; †P<0.001. CAD Coronary artery disease; CVD Cerebrovascular disease; PAD Peripheral arterial disease
Prevalence of risk factors in the REACH Registry
Hypercholesterolemia, hypertension, diabetes mellitus, modified metabolic syndrome, obesity and current/former smoking were common among Canadian REACH registrants (Table 1). The prevalence of diabetes, modified metabolic syndrome, hypertension, abdominal obesity, body mass index (BMI)-based obesity and current smoking were significantly greater among patients enrolled with risk factors only than among those enrolled with a history of symptomatic vascular disease (P<0.001). The symptomatic patients were a relatively homogeneous group with respect to the prevalence of associated risk factors, although there were some notable differences. For example, compared with all symptomatic patients, current smoking (P<0.001) and diabetes (P<0.05) were more common in patients with PAD, and hypertension (P<0.001) was more common in patients with CVD.
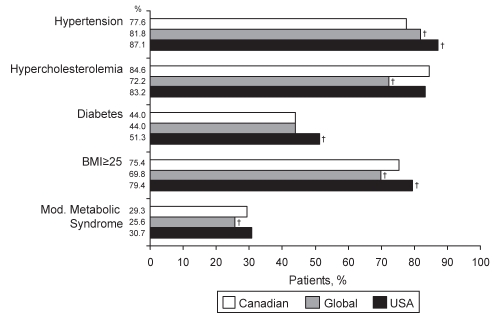
Hypercholesterolemia, as assessed by current treatment with lipid-lowering therapy, was significantly more common among Canadian (84.6%) and USA (83.2%) patients than among global patients (72.2%; P<0.001) (Figure 2). The prevalence of hypertension among Canadian patients was significantly lower than among USA and global patients (77.6%, 87.1% and 81.8%, respectively; P<0.001 for both) (Figure 2). Overweight/obese patients (BMI 25 kg/m2 or greater) accounted for 75.4% of the Canadian REACH population, significantly more than the global population (69.8%; P<0.001) and significantly less than the USA population (79.4%; P<0.001) (Figure 2). The prevalences of diabetes and modified metabolic syndrome were similar in all three cohorts, although diabetes was more common in the USA and modified metabolic syndrome was less common globally (P<0.001 for each) (Figure 2). However, differences in the prevalence of diabetes among Canadian, USA and global REACH patients disappeared when only patients with a BMI of 25 kg/m2 or less were analyzed (data not shown). All differences in risk factor prevalence persisted when the symptomatic population was analyzed independently (Figure S1 [available online at www.pulsus.com]).
Figure 2).
Prevalence of atherothrombotic risk factors among Canadian, global and United States (USA) patients enrolled in the REduction of Atherothrombosis for Continued Health (REACH) Registry. All values are adjusted for age and sex. For definitions of variables, refer to Table 1. †P<0.001. BMI Body mass index; Mod Modified
Within Canada, significantly more patients from Quebec and the Maritimes (P<0.001 for each) and significantly fewer patients from British Columbia and the Prairies (P<0.05 for each) were current smokers (Table 2). Other significant regional differences included more patients from the Maritimes with metabolic syndrome (P<0.05) and diabetes (P<0.001) and more patients from the Prairies with BMI-based and abdominal obesity (P<0.05 for each). The prevalence of obesity was also high in the Maritimes. Inter-Canadian differences in the risk factor profiles of symptomatic patients can be found in Table S1 (available online at www.pulsus.com).
TABLE 2.
Risk factor prevalence among Canadian REduction of Atherothrombosis for Continued Health (REACH) Registry patients
| Canada (n=1976) | British Columbia (n=164) | Prairies* (n=382) | Ontario (n=787) | Quebec (n=509) | Maritimes†(n=134) | |
|---|---|---|---|---|---|---|
| Diabetes‡, % | 44.0 | 49.7 | 34.9§ | 45.7 | 40.6 | 61.9§ |
| Hypertension¶, % | 77.6 | 85.3** | 71.3** | 80.2** | 77.0 | 69.9** |
| Hypercholesterolemia, % | 84.6 | 88.7 | 79.5** | 85.4 | 87.2 | 83.1 |
| Abdominal obesity††, % | 53.5 | 48.8 | 58.9** | 49.0** | 50.3 | 61.2** |
| Overweight/obese (BMI ≥25 kg/m2), % | 75.4 | 75.7 | 82.0** | 75.1 | 70.3** | 78.9 |
| Smoking, % | ||||||
| Former‡‡ | 44.2 | 48.3 | 50.0 | 43.4 | 46.3 | 49.7 |
| Current§§ | 13.5 | 7.5** | 10.7** | 11.9** | 19.6§ | 19.0§ |
| Modified metabolic syndrome¶¶, % | 29.3 | 21.7** | 26.0 | 27.6 | 32.0 | 39.1** |
| Impaired fasting glucose***, % | 22.8 | 27.2 | 21.7 | 22.4 | 22.5 | 24.4 |
The values for hypertension, hypercholesterolemia, abdominal obesity, body mass index (BMI), smoking, modified metabolic syndrome and impaired glucose tolerance are adjusted for age and sex. The overall P-values across the regions were not significant for former smoking and impaired fasting glucose; therefore, no pair-wise comparisons were performed.
Alberta, Manitoba and Saskatchewan;
Newfoundland and Labrador, Prince Edward Island, Nova Scotia and New Brunswick;
Patients with type 1 or 2 diabetes currently treated with hypoglycemic agents or a history of diabetes;
P<0.001;
Patients with systolic blood pressure ≥150 mmHg despite therapy for ≥3 months or a history of hypertension;
0.001<P<0.05;
Men: waist circumference ≥102 cm; women: waist circumference ≥88 cm;
≥5 cigarettes/day (mean) >1 month before enrolment;
≥5 cigarettes/day (mean) within 1 month of enrolment;
Patients with ≥3 of the following: abdominal obesity, triglycerides ≥1.7 mmol/L, blood pressure ≥130/85 mmHg, fasting glucose >6.1 mmol/L;
Patients with fasting glucose >5.6 mmol/L and <7.0 mmol/L
Medication use in the symptomatic population
Table 3 describes medication use among patients with symptomatic vascular disease. This comparison was chosen to illustrate the use of preventive medication in the highest risk population. Canadian patients with hypertension were using at least one antihypertensive agent at a rate similar to comparable USA patients, and significantly greater than the same global cohort (P<0.05). No significant differences in the use of at least one hypoglycemic agent among patients with diabetes and vascular disease were noted, although Canadians were more likely to be taking biguanides and less likely to be taking insulin than their global and USA counterparts (P<0.001 for all comparisons). Canadian patients were more likely than global patients to be taking at least one lipid-lowering therapy (P<0.001), and statin use was significantly greater among Canadian patients than among either the USA or global populations (P<0.001 for each). Canadian patients were also more likely than either USA or global patients to be using antiplatelet therapy and were more likely to use acetylsalicylic acid but less likely to use other antiplatelet agents (P<0.001 for all comparisons). The percentage of patients taking a combination of an antiplatelet agent, statin and an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker was significantly greater in Canada than in the USA and globally (P<0.001 for each). The use of nonsteroidal anti-inflammatory drugs was less frequent among symptomatic Canadian patients than among symptomatic USA patients (P<0.001). All international differences persisted when the total population was analyzed (Table S2 [available online at www.pulsus.com]).
