Abstract
Nonpolio enterovirus infections are common in the neonatal period, accounting for a large portion of febrile illness during the summer months. Unlike older children and adults, some neonates with enterovirus infection progress to multisystem disease and death. Multiple clinical syndromes of varying severity are associated with neonatal enterovirus infection: asymptomatic viral shedding, nonspecific febrile illness, aseptic meningitis, hepatic necrosis and coagulopathy, and myocarditis. In the present paper, a typical case of neonatal febrile illness is presented and the English-language literature is reviewed with respect to enteroviral infection in early infancy. The virology, epidemiology, transmission, clinical features, diagnosis and treatment of neonatal enteroviral infection are presented. Although the majority of infections in the neonate are benign, timely diagnosis in the febrile neonate will expedite efficient management. Clinicians also need to recognize the clinical manifestations and risk factors for severe disease to anticipate complications and implement intensive management of infants at high risk of adverse outcomes.
Keywords: Enterovirus, Infant, Neonate
Abstract
Les infections à entérovirus non poliomyélitiques sont courantes pendant la période néonatale, et elles sont responsables d’une forte proportion des maladies fébriles pendant les mois d’été. Contrairement aux enfants plus âgés et aux adultes, certains nouveau-nés atteints d’une infection à entérovirus voient leur état se détériorer en maladie multisystémique et finissent par mourir. Des syndromes cliniques multiples de gravités variées s’associent à une infection à entérovirus néonatale : une excrétion virale asymptomatique, une maladie fébrile non spécifique, une méningite aseptique, une myocardite et une nécrose hépatique accompagnée d’une coagulopathie. Dans le présent article, un cas classique de maladie fébrile néonatale est présentée et la documentation scientifique de langue anglaise sur l′infection entérovirale pendant la première enfance est analysée. La virologie, l′épidémiologie, la transmission, les caractéristiques cliniques, le diagnostic et le traitement de l′infection entérovirale néonatale sont présentés. Bien que la majorité des infections du nouveau-né soient bénignes, un diagnostic rapide du nouveau-né fiévreux permet d’accélérer une prise en charge efficace. Les cliniciens doivent également reconnaître les manifestations cliniques et les facteurs de risque d’une maladie grave afin d’anticiper les complications et d’adopter une prise en charge intensive des nourrissons très vulnérables à des issues négatives.
CASE PRESENTATION
A six-day-old boy presented in June with lethargy and a rash. He was born at term by repeat elective Cesarian section to a 30-year-old G4P3 mother after an uneventful pregnancy. The infant’s birth weight was 3625 g and there were no perinatal complications. The mother and infant were discharged home on day 3 of life. On day 6, the infant became lethargic, would not wake for feeding, and had a weak suck and cry. His mother had developed fever, chills and nausea three days postpartum and had noted a rash on her abdomen that spread to her extremities. On examination, the baby had normal vital signs and was afebrile. Pertinent findings were hypotonia, delayed capillary refill time and a maculopapular rash over the trunk. Laboratory investigations revealed a white blood cell (WBC) count of 16.5×109/L, a hemoglobin concentration of 140 g/L and liver enzymes within normal limits. Examination of the cerebrospinal fluid (CSF) revealed a WBC count of 4×106/L, a red blood cell count of 19×106/L, protein of 0.75 g/L and glucose of 2.6 mmol/L. Bacterial cultures of blood, urine and CSF were performed. Empirical antimicrobial therapy was initiated with intravenous ampicillin, gentamicin and acyclovir, and discontinued after five days because all bacterial cultures were negative and the infant was clinically improving. The diagnosis of enteroviral meningitis was confirmed when the CSF viral culture yielded coxsackie virus group B after seven days of incubation. Coxsackie B was also isolated from cultures of stool and nasopharyngeal aspirate. CSF viral polymerase chain reaction (PCR) was not requested at initial sampling, and because the diagnosis was confirmed by viral culture and the infant was improving, a repeat collection was not performed for PCR testing. An electrocardiogram and cardiac clinical assessment were normal. An echocardiogram was not performed. The infant was discharged from hospital after nine days, with no apparent adverse outcome.
