Abstract
Primary cardiac lymphomas are rare extranodal lymphomas that should be distinguished from secondary cardiac involvement by disseminated non-Hodgkin’s lymphoma. Cardiac lymphomas often mimic other cardiac neoplasms, including myxomas and angiosarcomas, and often require multimodality cardiac imaging, in combination with endomyocardial biopsy, excisional biopsy or pericardial fluid cytology, to establish a definitive diagnosis. A 60-year-old immunocompetent man who presented with superior vena cava syndrome secondary to a right atrial, primary cardiac diffuse large B cell lymphoma (non-Hodgkin’s lymphoma) is described in the present article. The patient had no clinical evidence of disseminated lymphoma and was successfully treated with prompt surgical excision of his atrial mass, followed by anthracycline-based chemotherapy. The patient required multimodality cardiac imaging to accurately identify and plan surgical excision of his cardiac lymphoma. The therapeutic management and clinical and radiological features of primary cardiac lymphoma are reviewed.
Keywords: Atrium, Lymphoma, Pathology, SVC syndrome, Tumour
Abstract
Les lymphomes cardiaques primaires sont des lymphomes extranodaux rares qu’il faudrait distinguer d’une atteinte cardiaque secondaire par lymphome non hodgkinien disséminé. Les lymphomes cardiaques imitent souvent d’autres néoplasmes cardiaques, y compris les myxomes et les angiosarcomes, et exigent souvent une imagerie cardiaque multimode, conjointement avec une biopsie endomyocardique, une biopsie-exérèse ou une cytologie du liquide péricardique, afin de poser un diagnostic définitif. Un homme immunocompétent de 60 ans qui a consulté en raison d’un syndrome de compression de la veine cave supérieure secondaire à un lymphome cardiaque primaire diffus à grandes cellules bêta (lymphome non hodgkinien) est décrit dans le présent article. Le patient ne présentait aucune constatation clinique de lymphome disséminé et a reçu un traitement concluant par excision chirurgicale rapide de la masse auriculaire, suivi d’une chimiothérapie à l’anthracycline. Il a dû subir une imagerie cardiaque multimode pour repérer avec précision et planifier l’excision chirurgicale du lymphome cardiaque. L’auteur examine la prise en charge thérapeutique et les caractéristiques cliniques et radiologiques du lymphome cardiaque primaire.
Primary cardiac lymphoma (PCL) is a rare cardiac neoplasm comprising 1.3% of cardiac tumours and approximately 0.5% of all extranodal lymphomas (1). By definition, PCLs are non-Hodgkin’s lymphomas that involve only the heart and/or pericardium (1). Diagnosis is ultimately confirmed by tumour biopsy or pericardial fluid cytology (1–3). Early diagnosis and therapy are key to establishing good clinical outcomes. An immunocompetent patient with a rare, primary cardiac diffuse large B cell lymphoma who presented with superior vena cava (SVC) syndrome is described in the present study. An integrated radiological approach helped define disease extent both pre- and postoperatively.
CASE PRESENTATION
A 60-year-old immunocompetent man presented to his family physician with a two-week history of face and neck swelling and erythema. The patient’s medical history was unremarkable. There was no history of chest pain, dyspnea or fever. The patient’s cardiac examination revealed no murmurs or extra heart sounds.
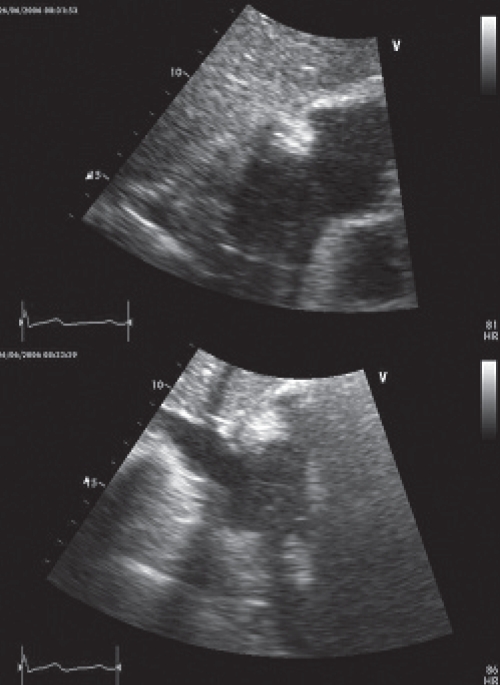
A chest computed tomography (CT) scan revealed heterogeneity of the right atrium and raised concerns of a possible mass. A transthoracic echocardiogram was performed and revealed a right atrial, soft tissue density measuring 3.5 cm × 4.5 cm (Figure 1). The mass appeared to partially occlude SVC flow, but did not appear to extend into either vena cavae. The patient’s remaining echocardiographic parameters were normal. During the scan, the patient demonstrated worsening facial plethora when lying supine, and an urgent surgical referral for SVC syndrome was made.