TABLE 3.
Medication use among Canadian, United States (USA) and global participants in the international Canadian REduction of Atherothrombosis for Continued Health (REACH) Registry who have symptomatic vascular disease (coronary artery disease, cerebrovascular disease or peripheral arterial disease)
| Canada, % | Global, % | USA, % | P (Canada versus global) | P (Canada versus USA) | |
|---|---|---|---|---|---|
| Symptomatic patients with a history of hypertension | (n=1208) | (n=44,612) | (n=16,336) | ||
| ≥1 antihypertensive agent | 99.6 | 98.8 | 99.0 | <0.05 | NS |
| Beta-blockers | 60.0 | 55.0 | 61.2 | <0.001 | NS |
| Angiotensin-converting enzyme inhibitors | 60.7 | 50.4 | 50.0 | <0.001 | <0.001 |
| Diuretics | 44.6 | 44.7 | 51.1 | NS | <0.001 |
| Calcium channel blockers | 42.0 | 38.9 | 35.4 | <0.05 | <0.001 |
| Angiotensin receptor blockers | 24.6 | 24.5 | 25.7 | NS | NS |
| Other antihypertensive agents | 5.5 | 10.7 | 13.0 | <0.001 | <0.001 |
| Symptomatic patients with a history of diabetes mellitus | (n=574) | (n=20,768) | (n=8278) | ||
| ≥1 diabetic medication | 87.0 | 88.0 | 89.5 | NS | NS |
| Sulfonylureas | 40.9 | 43.3 | 44.0 | NA | NA |
| Biguanides | 54.9 | 37.8 | 38.3 | <0.001 | <0.001 |
| Insulin | 22.1 | 28.7 | 31.7 | <0.001 | <0.001 |
| Thiazolidinediones | 17.3 | 15.4 | 30.1 | NS | <0.001 |
| Other diabetic medications | 5.8 | 10.8 | 8.1 | <0.001 | NS |
| All symptomatic patients | (n=1631) | (n=55,814) | (n=19,127) | ||
| ≥1 lipid-lowering agent | 86.4 | 73.8 | 83.7 | <0.001 | <0.05 |
| Statins | 82.5 | 68.8 | 77.7 | <0.001 | <0.001 |
| Other lipid-lowering agents | 7.5 | 11.1 | 19.2 | <0.001 | <0.001 |
| ≥1 antiplatelet agent | 88.9 | 84.1 | 82.7 | <0.001 | <0.001 |
| Acetylsalicylic acid | 81.8 | 71.1 | 75.9 | <0.001 | <0.001 |
| Other antiplatelet agents | 20.1 | 28.8 | 25.9 | <0.001 | <0.001 |
| Any 2 antiplatelet agents | 12.8 | 15.7 | 19.1 | <0.05 | <0.001 |
| Triple therapy* | 58.4 | 40.3 | 44.6 | <0.001 | <0.001 |
| Oral anticoagulants | 11.9 | 13.8 | 16.5 | NS | <0.001 |
| Nonsteroidal anti-inflammatory drugs | 9.4 | 10.7 | 17.0 | <0.001 | <0.001 |
All values are adjusted for age and sex.
Triple therapy includes an antiplatelet agent, a statin and either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker. NA Overall P-value was insignificant, so no pair-wise comparisons were performed; NS Overall P-value was significant, but the individual pair-wise comparison was not
Regionally, only the use of lipid-lowering agents differed among symptomatic patients in Canada. Patients from the Prairies were taking statins at a lower rate than other Canadian regions (76.4% versus 82.5%; P<0.05), while those from British Columbia used other lipid-lowering agents more often (16.3% versus 7.5%; P<0.001). Differences in the use of this class of medication extended to the entire Canadian cohort. The use of at least one antiplatelet agent also differed in the overall Canadian cohort, with patients from Quebec more likely to be taking at least one antiplatelet agent (90.8% versus 84.9%; P<0.001) and patients from the Maritimes less likely to be doing so (75.9% versus 84.9%; P<0.05).
Risk factor control
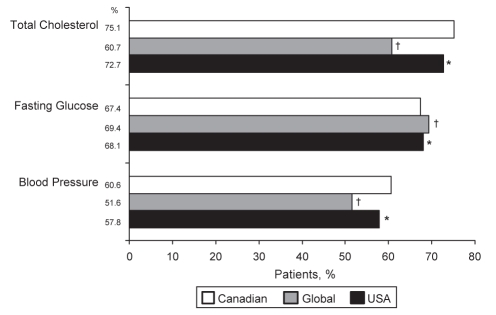
The mean baseline levels of fasting glucose, total cholesterol, and systolic and diastolic BPs were mostly similar among the Canadian, USA and global REACH populations, with some significant differences (Table S3 [available online at www.pulsus.com]). Namely, Canadian patients had a lower mean total cholesterol (4.5±0.03 mmol/L versus 4.9±0.01 mmol/L; P<0.001) and systolic BP (133.9±0.44 mmHg versus 137.8±0.07 mmHg; P<0.001) than global patients, and a lower mean total cholesterol (4.5±0.03 mmol/L versus 4.6±0.01 mmol/L; P<0.001) than USA patients. Based on a single baseline measurement, 60.6% of all Canadian REACH patients had BPs of 140/90 mmHg or less (130/80 mmHg or less for those with diabetes), 67.4% had a fasting glucose lower than 7.0 mmol/L, and 75.1% had a total cholesterol level lower than 5.2 mmol/L. Compared with global patients, significantly more Canadian patients were at target levels for BP (P<0.001) and total cholesterol (P<0.001) (Figure 3). There were similar numbers of USA and Canadian patients at target for fasting glucose, but significantly more Canadians were at target for BP and total cholesterol (P<0.05 for each) compared with USA patients.
Figure 3).