INTRODUCTION
The preceding case report illustrates a typical presentation of a clinically important infection. Nonpolio enterovirus (EV) infections are common in the neonate and are potentially fatal. The presentation and prognosis of EV infections differ in many respects between the neonate and the older child or adult. The following discussion summarizes aspects of the virology, epidemiology, transmission, clinical features, diagnosis and treatment of EV infection in the young infant, based on a review of the English-language literature. Pertinent articles were identified through a MEDLINE search (key words: ‘enterovirus’, ‘neonate’ or ‘infant’) and through the literature referenced in key studies.
Although several textbook chapters and review articles of EV infection emphasize the severity of this disease in the newborn period (1–3), EV infections are, in fact, common in the neonatal period, and most cases are either asymptomatic or minimally symptomatic and without long-term sequelae. This diagnosis should be strongly considered in the sick or febrile infant when these viruses are circulating in the community, usually during the summer and fall. When this diagnosis is considered, it can be confirmed in a timely fashion with the new molecular diagnostic tests. In a minority of cases, characterized by hepatic or cardiac involvement, EV infection may be severe or fatal. Recognizing the risk factors for severe illness will help clinicians institute early and aggressive management in the sickest patients. The present review will aid clinicians to appreciate the epidemiology, spectrum of clinical manifestations, current diagnostic testing and risk factors for this potentially life-threatening disease.
VIROLOGY
EVs are nonenveloped, single-stranded RNA viruses in the family Picornaviridae. They include the polioviruses, coxsackie viruses, echoviruses and EVs, with each group composed of multiple serotypes. Clinical manifestations of neonatal infection with the different EV strains are indistinguishable, with the exception of the polioviruses, which can cause a characteristic transverse myelitis, and coxsackie virus group B, which is usually implicated in cases of EV myocarditis (2). Echovirus serotype 11 and coxsackie virus group B are responsible for a large portion of severe neonatal infections (4,5). The present review focuses on nonpolio EV infections, which are clinically and epidemiologically distinct from disease caused by poliovirus.
EVs are able to withstand acidic gastric pH and survive at room temperature for several days, allowing efficient fecal-oral transmission. Pathogenesis involves entry through oral or respiratory epithelium, replication in lymphoid tissue and dissemination through the blood to produce manifold clinical presentations after an incubation period of two to 10 days (2).
EPIDEMIOLOGY
EV infections are common in the neonatal period (6–8). The best estimate of incidence comes from a prospective epidemiological survey from Rochester, New York, USA, which found that 12.8% of all newborns tested positive for EV from throat or stool cultures during a typical EV season (June to October) (6). Despite this surprisingly high incidence, 79% of infections were asymptomatic. The remaining 21% of infections were characterized by lethargy and fever, and all infants were admitted to hospital, representing a hospitalization rate due to EV infection of at least seven per 1000 live births (6). To put this number in perspective, EVs accounted for more hospitalizations than group B streptococcus, herpes simplex virus and cytomegalovirus combined during the same time period (6). Other studies cite incidence rates of 26 to 50 per 100,000 live births (5,9); however, these figures likely represent underestimates of the true disease burden. These retrospective analyses, based on laboratory records of EV isolates, probably fail to capture a significant number of cases of clinically unrecognized, non-specific EV infection. EV is a frequent etiological agent of nonspecific febrile illness in the young infant, accounting for 47% to 63% of cases requiring hospitalization to rule out bacterial sepsis (7,8). In summary, EV infection is common in the neonate; however, many infections are asymptomatic and many others present as a nonspecific febrile illness.
In temperate climates, a distinct seasonal pattern of EV infection exists, with 64% to 73% of infections occurring between May and September (9,10). Therefore, clinicians should be particularly mindful of this diagnosis when EVs are circulating in their community, usually during the summer and fall months.
TRANSMISSION
Newborn EV infection may be acquired vertically from an infected mother in utero, at the time of delivery or postnatally. Alternatively, EV may be transmitted from community sources or through nosocomial spread after birth. Approximately 11% to 22% of serious EV infections are acquired transplacentally, as evidenced by the onset of disease within the first two days of life and the isolation of virus from amniotic fluid or cord blood (4,5). The dominant mode of transmission of serious neonatal infection (in 63% of serious neonatal echovirus infections) is likely at the time of delivery through contact with maternal blood, fecal material, or vaginal or cervical secretions (4).