Figure 1).
Transthoracic echocardiogram with subcostal views showing a homogenous, immobile, soft tissue density measuring 3.5 cm × 4.5 cm within the right atrium. The mass appears to partially occlude superior vena cava flow but does not appear to extend into either vena cavae
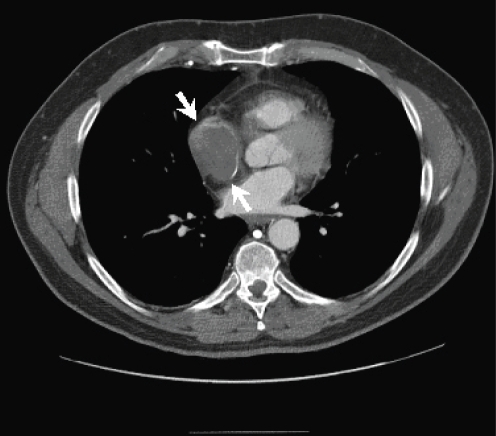
Because there was concern that the patient could not tolerate the extended supine position required for magnetic resonance imaging (MRI), a CT with contrast was performed, which revealed a 4.9 cm tumour that was nearly filling the right atrium (Figure 2). There was no extension into the SVC. All other cardiac structures were uninvolved.
Figure 2).
Computed tomography scan demonstrating a 4.9 cm right atrial mass (arrows) that nearly fills the atrial chamber. A thin rim of contrast enhancement surrounding the right atrial mass is also seen. There is no evidence of mediastinal lymphadenopathy or pulmonary/skeletal disease
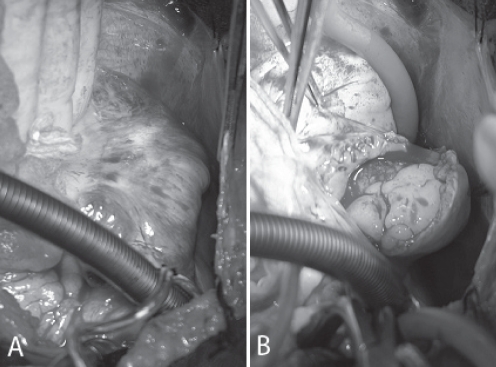
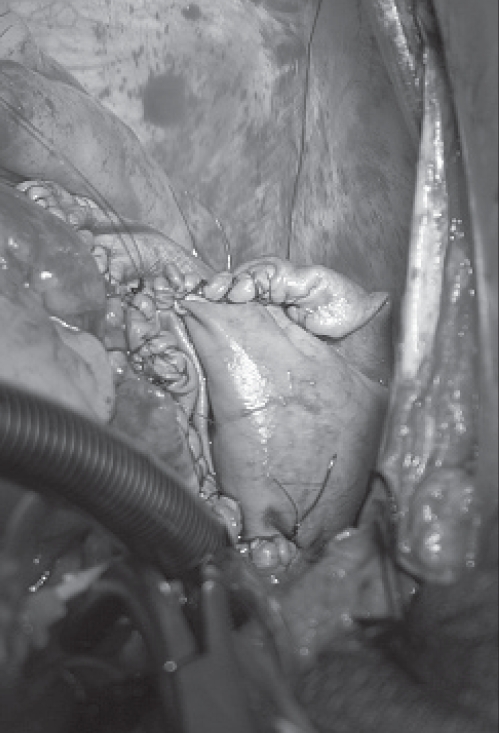
Due to pending SVC obstruction, the patient underwent urgent surgical excision of the atrial mass. At surgery, the tumour immediately bulged out through the sclerotic right atrium (Figure 3A) and revealed an anteromedial atrial attachment (Figure 3B). Pericardial disease was not identified. Following surgical excision, there appeared to be residual tumour along the posterosuperior atrial resection margin. However, further safe excision was not possible, and a bovine pericardial patch was anastomosed to the extensive resection margin (Figure 4).