Percentage of Canadian, global and United States (USA) patients in the REduction of Atherothrombosis for Continued Health (REACH) Registry at target levels for total cholesterol (<5.2 mmol/L), fasting glucose (<7.0 mmol/L) and blood pressure (≤140/90 mmHg for nondiabetic patients and ≤130/80 mmHg for diabetic patients). All values are adjusted for age and sex. *0.001<P<0.05; †P<0.001
Mean baseline levels of fasting glucose, total cholesterol, and systolic and diastolic BPs were similar across Canada, with few differences (Table S4 [available online at www.pulsus.com]). Compared with the overall Canadian population, fewer patients from the Maritimes had fasting glucose levels lower than 7.0 mmol/L (49.4% versus 67.4%; P<0.001), and more patients from the Prairies (67.4% versus 60.6%; P<0.05) and fewer from Ontario (57.9% versus 60.6%; P<0.05) had BPs of 140/90 mmHg or lower (130/80 mmHg or lower for those with diabetes) (Table S5 [available online at www.pulsus.com]).
DISCUSSION
Baseline results from the 1976 Canadian patients of the international REACH Registry provide a snapshot of real-world outpatients either with or at-risk for atherothrombotic vascular disease in Canada, thus revealing trends in disease epidemiology and management. Aside from a few notable differences, the risk factor profiles of symptomatic patients were similar regardless of the vascular bed affected. The significantly greater prevalence of diabetes, hypertension and current smoking among patients with risk factors only reflects their role as qualifying enrolment criteria for these patients. The majority (68.9%) of the Canadian cohort had CAD, and 12.6% of patients had polyvascular disease (ie, manifestations in more than one vascular bed [diffuse vascular disease]) (data not shown). The relatively high prevalence of polyvascular disease, as well as the similar risk factor profiles of patients with CAD, CVD and PAD, underscores the systemic nature of atherosclerotic disease and the importance of appropriate risk factor control for preventing not only recurrent events in the same vascular bed but in other beds as well. Notable exceptions to disease-risk factor associations were observed in the Canadian and global cohorts. For example, the observation that there were significantly more PAD patients who were current smokers or had diabetes is consistent with several studies suggesting that these are the strongest risk factors for symptomatic PAD (11). Similarly, the highest rates of hypertension were noted in patients with CVD, consistent with the well-known association between hypertension and stroke (12–14).
Although the overall risk factor profiles of Canadian, USA and global REACH patients were similar, there were differences. Notably, fewer Canadians were enrolled with hypertension. These results are in agreement with the findings of Joffres et al (15), who found that rates of hypertension in the Canadian Heart Health Survey were lower than those in the Third National Health and Nutrition Evaluation Survey (NHANES III) (USA). Perhaps not surprisingly, more Canadian and USA patients were overweight or obese than global patients, although compared with the USA cohort, fewer Canadian patients had class II (BMI 35 kg/m2 to less than 40 kg/m2) or III (BMI 40 kg/m2 or greater) obesity (Table S6 [available online at www.pulsus.com]). The higher prevalence of abdominal obesity in the Canadian and USA cohorts may explain why the prevalence of modified metabolic syndrome was also higher among these patients than worldwide. Canadian and USA REACH patients reported significantly higher rates of hypercholesterolemia than global patients. However, because the definition of hypercholesterolemia used in the REACH Registry was based on the use of lipid-lowering medication (2), the higher rates of hypercholesterolemia among Canadian and USA REACH patients may be due to the greater use of lipid-lowering therapies, particularly statins, in Canada and the USA. Consistent with results from the Canadian Heart Health Survey (16), while the risk factor profiles of patients among the different Canadian regions were similar, there were significant differences in the rates of current smoking, abdominal obesity, diabetes and metabolic syndrome. Provincial governments may wish to take note of these differences to optimize the allocation of funds to appropriate risk management strategies. Interestingly, although symptomatic patients in the Prairies had an increased prevalence of obesity compared with other Canadian regions, there was no corresponding increase in hypertension or diabetes/impaired fasting glucose. Indeed, the prevalence of these conditions in the Prairies was 5% to 10% below the national average, which is both clinically and statistically significant. We have no explanation for this observation, although it is important to note because it may affect future analyses performed on this cohort.
Aggressive risk factor management with pharmacological therapies is critical to improving survival and quality of life and reducing the risk of ischemic events (17–21); therefore, it is a critical component of consensus clinical practice guidelines worldwide (7,8,10,11,22–24). Based on the results of the REACH Registry, Canadian physicians seem to be more aggressive in managing atherothrombotic risk factors than global physicians because the use of antihypertensive, antiplatelet and lipid-lowering therapies was significantly greater among Canadian patients. In turn, the more aggressive use of pharmacological therapies seems to be effective in controlling risk factors because significantly more Canadian patients were at target levels for total cholesterol and BP than global patients. It may be speculated that the greater proportion of patients at goal for these risk factors may be a product of the universality of the Canadian health care system, a broad reimbursement scheme for most risk-reducing medications and the focus on primary care. Primary care physicians likely see their patients more often and over a longer time span than a specialist, allowing them to more closely monitor and adjust therapeutic regimens.
By utilizing medication usage patterns reported by Canadian patients in the REACH Registry as a surrogate marker, we may speculate on the factors that influence current clinical practice. The prevalent use of biguan ides likely reflects the Canadian Diabetes Association evidence-based recommendation (7) to use metformin as the first-line treatment for patients with type 2 diabetes. The low use of thiazolidinediones may be explained by their omission from provincial formulary lists at the time of enrolment, while the low use of insulin among Canadian REACH subjects may be explained by the reluctance of Canadian general practitioners, who comprised 75% of the Canadian REACH physicians, to prescribe insulin except as a last resort (25). The use of antihypertensive agents among Canadian REACH patients seems to reflect current Canadian Hypertension Education Program guidelines; the most commonly used antihypertensive agents were beta-blockers and ACE inhibitors, the combination of which is recommended as initial therapy for patients with CAD (26), the patient group that comprises almost 70% of the Canadian REACH cohort. The fact that 88.9% of symptomatic patients from Canada were taking at least one antiplatelet agent, 92% of whom were taking acetylsalicylic acid, suggests that Canadian physicians adhere to recommendations set forth by the CCS guidelines on the evaluation and management of chronic ischemic heart disease (27). Physicians also seem to be cognizant of Canadian guidelines for the treatment and management of dyslipidemia because the vast majority of patients were taking a statin (10). The significantly lower use of non-steroidal anti-inflammatory drugs in Canada suggests that the greater use of risk-reducing medications by Canadian patients is a result of Canadian physicians selectively prescribing appropriate risk-reducing medications and not a consequence of generalized overprescribing.