Nosocomial transmission was well documented in a literature review (4) of 16 nursery outbreaks involving 206 neonates, and appears to result in less severe disease. Mortality among index cases is higher than that of secondary cases in nursery outbreaks (4), and studies have consistently shown that infection within the first week of life (probably through vertical transmission) is associated with higher mortality than disease of later onset (probably through postnatal transmission) (4,5,10,11). The infant acquiring EV from an infected mother is at particularly high risk of severe infection. The close contact with contaminated secretions, the high inoculum size, maternal and transplacental viremia, a lack of transplacentally acquired antibody and impaired neonatal immunity may all contribute to this increased susceptibility of the neonate born to an infected mother.
CLINICAL SYNDROMES
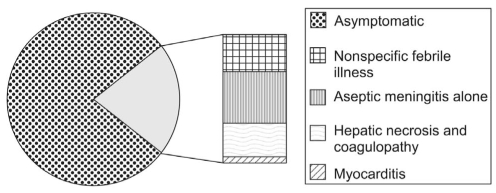
Nonpolio EV infection occurs in a spectrum of illness ranging from asymptomatic viral shedding to fatal multi-system disease. The majority (79%) of EV infections are asymptomatic, and most symptomatic neonates are hospitalized (6). The approximate distribution of clinical manifestations among hospitalized infants is reflected in a recently published retrospective series (10) of 146 hospitalized patients completed over a nine-year period in Taiwan, in which 30% of patients had a clinical syndrome of non-specific febrile illness, 40% had aseptic meningitis, and 30% had severe infection or hepatic necrosis and coagulopathy (HNC). No fatalities occurred among infants with nonspecific febrile illness or isolated aseptic meningitis, whereas the mortality rate in the group with HNC was 24%. In the subgroup of seven patients who also had myocarditis, the mortality rate reached 71% (10). These data illustrate that the common manifestations of EV infection have a low mortality, while the potentially fatal complications (ie, HNC and myocarditis) are much less common. Figure 1 summarizes the data from these two studies to illustrate the relative incidence of various neonatal EV syndromes.
Figure 1.
Spectrum of neonatal enterovirus infections. Adapted from references 6 and 10
Nonspecific febrile illness
EVs play an important and perhaps under-recognized etiological role in the clinical syndrome of neonatal nonspecific febrile illness. In one series (7), among 58 infants younger than 30 days of age who presented to the emergency room with a febrile illness requiring blood culture or lumbar puncture, 47% had EV as the sole pathogen. In another study (8), EVs were responsible for 63% of 110 hospitalizations of infants younger than three months of age during the summer and fall months. Symptoms and signs of EV-positive patients (eg, fever, irritability, poor feeding, cough, lethargy and rash) were not significantly different from those of patients who tested negative (8) and were indistinguishable from the features of bacterial sepsis. Reflecting this overlap of clinical findings, 94% of EV-positive patients were treated with antibiotics and 97% were hospitalized (8). The prognosis is favourable, as shown in one study (10) where no deaths or long-term sequelae occurred among 42 patients after nonspecific febrile illness. Thus, the clinical importance of this common entity is the medical and economic burden related to the hospitalization and unnecessary antibiotic treatment of these febrile infants.
Central nervous system infection
Enteroviral meningitis or meningoencephalitis is a relatively common clinical syndrome with a good prognosis if seen in isolation but with significant mortality when accompanied by multisystem disease. Case definitions in the literature include isolation of EV from a CSF sample, or CSF pleocytosis and negative CSF bacterial culture with identification of EV from another site (10,12). Combining the data from three studies (4,10,13), a total of nine deaths occurred among 143 patients with enteroviral meningitis. In seven cases, another organ system disease was present (pneumonia in one and myocarditis in six). In the largest of these studies (10), no deaths occurred in the 61 patients with aseptic meningitis alone. In summary, enteroviral central nervous system (CNS) disease carries a low risk of mortality, except when associated with other organ system involvement.