Figure 3).
A Future right atriotomy site demonstrating surface sclerosis. B Incision into the right atrium, 1 cm lateral to the atrioventricular junction, and the course of the right coronary artery revealed an atrial mass that immediately bulged out through the atriotomy. The mass had an anteromedial attachment and did not invade the vena cavae or other cardiac structures
Figure 4).
Bovine pericardial patch anastamosed to the extensive resection margin
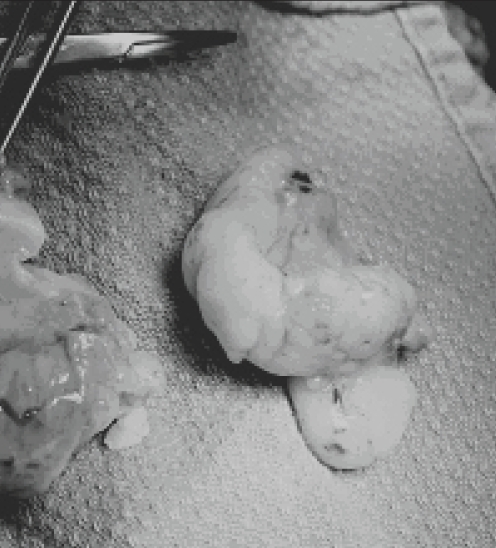
The excised atrial mass was soft and homogeneous, measured 5.5 cm in greatest dimensions and exhibited a ‘fish-flesh’ gross appearance, suggestive of a lymphoproliferative disorder (Figure 5). Histopathological examination revealed complete destructive replacement of the myocardial wall by a diffuse large B cell lymphoma that demonstrated plasmacytoid differentiation (non-Hodgkin’s lymphoma). This primary myocardial diffuse large B cell lymphoma was characterized by morphology, immunohistochemistry, conventional cytogenetics and comparative genomic hybridization as described by Schell et al (4). No intraoperative frozen section analysis was performed. However, a pathologist was immediately consulted on surgical excision of the cardiac mass to grossly evaluate the neoplasm and select sections of tumour for cytogenetic analysis (fresh tumour placed in tissue culture medium) and snap freezing in isopentane and liquid nitrogen for future immunohistochemical and molecular studies. Separately submitted resection margins, including margins from the SVC, inferior vena cava and aorta, were all negative for malignancy. The posterosuperior atrial resection margin, which was suspected to be involved by lymphoma intraoperatively, was not biopsied because there was substantial risk of cardiac perforation.
Figure 5).
Excised primary cardiac lymphoma demonstrating a homogeneous ‘fish-flesh’ gross appearance that is often typical of lymphoproliferative disorders. Histopathological examination confirmed the tumour to be a diffuse, large B cell lymphoma with plasmacytoid differentiation
Because there was concern of residual disease, postoperative cardiac MRI was performed. Multiplanar, multisequence MRI, including cine studies and postgadolinium imaging, revealed focal residual disease involving the posterosuperior, lateral right atrial wall. Staging CT scans of the head, neck, chest, abdomen and pelvis did not reveal any abnormalities. A bone marrow biopsy and lumbar puncture were negative for lymphoma.
The patient underwent six cycles of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone chemotherapy. He also received one dose of high-dose methotrexate following cycle 3 of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone chemotherapy to ensure adequate central nervous system penetration of chemotherapy. Considering the risk of arrhythmia and/or cardiac perforation, due to lysis of residual neoplastic disease, the patient was admitted to the coronary care unit for monitoring during chemotherapy initiation. Although the patient developed an episode of febrile neutropenia, no cardiac complications were encountered during his chemotherapy. Symptoms of SVC syndrome were alleviated immediately following surgery. Serial echocardiography, MRI and positron emission tomography scanning have demonstrated complete tumour remission, and the patient is well 22 months after his initial surgery.
DISCUSSION
Primary cardiac lymphomas are rare neoplasms and comprise 1.3% of all cardiac tumours (1). To date, fewer than 90 tumours have been described in the English literature, mostly as single case reports (3). Primary cardiac lymphomas must be distinguished from the most common benign tumours of the heart, myxoma and other cardiac neoplasms, including angiosarcoma – the most common primary malignancy of the heart. Because PCLs are often aggressive neoplasms, prompt diagnosis and therapy (chemotherapy and/or surgery) are key successful clinical outcomes.