Additional evidence supporting the aggressive treatment of risk factors by Canadian physicians can be found by examining the proportion of symptomatic patients taking an antiplatelet agent, a statin and either an ACE inhibitor or angiotensin receptor blocker (‘triple therapy’). Both the American College of Cardiology/American Heart Association guidelines for secondary prevention (24) and the CCS consensus statement on PAD (11) recommend the use of triple therapy for all patients with established atherosclerotic vascular disease. The fact that significantly more symptomatic Canadian patients were taking triple therapy compared with symptomatic USA and global patients (P<0.001 for each) further confirms the high awareness of guidelines among Canadian physicians and their willingness to use aggressive pharmacological therapy.
While it is encouraging that a greater proportion of Canadian patients were being treated with risk-reducing medications and were more likely to be at appropriate target levels based on single baseline measurements, there is still room for improvement. Appropriate risk factor control by as many patients as possible is critical because poorly controlled risk factors are associated with adverse clinical events. For example, in a population-based study of hypertension control (28), 95% of strokes occurred in patients with uncontrolled hypertension, and the population-attributable risk of stroke in people with uncontrolled hypertension despite antihypertensive treatment was 45%.
Steps must be taken to reduce the number of Canadian patients not at treatment goals for prominent cardiovascular risk factors. First, in the absence of contraindications, it is critical that appropriate pharmacological therapy be initiated in the 17.8% of patients with a total cholesterol level of 5.2 mmol/L or greater, the 12.8% of patients with a fasting glucose of 7.0 mmol/L or greater, and the 3.1% of patients with a BP of 140/90 mmHg or greater who were not taking any therapy. However, it is equally important that therapy be escalated in patients already taking medication but not at target goals. Aside from including additional pharmacological therapies, physicians should be willing to up-titrate medication doses to help patients achieve target goals, as data from two large Canadian outpatient-based registries of patients with, or at risk for, cardiovascular disease showed that the median dose of statins and ACE inhibitors used by registry patients was only 25% to 50% of the target doses used in long-term clinical trials (29). Additionally, physicians should increase their efforts to encourage patients to control their weight because three-quarters of Canadian patients failed to meet the target BMI of less than 25 kg/m2, and roughly one-half had a waist circumference that was larger than recommended (less than 88 cm for women and less than 102 cm for men). The need for appropriate weight reduction strategies is particularly important in the Prairies because significantly more patients from this region presented with abdominal obesity. Finally, while rates of current smoking were generally low across Canada, physicians in Quebec should continue to stress the risks of smoking and encourage its cessation because the largest proportion of current smokers was found in this province.
While there is still room for improvement in levels of risk factor control, it is encouraging that the high-risk Canadian REACH cohort was treated more aggressively than previously reported Canadian cohorts at lower risk. In the Canadian REACH cohort, the rate of untreated dyslipidemia was only 25% compared with 63% in a cohort of slightly younger primary care patients with dyslipidemia (higher percentage of women) recruited between 2000 and 2003 (30). Notably, rates of risk factor control and medication use were remarkably similar among Canadian patients enrolled in REACH and similar patient populations enrolled in the Canadian Vascular Protection (VP) Registry and the Guidelines Oriented Approach to Lipid Lowering (GOALL) Registry, which recruited more than 9000 patients between 2001 and 2004 (29,31). The concordance between the REACH and VP/GOALL patients validates the Canadian REACH cohort as representative of current, real-world Canadian clinical practice in patients with, or at risk for, atherosclerotic cardiovascular disease.
Limitations
Limitations to the present analysis include the nonrandomized selection and participation of physicians. Because physicians who accepted the invitation to participate in the REACH Registry may be more inclined to research and more informed on recent advances, they may be more likely than other physicians to provide better care. Therefore, the present analysis may overestimate the treatment of patients with, or at risk for, atherothrombotic vascular disease, thus underestimating the treatment gap. Although physicians were instructed to recruit consecutive patients, there was no mechanism to ensure that this occurred, and therefore, no means to measure the impact of any recruiter bias. Factors such as patient preference, noncompliance, unknown contraindications to medication, and cost issues were not recorded; therefore, their impact on reported medication usage and achievement of target goals is unknown. While measures of total cholesterol were recorded, there were no measures of low-density lipoprotein or HDL cholesterol, and hyperlipidemia guideline targets are largely based on these measures (10,32). Instead of defining impaired fasting glucose as 6.1 mmol/L to 6.9 mmol/L as per the 2003 Canadian Diabetes Association guidelines (7), the same criteria used in the global REACH baseline manuscript, which is also consistent with the CCS definition of the glycemic criteria for metabolic syndrome (10), were applied. The percentage of patients considered to be at target levels for BP, fasting blood glucose and total cholesterol were based on single measurements. While glycosylated hemoglobin levels are often used as criteria for defining diabetes control (7), they were not measured as part of the REACH Registry.
Overall, baseline data from the REACH Registry are similar to other, larger registries of Canadian patients with or at risk for cardiovascular disease and show that Canadian patients have a slightly different risk factor profile and are more likely than global and USA patients to be at target levels for BP and total cholesterol. While medication use and risk factor control in Canada compared favourably with USA and international REACH registrants, there is still room for improvement because not all patients eligible for risk-reducing medications are receiving them, and not all patients taking medications are at target levels. Future follow-up assessments will allow for comparisons of cardiovascular outcomes for different patient subgroups and define predictors of risk for subsequent atherothrombotic events. They may also help confirm that the more aggressive risk factor management in Canada correlates with fewer adverse cardiovascular events.
TABLE S1.
Age- and sex-adjusted risk factor prevalence among symptomatic Canadian REduction of Atherothrombosis for Continued Health (REACH) Registry patients
| Canada (n=1631) | British Columbia (n=134) | Prairies* (n=327) | Ontario (n=632) | Quebec (n=435) | Maritimes†(n=103) | |
|---|---|---|---|---|---|---|
| Diabetes‡, % | 35.3 | 42.6 | 27.8§ | 35.7 | 33.5 | 53.0¶ |
| Hypertension**, % | 74.9 | 84.1§ | 67.8§ | 77.5§ | 74.6 | 68.8 |
| Hypercholesterolemia, % | 84.1 | 91.8§ | 78.3¶ | 83.8 | 87.0 | 86.4 |
| Abdominal obesity††, % | 49.7 | 47.7 | 56.5§ | 44.2§ | 46.5 | 55.5 |
| Overweight (BMI 25 kg/m2 to <30 kg/m2), % | 41.8 | 45.0 | 44.2 | 42.9 | 38.0 | 43.9 |
| Obesity, % | ||||||
| Class I (BMI 30 kg/m2 to <35 kg/m2) | 22.5 | 19.9 | 26.3 | 21.3 | 22.9 | 19.2 |
| Class II (BMI 35 kg/m2 to <40 kg/m2) | 6.2 | 5.3 | 7.2 | 4.8 | 5.6 | 10.1 |
| Class III (BMI ≥40 kg/m2) | 2.4 | 3.4 | 1.8 | 2.6 | 1.4 | 1.9 |
| Smoking, % | ||||||
| Former‡‡ | 47.6 | 54.5 | 55.3 | 46.0 | 48.0 | 53.6 |
| Current§§ | 12.6 | 5.4§ | 8.3§ | 11.1 | 19.1¶ | 16.0 |
| Modified metabolic syndrome¶¶, % | 24.6 | 19.2 | 21.2 | 21.9 | 28.2§ | 36.2§ |
| Impaired fasting glucose***, % | 22.9 | 25.8 | 22.8 | 21.6 | 22.6 | 27.9 |
The overall P-values across the regions were not significant for the following categories: overweight, obesity, former smoker and impaired fasting glucose; therefore, no pair-wise comparisons were performed.