Several studies have addressed the question of whether enteroviral meningitis early in life leads to neurodevelopmental sequelae. The first study (14) compared 19 children (aged one to 64 months) with CNS EV infection with matched control subjects at a mean age at evaluation of approximately five years. Among the 13 children whose illness occurred in the first year of life, the mean IQ was statistically lower than that of the control group (97 versus 115, respectively). However, the clinical significance of this difference is questionable because the group with enteroviral meningitis had intelligence scores within the normal range (14). The second study (12) compared nine infants with enteroviral meningitis with matched control subjects and found no difference in the mean IQ level as assessed by the Stanford-Binet Intelligence Scale or the Bayley Scale of Infant Development at a mean age at follow-up of 45 months (12). A third study (13) compared 33 survivors of neonatal enteroviral meningitis to 31 of their siblings. None of the survivors had adverse neurological sequelae and they performed as well as their siblings on tests of cognitive, perceptual-motor, language, memory and emotional-behavioural functions (13). While there is a lack of convincing evidence of developmental delay following early enteroviral meningitis, larger studies adjusting for infection severity (ie, multisystem involvement) would help clarify the long-term prognosis for this group of patients.
Severe neonatal disease: HNC and myocarditis
Although most EV infections in infancy have a benign outcome, there exists the potential for serious adverse outcome and death in this age group. Two retrospective studies (4,5) document mortality attributable to EV infection in the neonate: a case series and literature review of 41 fatal neonatal coxsackie B infections (5), and a literature review of 61 serious neonatal echovirus infections with an 83% case-fatality rate (4). While multiple organ systems may be involved (including the CNS, liver, heart, lung, pancreas and adrenal glands), the terminal events are usually cardiovascular collapse and/or hemorrhagic phenomena (4,5,15). Therefore, the following discussion will focus on the clinical syndromes of HNC and myocarditis, both of which have a high mortality. While these syndromes are explored separately, it should be noted that multiple organ systems may be involved simultaneously: in one series, 50% of patients with HNC had concurrent meningitis and 17% had concurrent myocarditis (10).
Case definitions in the literature of EV-associated HNC include the following criteria: serum transaminase levels greater than three times the normal level, platelet count less than 100×109/L, prothrombin time greater than two times the control value, or evidence of hepatic necrosis or extensive hemorrhage on autopsy (5,10,15). Most cases of HNC present within the first week of life, earlier in onset than the syndromes of nonspecific febrile illness and aseptic meningitis (10). At presentation, clinical findings of HNC are nonspecific. In a case series (15) of 16 patients with HNC, prominent symptoms included jaundice (69%), lethargy (69%), decreased feeding (56%), fever (50%) and decreased perfusion (50%). Hemorrhagic complications occur frequently, including intracranial bleeding (38% in one series) (15) and pulmonary hemorrhage (5). The reported case fatality rate among infants with HNC is 24% to 31% (10,15). Factors significantly associated with death in HNC syndrome are an elevated bilirubin level (reflecting more severe hepatic dysfunction) and concurrent myocarditis (10). Among survivors of HNC, normal liver function tests, platelet count, coagulation profile, height and weight were observed at follow-up nine to 48 months after their illness (15).
EV myocarditis in the infant heralds a dismal prognosis (5,10,15). In one EV study (10), it was defined as an ejection fraction of less than 50% on echocardiography, arrhythmia and elevation of the cardiac fraction of creatinine kinase. Unlike other clinical syndromes that can be caused by multiple EV strains, the etiological agent of EV myocarditis is usually coxsackie virus group B (2). The clinical course of fatal infection follows one of three patterns: rapid progression of illness to death at two to 17 days of age; a diphasic illness with initial improvement, followed by marked deterioration and death at eight to 14 days of age; or progressive disease terminating in death at two to 28 days of age (5). In two independent series (10,15), the mortality associated with myocarditis was 71% (five of seven) and 100% (five of five).