Cardiac tumours have been called the great mimickers and can present with a variety of symptoms. There are no pathognomonic features of PCL, and patients may be investigated for arrhythmia, chest pain, heart failure, pericardial effusion and syncope (2,3). Various imaging modalities may be used for initial diagnosis including chest radiograph, echocardiography, CT and MRI (2,3). Diagnosis is ultimately confirmed by endomyocardial or excisional biopsy or pericardial fluid cytology (1–3). Cardiac involvement by disseminated lymphoma should be distinguished from PCL, with the former being documented in approximately 20% of autopsy cases (5). More than two-thirds of patients with PCL demonstrate right atrial involvement; PCL should be included in the differential diagnosis of any right atrial mass, especially if there is a history of refractory pericardial effusion (1–3,5).
On echocardiography, cardiac lymphomas most commonly manifest as circumscribed nodular myocardial masses, often with an associated pericardial effusion (2). Cardiac lymphomas may also present as ill-defined, infiltrative masses (2,5), as in our case, which was difficult to detect by echocardiography and was only visualized in subcostal views. In the largest series of PCLs (3), all patients with a cardiac mass were identified by echocardiography. Because time to diagnosis is a major prognostic factor for patients with PCL, adequate radiological imaging is vital to prompt biopsy and diagnosis (2,3).
The initial characterization of a cardiac neoplasm is best initiated using an integrated imaging approach due to the highly variable gross pathological features of cardiac tumours. Our case fulfilled the 2006 appropriateness criteria for evaluation of intra- and extracardiac structures by cardiac CT and MRI because of the technically limited images obtained from echocardiography and the need to evaluate possible pericardial involvement.
A multimodality imaging approach for PCLs is necessary because these tumours demonstrate variable radiological features, including variable contrast enhancement patterns (2,5). On MRI, cardiac lymphomas may demonstrate high- or low-signal intensity; on CT, they may demonstrate increased or decreased contrast enhancement and may appear similar or less dense than cardiac muscles (5). Pericardial thickening with or without pericardial effusion may also be the only abnormalities in PCLs (2,5).
The majority of PCLs (80% of published cases) are clinically aggressive diffuse large B cell lymphomas (2,3,5). Rare T cell lymphomas have been reported (3). Late diagnosis appears to be the major cause of poor outcomes in PCL (2). Early clinical suspicion of PCLs is important to improving patient outcomes. While anthracycline-based systemic chemotherapy is the only effective treatment that can offer a potential cure (3), there is a definite role for surgical excision of PCLs when patients have a false-negative biopsy and when patients are at risk for life-threatening hemodynamic compromise, as occurred in our patient (2).
CONCLUSIONS
Although PCLs are rare neoplasms, they should be included in the differential diagnosis of cardiac tumours, especially ones that present in the right atrium and demonstrate evidence of tissue invasion (1–3). Early recognition of PCLs with prompt anthracycline-based chemotherapy provides patients with the greatest potential for cure.
Acknowledgments
The authors thank Mr TR for making the present study possible.
REFERENCES
- 1.Burke A, Virmani R. Atlas of Tumor Pathology (Third series, fascicle 16): Tumors of the Heart and Great Vessels. Washington: Armed Forces Institute of Pathology; 1996. pp. 171–9. [Google Scholar]
- 2.Ceresoli GL, Ferreri AJM, Bucci E, Ripa C, Ponzoni M, Villa E. Primary cardiac lymphoma in immunocompetent patients: Diagnostic and therapeutic management. Cancer. 1997;80:1497–506. doi: 10.1002/(sici)1097-0142(19971015)80:8<1497::aid-cncr18>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Nascimento AF, Winters GL, Pinkus GS. Primary cardiac lymphoma: Clinical, histologic, immunophenotypic, and genotypic features of 5 cases of a rare disorder. Am J Surg Pathol. 2007;31:1344–50. doi: 10.1097/PAS.0b013e3180317341. [DOI] [PubMed] [Google Scholar]
- 4.Schell AJ, Xu Y, Baetz T, et al. Primary cardiac lymphoma: Molecular cytogenetic characterization of a rare entity. Cardiovasc Pathol. 2009;18:92–9. doi: 10.1016/j.carpath.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Rolla G, Galligaris-Cappio F, Burke AP. Cardiac lymphomas. In: Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, editors. World Health Organization Classification of Tumours Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. 1st edn. Lyon: IARC Press; 2004. pp. 282–3. [Google Scholar]