Alberta, Manitoba and Saskatchewan;
Newfoundland and Labrador, Prince Edward Island, Nova Scotia and New Brunswick;
Patients with type 1 or 2 diabetes currently treated with hypoglycemic agents or a history of diabetes;
0.001<P<0.05;
P<0.001;
Patients with systolic blood pressure ≥150 mmHg despite therapy for ≥3 months or a history of hypertension;
Men: waist circumference ≥102 cm; women: waist circumference ≥88 cm;
≥5 cigarettes/day (mean) >1 month before enrolment;
≥5 cigarettes/day (mean) within 1 month of enrolment;
Patients with ≥3 of the following: abdominal obesity, triglycerides ≥1.7 mmol/L, blood pressure ≥130/85 mmHg, fasting glucose >6.1 mmol/L;
Patients with fasting glucose >5.6 mmol/L and <7.0 mmol/L. BMI Body mass index
TABLE S2.
Age- and sex-adjusted medication use among Canadian, United States (USA) and global participants in the international Canadian REduction of Atherothrombosis for Continued Health (REACH) Registry
| Canada, % | Global, % | USA, % | P (Canada versus global) | P (Canada versus USA) | |
|---|---|---|---|---|---|
| Patients with a history of hypertension | (n=1513) | (n=55,824) | (n=22,493) | ||
| ≥1 antihypertensive agent | 99.7 | 98.8 | 99.0 | <0.05 | <0.05 |
| Beta-blockers | 52.4 | 50.3 | 53.1 | NS | NS |
| Angiotensin-converting enzyme Inhibitors | 60.9 | 50.0 | 50.4 | <0.001 | <0.001 |
| Diuretics | 47.3 | 45.9 | 51.9 | NS | <0.001 |
| Calcium channel blockers | 41.4 | 38.8 | 35.5 | <0.05 | <0.001 |
| Angiotensin receptor blockers | 27.0 | 26.5 | 27.8 | NS | NS |
| Other antihypertensive agents | 5.9 | 11.1 | 13.0 | <0.001 | <0.001 |
| Patients with a history of diabetes mellitus | (n=864) | (n=30,043) | (n=13,223) | ||
| ≥1 diabetic medication | 90.0 | 90.3 | 91.4 | NS | NS |
| Sulfonylureas | 44.4 | 45.0 | 45.3 | NA | NA |
| Biguanides | 58.9 | 42.0 | 42.9 | <0.001 | <0.001 |
| Insulin | 20.3 | 27.0 | 28.4 | <0.001 | <0.001 |
| Thiazolidinediones | 20.6 | 17.9 | 32.3 | <0.05 | <0.001 |
| Other diabetic medications | 6.4 | 11.5 | 8.3 | <0.001 | NS |
| All patients | (n=1976) | (n=68,236) | (n=25,763) | ||
| ≥1 lipid-lowering agent | 85.9 | 75.1 | 84.4 | <0.001 | NS |
| Statins | 81.6 | 69.3 | 77.5 | <0.001 | <0.001 |
| Other lipid-lowering agents | 7.9 | 12.0 | 19.1 | <0.001 | <0.001 |
| ≥1 antiplatelet agent | 84.9 | 78.6 | 77.4 | <0.001 | <0.001 |
| Acetylsalicylic acid | 78.9 | 67.2 | 71.7 | <0.001 | <0.001 |
| Other antiplatelet agents | 16.7 | 24.7 | 20.6 | <0.001 | <0.001 |
| Any 2 antiplatelet agents | 10.6 | 13.2 | 14.8 | <0.001 | <0.001 |
| Triple therapy* | 56.4 | 38.7 | 42.9 | <0.001 | <0.001 |
| Oral anticoagulants | 10.8 | 12.3 | 13.9 | NS | <0.001 |
| Nonsteroidal anti-inflammatory drugs | 10.2 | 11.6 | 18.0 | NS | <0.001 |
Triple therapy includes an antiplatelet agent, a statin, and either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker. NA Overall P-value was insignificant, so no pair-wise comparisons were performed; NS Overall P-value was significant, but the individual pair-wise comparison was not
TABLE S3.
Age- and sex-adjusted baseline levels of glucose, total cholesterol, and systolic and diastolic blood pressures (BPs) among Canadian, United States (USA) and global patients in the REduction of Atherothrombosis for Continued Health (REACH) Registry
| Canada (n=1976) | Global (n=68,236) | USA (n=25,763) | P (Canada versus global) | P (Canada versus USA) | |
|---|---|---|---|---|---|
| Fasting glucose, mmol/L | 6.8±0.06 | 6.7±0.01 | 6.8±0.02 | NS | NS |
| Total cholesterol, mmol/L | 4.5±0.03 | 4.9±0.01 | 4.6±0.01 | <0.001 | <0.001 |
| Systolic BP, mmHg | 133.9±0.44 | 137.8±0.07 | 133.5±0.12 | <0.001 | NS |
| Diastolic BP, mmHg | 76.2±0.25 | 78.4±0.04 | 75.5±0.07 | <0.001 | <0.05 |
Data presented as mean ± SEM. NS Overall P-value was significant, but the individual pair-wise comparison was not
TABLE S4.