Risk factors for severe disease
Because EV infections vary greatly in severity, understanding the risk factors for severe infection may help clinicians identify infants at risk for adverse outcomes to institute early and aggressive management. In the largest case series to date (10), involving 146 patients with EV infection and 42 cases of HNC, features independently and significantly associated with severe infection or HNC were prematurity (37 weeks or less), maternal history of illness, early age of onset of illness (younger than seven days of age), higher WBC count (15×109/L or greater) and low hemoglobin (107 g/L or lower) (10). Maternal illness and early-onset disease were identified as risk factors in other studies (4,15), which reflects the higher mortality associated with vertical as opposed to postnatal transmission. An elevated WBC count may reflect a higher degree of inflammation and consequent hepatic and/or myocardial damage, and low hemoglobin may signal a bleeding diathesis. Table 1 outlines these risk factors along with the odds ratio for severe (HNC) versus milder (febrile illness and aseptic meningitis) disease.
TABLE 1.
Risk factors for severe enteroviral disease
| Risk factor | OR (95% CI)* | P |
|---|---|---|
| Prematurity | 6.6 (1.5–29) | 0.012 |
| Maternal illness | 6.0 (1.2–29) | 0.027 |
| Onset before one week of age | 49 (8.4–290) | 0.001 |
| Elevated white blood cell count | 6.8 (1.1–27) | 0.006 |
| Low hemoglobin | 29 (5.2–160) | 0.001 |
For hepatic necrosis and coagulopathy versus febrile illness or aseptic meningitis. Data from reference 10
DIAGNOSIS
Accurate and timely diagnosis of EV infection can be helpful in excluding other conditions, such as bacterial sepsis, thereby reducing unnecessary antibiotic use and hospital admission. Moreover, early diagnosis may help clinicians anticipate complications in those infants initially presenting with severe disease. The specific diagnosis is best confirmed by identification of EV from clinical samples by nucleic acid amplification or viral culture. Infected infants carry high viral loads and shed virus from mucosal surfaces; thus, EV is readily isolated from throat swabs or stool samples (1). In more serious infections, EV may be identified in blood, CSF, urine, ascitic fluid, biopsy or autopsy specimens (16).
Assays using PCR to amplify viral nucleic acid for detection have revolutionized EV diagnosis. The technique is rapid, sensitive and specific, and requires small volumes of clinical material (1). Because EVs share common genomic sequences, PCR-based assays will detect almost all serotypes (17). PCR has gained acceptance as the diagnostic modality of choice over viral culture and serology by virtue of its rapidity and sensitivity (17). While there are commercial assays available, their use is not standard and many laboratories use PCR techniques developed ‘in-house’. Therefore, the sensitivity and specificity may vary between diagnostic laboratories.
Compared with PCR, the sensitivity of viral culture is approximately 75%, probably because viral RNA is more stable to specimen collection, transportation and handling than are viable viral particles (17). While EVs grow rapidly in cell culture (three to seven days) relative to other viruses, this time frame is still too protracted to be useful for clinical management. Nonetheless, viral culture enables the virus serotype to be identified, which may be important in epidemiological investigations (1).
Although the serological detection of EV is possible because neonates mount a humoral antibody response (1), the clinical utility of serology is limited and it is not recommended for diagnosis. The large number of possible EV serotypes to be tested and the need for acute and convalescent specimens (1) make serology impractical for acute diagnostic purposes.
TREATMENT
Specific therapies available for the treatment of EV infection include intravenous immune globulin (IVIG) and the antiviral agent pleconaril. However, evidence for their efficacy in the neonatal population is scant. There are case reports of clinical success using IVIG both for prophylaxis against EV infection during nursery outbreaks and for treatment of symptomatic newborns (18). On the other hand, randomized administration of IVIG to nine neonates with EV infection did not reduce the daily incidence of viremia and viruria compared with a control group (19).