Age- and sex-adjusted baseline levels of glucose, total cholesterol, and systolic and diastolic blood pressures (BPs) among Canadian regions in the Canadian REduction of Atherothrombosis for Continued Health (REACH) Registry
| Canada (n=1976) | British Columbia (n=164) | Prairies* (n=382) | Ontario (n=787) | Quebec (n=509) | Maritimes†(n=134) | |
|---|---|---|---|---|---|---|
| Fasting glucose, mmol/L | 6.8±0.06 | 6.5±0.22 | 6.9±0.14 | 6.8±0.10 | 6.6±0.12‡ | 7.6±0.22§ |
| Total cholesterol, mmol/L | 4.5±0.03 | 4.4±0.09 | 4.6±0.06 | 4.5±0.04 | 4.5±0.05 | 4.5±0.10 |
| Systolic BP, mmHg | 133.9±0.44 | 131.4±1.40 | 131.6±0.91‡ | 134.2±0.64 | 136.1±0.79§ | 130.3±1.54‡ |
| Diastolic BP, mmHg | 76.2±0.25 | 75.9±0.75 | 75.5±0.49 | 76.5±0.34 | 76.7±0.42 | 74.9±0.83 |
Data presented as mean ± SEM. The overall P-values across the regions were not significant for diastolic BP; therefore, no pair-wise comparisons were performed.
Alberta, Manitoba and Saskatchewan;
Newfoundland and Labrador, Prince Edward Island, Nova Scotia and New Brunswick;
0.001<P<0.05;
P<0.001
TABLE S5.
Age- and sex-adjusted percentage of Canadian REduction of Atherothrombosis for Continued Health (REACH) Registry patients at target levels for fasting glucose, total cholesterol and blood pressure by region
| Canada (n=1976) | British Columbia (n=164) | Prairies* (n=382) | Ontario (n=787) | Quebec (n=509) | Maritimes†(n=134) | |
|---|---|---|---|---|---|---|
| Fasting glucose <7.0 mmol/L, % | 67.4 | 75.4 | 68.5 | 68.6 | 68.4 | 49.4‡ |
| Total cholesterol <5.2 mmol/L, % | 75.1 | 83.7 | 71.5 | 75.9 | 75.9 | 77.9 |
| Blood pressure ≤140/90 mmHg for nondiabetic patients and ≤130/80 mmHg for diabetic patients, % | 60.6 | 67.1 | 67.4§ | 57.9§ | 59.2 | 61.7 |
The overall P-values across the regions were not significant for total cholesterol; therefore, no pair-wise comparisons were performed.
Alberta, Manitoba and Saskatchewan;
Newfoundland and Labrador, Prince Edward Island, Nova Scotia and New Brunswick;
P<0.001;
0.001<P<0.05
TABLE S6.
Age- and sex-adjusted prevalence of body mass index (BMI)-based class of obesity in Canadian, global and United States (USA) patients in the Canadian REduction of Atherothrombosis for Continued Health (REACH) Registry
| Canada (n=1976) | Global (n=68,236) | USA (n=25,763) | P (Canada versus global) | P (Canada versus USA) | |
|---|---|---|---|---|---|
| Class I (BMI 30 kg/m2 to <35 kg/m2), % | 22.9 | 19.8 | 24.6 | <0.001 | NS |
| Class II (BMI 35 kg/m2 to <40 kg/m2), % | 6.6 | 6.6 | 10.0 | NS | <0.001 |
| Class III (BMI ≥40 kg/m2), % | 2.8 | 3.5 | 5.9 | NS | <0.001 |
NS Overall P-value was significant, but the individual pair-wise comparison was not
Acknowledgments
The authors thank John Stewart MSc, for his thoughtful contributions to the manuscript.
Footnotes
CANADIAN REACH REGISTRy STEERING COMMITTEE MEMBERS: Alan Bell (Clinical Lecturer, University of Toronto, Toronto, Ontario), Eric Cohen (Sunnybrook College Health Sciences Centre, Toronto), Shaun Goodman (St Michael’s Hospital, Toronto), Robert Herman (University of Calgary, Calgary, Alberta), Michael Hill (University of Calgary, Foothills Hospital, Calgary), Pravin Mehta (Laxmi Centre, Winnipeg, Manitoba), Andre Roussin (Notre-Dame Hospital, Montreal, Quebec) and Graham Turpie (Hamilton Health Sciences, Hamilton, Ontario).
SOURCE OF SUPPORT: sanofi-aventis and Bristol-Myers Squibb.
REFERENCES
- 1.Mathers CD, Loncar D.Updated projections of global mortality and burden of disease, 2002–2030: Data sources, methods and results<http://www.who.int/healthinfo/statistics/bod_projections2030_paper.pdf> (Version current at November 2006).
- 2.Ohman EM, Bhatt DL, Steg PG, et al. The REduction of Atherothrombosis for Continued Health (REACH) Registry: An international, prospective, observational investigation in subjects at risk for atherothrombotic events – study design. Am Heart J. 2006;151:786.e1–10. doi: 10.1016/j.ahj.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–9. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 5.Statistics Canada Population by year, by province and territory<http://www40.statcan.ca/l01/cst01/demo02a.htm> (Version current at March 6, 2008).
- 6.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 7.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 2003 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes. 2003;27:S1–S152. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 8.NCEP Expert Panel Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 9.Khan NA, McAlister FA, Rabkin SW, et al. The 2006 Canadian Hypertension Education Program recommendations for the management of hypertension: Part II – therapy. Can J Cardiol. 2006;22:583–93. doi: 10.1016/s0828-282x(06)70280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McPherson R, Frohlich J, Fodor G, Genest J, Canadian Cardiovascular Society Canadian Cardiovascular Society position statement – recommendations for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease. Can J Cardiol. 2006;22:913–27. doi: 10.1016/s0828-282x(06)70310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abramson BL, Huckell V, Anand S, et al. Canadian Cardiovascular Society Consensus Conference: Peripheral arterial disease – executive summary. Can J Cardiol. 2005;21:997–1006. [PubMed] [Google Scholar]
- 12.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 13.Weber MA. Managing the patient at risk for a second stroke. J Hypertens Suppl. 2005;23:S41–7. doi: 10.1097/01.hjh.0000165627.64686.cc. [DOI] [PubMed] [Google Scholar]
- 14.Canadian Stroke Network and the Heart and Stroke Foundation of Canada: Canadian Stroke Strategy Canadian Best Practice Recommendations for Stroke Care 2006. <http://www.canadianstrokestrategy.ca/eng/resourcestools/documents/StrokeStrategyManual.pdf> (Version current at April 9, 2008).
- 15.Joffres MR, Hamet P, MacLean DR, L’Italien GJ, Fodor G. Distribution of blood pressure and hypertension in Canada and the United States. Am J Hypertens. 2001;14:1099–105. doi: 10.1016/s0895-7061(01)02211-7. [DOI] [PubMed] [Google Scholar]
- 16.Tanuseputro P, Manuel DG, Leung M, Nguyen K, Johansen H. Risk factors for cardiovascular disease in Canada. Can J Cardiol. 2003;19:1249–59. [PubMed] [Google Scholar]
- 17.Antithrombotic Trialists Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 19.Fox KM. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: Randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362:782–8. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 20.Tran H, Anand SS. Oral antiplatelet therapy in cerebrovascular disease, coronary artery disease, and peripheral arterial disease. JAMA. 2004;292:1867–74. doi: 10.1001/jama.292.15.1867. [DOI] [PubMed] [Google Scholar]
- 21.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 22.Erdine S, Ari O, Zanchetti A, et al. ESH-ESC guidelines for the management of hypertension. Herz. 2006;31:331–8. doi: 10.1007/s00059-006-2829-3. [DOI] [PubMed] [Google Scholar]
- 23.Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: Executive summary. Eur Heart J. 2007;28:2375–414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- 24.Smith SC, Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: Endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006;113:2363–72. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
- 25.Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: Results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673–9. doi: 10.2337/diacare.28.11.2673. [DOI] [PubMed] [Google Scholar]
- 26.Campbell NR, Hemmelgarn B, Tremblay G, Canadian Hypertension Education Program 2008Canadian Hypertension Education Program Recommendations: The Scientific Summary – an Annual Update<http://hypertension.ca/chep/recommendations/summaries/evidence-based-discussion/> (Version current at March 21, 2008).
- 27.Canadian Cardiovascular Society Consensus Conference on the Evaluation and Management of Chronic Ischemic Heart Disease<http://www.ccs.ca/download/consensus_conference/consensus_conference_archives/1997_CC_Evaluation_and_Management_CIHD.pdf> (Version current at April 15, 2008).
- 28.Li C, Engstrom G, Hedblad B, Berglund G, Janzon L. Blood pressure control and risk of stroke: A population-based prospective cohort study. Stroke. 2005;36:725–30. doi: 10.1161/01.STR.0000158925.12740.87. [DOI] [PubMed] [Google Scholar]
- 29.Hackam DG, Leiter LA, Yan AT, et al. Missed opportunities for the secondary prevention of cardiovascular disease in Canada. Can J Cardiol. 2007;23:1124–30. doi: 10.1016/s0828-282x(07)70882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrella RJ, Merikle E, Jones J. Prevalence and treatment of dyslipidemia in Canadian primary care: A retrospective cohort analysis. Clin Ther. 2007;29:742–50. doi: 10.1016/j.clinthera.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Yan AT, Yan RT, Tan M, et al. Contemporary management of dyslipidemia in high-risk patients: Targets still not met. Am J Med. 2006;119:676–83. doi: 10.1016/j.amjmed.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1.
Age- and sex-adjusted risk factor prevalence among symptomatic Canadian REduction of Atherothrombosis for Continued Health (REACH) Registry patients
| Canada (n=1631) | British Columbia (n=134) | Prairies* (n=327) | Ontario (n=632) | Quebec (n=435) | Maritimes†(n=103) | |
|---|---|---|---|---|---|---|
| Diabetes‡, % | 35.3 | 42.6 | 27.8§ | 35.7 | 33.5 | 53.0¶ |
| Hypertension**, % | 74.9 | 84.1§ | 67.8§ | 77.5§ | 74.6 | 68.8 |
| Hypercholesterolemia, % | 84.1 | 91.8§ | 78.3¶ | 83.8 | 87.0 | 86.4 |
| Abdominal obesity††, % | 49.7 | 47.7 | 56.5§ | 44.2§ | 46.5 | 55.5 |
| Overweight (BMI 25 kg/m2 to <30 kg/m2), % | 41.8 | 45.0 | 44.2 | 42.9 | 38.0 | 43.9 |
| Obesity, % | ||||||
| Class I (BMI 30 kg/m2 to <35 kg/m2) | 22.5 | 19.9 | 26.3 | 21.3 | 22.9 | 19.2 |
| Class II (BMI 35 kg/m2 to <40 kg/m2) | 6.2 | 5.3 | 7.2 | 4.8 | 5.6 | 10.1 |
| Class III (BMI ≥40 kg/m2) | 2.4 | 3.4 | 1.8 | 2.6 | 1.4 | 1.9 |
| Smoking, % | ||||||
| Former‡‡ | 47.6 | 54.5 | 55.3 | 46.0 | 48.0 | 53.6 |
| Current§§ | 12.6 | 5.4§ | 8.3§ | 11.1 | 19.1¶ | 16.0 |
| Modified metabolic syndrome¶¶, % | 24.6 | 19.2 | 21.2 | 21.9 | 28.2§ | 36.2§ |
| Impaired fasting glucose***, % | 22.9 | 25.8 | 22.8 | 21.6 | 22.6 | 27.9 |
The overall P-values across the regions were not significant for the following categories: overweight, obesity, former smoker and impaired fasting glucose; therefore, no pair-wise comparisons were performed.
Alberta, Manitoba and Saskatchewan;
Newfoundland and Labrador, Prince Edward Island, Nova Scotia and New Brunswick;
Patients with type 1 or 2 diabetes currently treated with hypoglycemic agents or a history of diabetes;
0.001<P<0.05;
P<0.001;
Patients with systolic blood pressure ≥150 mmHg despite therapy for ≥3 months or a history of hypertension;
Men: waist circumference ≥102 cm; women: waist circumference ≥88 cm;
≥5 cigarettes/day (mean) >1 month before enrolment;
≥5 cigarettes/day (mean) within 1 month of enrolment;
Patients with ≥3 of the following: abdominal obesity, triglycerides ≥1.7 mmol/L, blood pressure ≥130/85 mmHg, fasting glucose >6.1 mmol/L;
Patients with fasting glucose >5.6 mmol/L and <7.0 mmol/L. BMI Body mass index
TABLE S2.