Pleconaril prevents picornaviral replication by inhibiting viral uncoating and by blocking viral attachment to host cell receptors (18). In children and adults, pleconaril shortens the duration of enteroviral meningitis (18), and has been used to treat chronic EV infection, viral myocarditis and vaccine-associated poliomyelitis in immunocompromised patients (17). Three studies (20–22) were identified that examined the use of pleconaril for the treatment of EV infection in infants. In one uncontrolled case series (20) comprising six neonates with overwhelming EV infection treated with pleconaril, complete recovery was observed in four infants, developmental delay in one infant and death in one infant. In another small series (21), two of three neonates with life-threatening enteroviral hepatitis recovered fully after treatment with pleconaril. A randomized, placebo-controlled trial (22) of pleconaril in 20 infants younger than 12 months of age with enteroviral meningitis did not demonstrate a difference between treatment and control groups in terms of the duration of hospitalization, symptoms, or EV positivity by PCR or viral culture. However, the study was discontinued before adequate statistical power was achieved because of low accrual rates.
Therapy with IVIG and/or pleconaril in the neonate with EV infection is of unproven efficacy but may be considered among infants with severe, life-threatening disease.
CASE STUDY REVISITED
The initially nonspecific clinical presentation resembling bacterial sepsis and subsequent treatment with antibiotics is typical of EV infection. As expected in CNS infection without accompanying hepatic or cardiac involvement, the disease followed a benign course. Clinical signs of myocarditis were not observed, although the strain of virus isolated (coxsackie virus group B) is the one usually responsible for this complication. The onset of disease within the first week of life and the history of maternal fever and rash were risk factors for severe disease in the present case. However, the virus may have been transmitted postnatally rather than vertically, given that maternal symptoms began three days postpartum, followed by illness in the baby on day 6 of life. This may account for the milder disease observed. Furthermore, although the WBC count was elevated (16.5×109/L), there was no evidence of hepatic inflammation (the infant had normal liver enzymes) and the hemoglobin was within normal limits (140 g/L), indicating the absence of hemorrhagic phenomena. There was no evidence of cardiac disease. Therefore, this case is illustrative of the typical progress and favourable outcome of enteroviral meningitis without concurrent HNC or myocarditis in the neonate.
CONCLUSIONS
Nonpolio EVs are important pathogens in the neonatal period. They account for a significant portion of febrile illness requiring hospitalization in this age group, particularly during the summer months. The spectrum includes the common but generally benign syndromes of asymptomatic infection, nonspecific febrile illness and aseptic meningitis, as well as the uncommon but potentially fatal syndromes of HNC and myocarditis. An awareness of the seasonality and the various clinical syndromes associated with EV infection, as well as the appropriate diagnostic testing, will help clinicians to recognize this common viral infection in a timely manner. The recognition of the risk factors for severe disease will help clinicians to exercise vigilance when managing neonates at high risk of an adverse outcome.
ACKNOWLEDGEMENT
Special thanks to Izabela Sztukowski for her help in preparing the manuscript.
REFERENCES
- 1.Modlin J. Coxsackievirus and echovirus disease in the newborn infant. In: Mandell GL, Bennet JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 5th edn. Philadelphia: Churchill Livingstone; 2000. pp. 1910–1. [Google Scholar]
- 2.Zaoutis T, Klein JD. Enterovirus infections. Pediatr Rev. 1998;19:183–91. doi: 10.1542/pir.19-6-183. [DOI] [PubMed] [Google Scholar]
- 3.Casey J, Pichichero ME. Nonpolio enterovirus infections: A new era. Contemp Pediatr. 2001;18:82–8. [Google Scholar]