Age- and sex-adjusted medication use among Canadian, United States (USA) and global participants in the international Canadian REduction of Atherothrombosis for Continued Health (REACH) Registry
| Canada, % | Global, % | USA, % | P (Canada versus global) | P (Canada versus USA) | |
|---|---|---|---|---|---|
| Patients with a history of hypertension | (n=1513) | (n=55,824) | (n=22,493) | ||
| ≥1 antihypertensive agent | 99.7 | 98.8 | 99.0 | <0.05 | <0.05 |
| Beta-blockers | 52.4 | 50.3 | 53.1 | NS | NS |
| Angiotensin-converting enzyme Inhibitors | 60.9 | 50.0 | 50.4 | <0.001 | <0.001 |
| Diuretics | 47.3 | 45.9 | 51.9 | NS | <0.001 |
| Calcium channel blockers | 41.4 | 38.8 | 35.5 | <0.05 | <0.001 |
| Angiotensin receptor blockers | 27.0 | 26.5 | 27.8 | NS | NS |
| Other antihypertensive agents | 5.9 | 11.1 | 13.0 | <0.001 | <0.001 |
| Patients with a history of diabetes mellitus | (n=864) | (n=30,043) | (n=13,223) | ||
| ≥1 diabetic medication | 90.0 | 90.3 | 91.4 | NS | NS |
| Sulfonylureas | 44.4 | 45.0 | 45.3 | NA | NA |
| Biguanides | 58.9 | 42.0 | 42.9 | <0.001 | <0.001 |
| Insulin | 20.3 | 27.0 | 28.4 | <0.001 | <0.001 |
| Thiazolidinediones | 20.6 | 17.9 | 32.3 | <0.05 | <0.001 |
| Other diabetic medications | 6.4 | 11.5 | 8.3 | <0.001 | NS |
| All patients | (n=1976) | (n=68,236) | (n=25,763) | ||
| ≥1 lipid-lowering agent | 85.9 | 75.1 | 84.4 | <0.001 | NS |
| Statins | 81.6 | 69.3 | 77.5 | <0.001 | <0.001 |
| Other lipid-lowering agents | 7.9 | 12.0 | 19.1 | <0.001 | <0.001 |
| ≥1 antiplatelet agent | 84.9 | 78.6 | 77.4 | <0.001 | <0.001 |
| Acetylsalicylic acid | 78.9 | 67.2 | 71.7 | <0.001 | <0.001 |
| Other antiplatelet agents | 16.7 | 24.7 | 20.6 | <0.001 | <0.001 |
| Any 2 antiplatelet agents | 10.6 | 13.2 | 14.8 | <0.001 | <0.001 |
| Triple therapy* | 56.4 | 38.7 | 42.9 | <0.001 | <0.001 |
| Oral anticoagulants | 10.8 | 12.3 | 13.9 | NS | <0.001 |
| Nonsteroidal anti-inflammatory drugs | 10.2 | 11.6 | 18.0 | NS | <0.001 |
Triple therapy includes an antiplatelet agent, a statin, and either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker. NA Overall P-value was insignificant, so no pair-wise comparisons were performed; NS Overall P-value was significant, but the individual pair-wise comparison was not
TABLE S3.
Age- and sex-adjusted baseline levels of glucose, total cholesterol, and systolic and diastolic blood pressures (BPs) among Canadian, United States (USA) and global patients in the REduction of Atherothrombosis for Continued Health (REACH) Registry
| Canada (n=1976) | Global (n=68,236) | USA (n=25,763) | P (Canada versus global) | P (Canada versus USA) | |
|---|---|---|---|---|---|
| Fasting glucose, mmol/L | 6.8±0.06 | 6.7±0.01 | 6.8±0.02 | NS | NS |
| Total cholesterol, mmol/L | 4.5±0.03 | 4.9±0.01 | 4.6±0.01 | <0.001 | <0.001 |
| Systolic BP, mmHg | 133.9±0.44 | 137.8±0.07 | 133.5±0.12 | <0.001 | NS |
| Diastolic BP, mmHg | 76.2±0.25 | 78.4±0.04 | 75.5±0.07 | <0.001 | <0.05 |
Data presented as mean ± SEM. NS Overall P-value was significant, but the individual pair-wise comparison was not
TABLE S4.
Age- and sex-adjusted baseline levels of glucose, total cholesterol, and systolic and diastolic blood pressures (BPs) among Canadian regions in the Canadian REduction of Atherothrombosis for Continued Health (REACH) Registry
| Canada (n=1976) | British Columbia (n=164) | Prairies* (n=382) | Ontario (n=787) | Quebec (n=509) | Maritimes†(n=134) | |
|---|---|---|---|---|---|---|
| Fasting glucose, mmol/L | 6.8±0.06 | 6.5±0.22 | 6.9±0.14 | 6.8±0.10 | 6.6±0.12‡ | 7.6±0.22§ |
| Total cholesterol, mmol/L | 4.5±0.03 | 4.4±0.09 | 4.6±0.06 | 4.5±0.04 | 4.5±0.05 | 4.5±0.10 |
| Systolic BP, mmHg | 133.9±0.44 | 131.4±1.40 | 131.6±0.91‡ | 134.2±0.64 | 136.1±0.79§ | 130.3±1.54‡ |
| Diastolic BP, mmHg | 76.2±0.25 | 75.9±0.75 | 75.5±0.49 | 76.5±0.34 | 76.7±0.42 | 74.9±0.83 |
Data presented as mean ± SEM. The overall P-values across the regions were not significant for diastolic BP; therefore, no pair-wise comparisons were performed.
Alberta, Manitoba and Saskatchewan;
Newfoundland and Labrador, Prince Edward Island, Nova Scotia and New Brunswick;
0.001<P<0.05;
P<0.001
TABLE S5.
Age- and sex-adjusted percentage of Canadian REduction of Atherothrombosis for Continued Health (REACH) Registry patients at target levels for fasting glucose, total cholesterol and blood pressure by region
| Canada (n=1976) | British Columbia (n=164) | Prairies* (n=382) | Ontario (n=787) | Quebec (n=509) | Maritimes†(n=134) | |
|---|---|---|---|---|---|---|
| Fasting glucose <7.0 mmol/L, % | 67.4 | 75.4 | 68.5 | 68.6 | 68.4 | 49.4‡ |
| Total cholesterol <5.2 mmol/L, % | 75.1 | 83.7 | 71.5 | 75.9 | 75.9 | 77.9 |
| Blood pressure ≤140/90 mmHg for nondiabetic patients and ≤130/80 mmHg for diabetic patients, % | 60.6 | 67.1 | 67.4§ | 57.9§ | 59.2 | 61.7 |
The overall P-values across the regions were not significant for total cholesterol; therefore, no pair-wise comparisons were performed.
Alberta, Manitoba and Saskatchewan;
Newfoundland and Labrador, Prince Edward Island, Nova Scotia and New Brunswick;
P<0.001;
0.001<P<0.05
TABLE S6.
Age- and sex-adjusted prevalence of body mass index (BMI)-based class of obesity in Canadian, global and United States (USA) patients in the Canadian REduction of Atherothrombosis for Continued Health (REACH) Registry
| Canada (n=1976) | Global (n=68,236) | USA (n=25,763) | P (Canada versus global) | P (Canada versus USA) | |
|---|---|---|---|---|---|
| Class I (BMI 30 kg/m2 to <35 kg/m2), % | 22.9 | 19.8 | 24.6 | <0.001 | NS |
| Class II (BMI 35 kg/m2 to <40 kg/m2), % | 6.6 | 6.6 | 10.0 | NS | <0.001 |
| Class III (BMI ≥40 kg/m2), % | 2.8 | 3.5 | 5.9 | NS | <0.001 |
NS Overall P-value was significant, but the individual pair-wise comparison was not