- 4.Modlin JF. Perinatal echovirus infection: Insights from a literature review of 61 cases of serious infection and 16 outbreaks in nurseries. Rev Infect Dis. 1986;8:918–26. doi: 10.1093/clinids/8.6.918. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan MH, Klein SW, McPhee J, Harper RG. Group B coxsackievirus infections in infants younger than three months of age: A serious childhood illness. Rev Infect Dis. 1983;5:1019–32. doi: 10.1093/clinids/5.6.1019. [DOI] [PubMed] [Google Scholar]
- 6.Jenista JA, Powell KR, Menegus MA. Epidemiology of neonatal enterovirus infection. J Pediatr. 1984;104:685–90. doi: 10.1016/s0022-3476(84)80944-0. [DOI] [PubMed] [Google Scholar]
- 7.Rotbart HA, McCracken GH, Jr, Whitley RJ, et al. Clinical significance of enteroviruses in serious summer febrile illnesses of children. Pediatr Infect Dis J. 1999;18:869–74. doi: 10.1097/00006454-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Dagan R, Hall CB, Powell KR, Menegus MA. Epidemiology and laboratory diagnosis of infection with viral and bacterial pathogens in infants hospitalized for suspected sepsis. J Pediatr. 1989;115:351–6. doi: 10.1016/s0022-3476(89)80831-5. [DOI] [PubMed] [Google Scholar]
- 9.Verboon-Maciolek MA, Krediet TG, van Loon AM, et al. Epidemiological survey of neonatal non-polio enterovirus infection in the Netherlands. J Med Virol. 2002;66:241–5. doi: 10.1002/jmv.2136. [DOI] [PubMed] [Google Scholar]
- 10.Lin TY, Kao HT, Hsieh SH, et al. Neonatal enterovirus infections: Emphasis on risk factors of severe and fatal infections. Pediatr Infect Dis J. 2003;22:889–94. doi: 10.1097/01.inf.0000091294.63706.f3. [DOI] [PubMed] [Google Scholar]
- 11.Abzug MJ, Levin MJ, Rotbart HA. Profile of enterovirus disease in the first two weeks of life. Pediatr Infect Dis J. 1993;12:820–4. doi: 10.1097/00006454-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Wilfert CM, Thompson RJ, Jr, Sunder TR, O’Quinn A, Zeller J, Blacharsh J. Longitudinal assessment of children with enteroviral meningitis during the first three months of life. Pediatrics. 1981;67:811–5. [PubMed] [Google Scholar]
- 13.Bergman I, Painter MJ, Wald ER, Chiponis D, Holland AL, Taylor HG. Outcome in children with enteroviral meningitis during the first year of life. J Pediatr. 1987;110:705–9. doi: 10.1016/s0022-3476(87)80006-9. [DOI] [PubMed] [Google Scholar]
- 14.Sells CJ, Carpenter RL, Ray CG. Sequelae of central-nervous-system enterovirus infections. N Engl J Med. 1975;293:1–4. doi: 10.1056/NEJM197507032930101. [DOI] [PubMed] [Google Scholar]
- 15.Abzug MJ. Prognosis for neonates with enterovirus hepatitis and coagulopathy. Pediatr Infect Dis J. 2001;20:758–63. doi: 10.1097/00006454-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Dagan R. Nonpolio enteroviruses and the febrile young infant: Epidemiologic, clinical and diagnostic aspects. Pediatr Infect Dis J. 1996;15:67–71. doi: 10.1097/00006454-199601000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Sawyer MH. Enterovirus infections: Diagnosis and treatment. Pediatr Infect Dis J. 1999;18:1033–40. doi: 10.1097/00006454-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Rotbart HA. Antiviral therapy for enteroviral infections. Pediatr Infect Dis J. 1999;18:632–3. doi: 10.1097/00006454-199907000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Abzug MJ, Keyserling HL, Lee ML, Levin MJ, Rotbart HA. Neonatal enterovirus infection: Virology, serology, and effects of intravenous immune globulin. Clin Infect Dis. 1995;20:1201–6. doi: 10.1093/clinids/20.5.1201. [DOI] [PubMed] [Google Scholar]
- 20.Rotbart HA, Webster AD Pleconaril Treatment Registry Group. Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin Infect Dis. 2001;32:228–35. doi: 10.1086/318452. [DOI] [PubMed] [Google Scholar]
- 21.Aradottir E, Alonso EM, Shulman ST. Severe neonatal enteroviral hepatitis treated with pleconaril. Pediatr Infect Dis J. 2001;20:457–9. doi: 10.1097/00006454-200104000-00022. [DOI] [PubMed] [Google Scholar]
- 22.Abzug MJ, Cloud G, Bradley J, et al. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Double blind placebo-controlled trial of pleconaril in infants with enterovirus meningitis. Pediatr Infect Dis J. 2003;22:335–41. doi: 10.1097/01.inf.0000059765.92623.70. [DOI] [PubMed] [Google Scholar]