Abstract
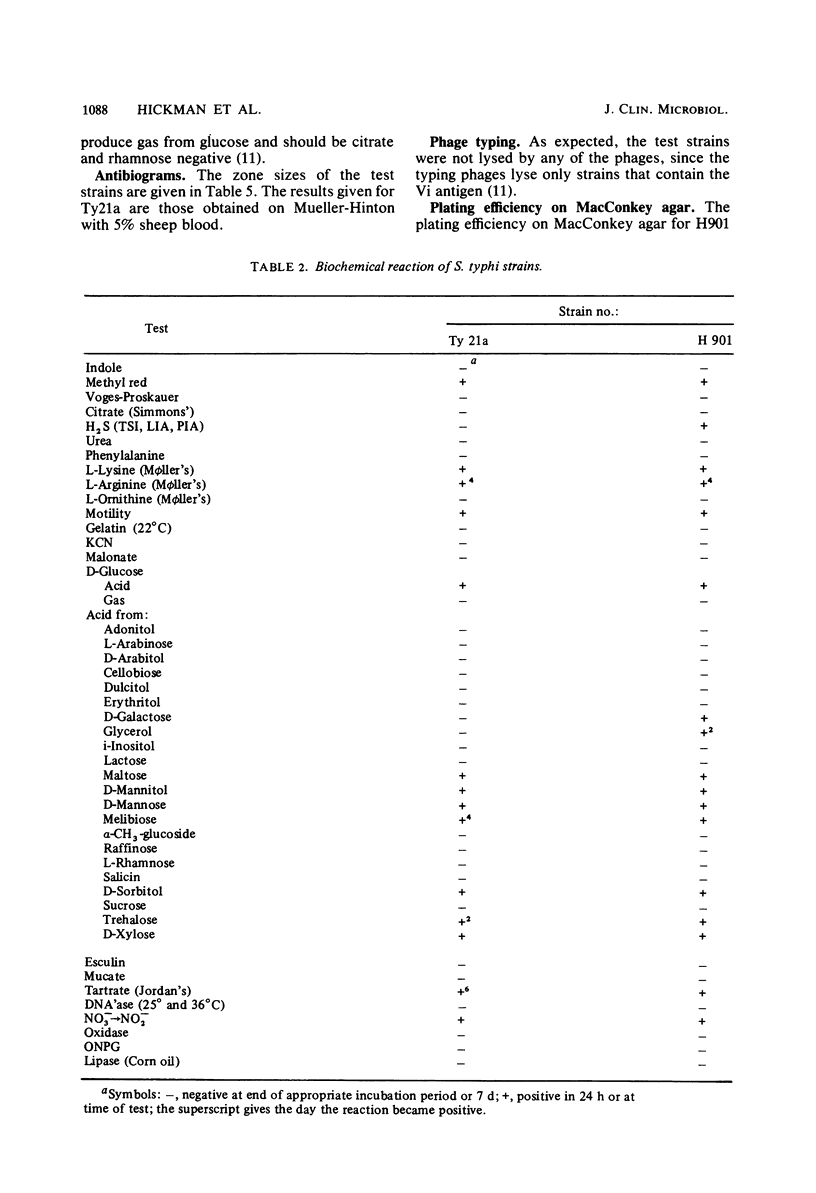
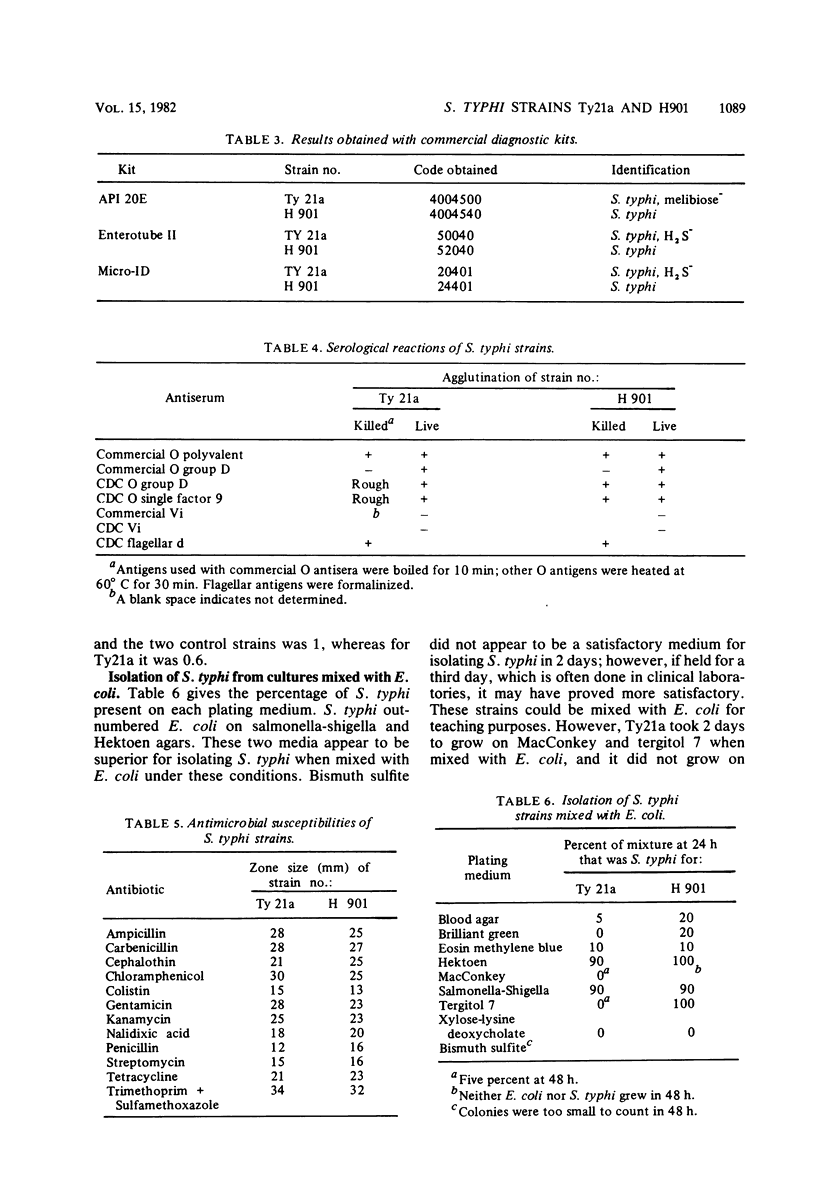
A total of 21 cases of laboratory-acquired typhoid fever associated with teaching and proficiency tests occurred in the United States during a 33-month period, prompting a search for less virulent strains of S. typhi which would be suitable for teaching purposes. Two strains were evaluated which are reported to have reduced virulence for mice. Strain Ty21a is a genetically constructed mutant that lacks the enzyme UDP-glucose-4-epimerase. This strain has reduced virulence for humans if grown under special laboratory conditions (in the presence of 0.1% d-galactose) and has been evaluated as a candidate for use as a live, oral vaccine. Strain H901 was originally isolated in Russia in 1918. It has not been tested in humans, but its nonmotile variant, O901, has been found to be somewhat less virulent for humans; however, it can cause infection with doses of 107 organisms. In teaching exercises, all strains should be treated as though they are fully virulent. Ty21a and H901 were satisfactory, but not ideal, for teaching purposes. Biochemically, they could be identified by conventional tests and by commercially available diagnostic systems, although Ty21a was H2S negative. Serologically, both strains posed problems. Both Ty21a and H901 were Vi antigen negative, and Ty21a was rough and grew poorly. Both strains were susceptible to antibiotics, including chloramphenicol, ampicillin, and trimethoprim-sulfameth-oxazole. When Ty21a and H901 were mixed with Escherichia coli and plated, Hektoen and salmonella-shigella agars were most useful for their recovery. The appearance of Ty21a and H901 on differential plating media was typical, although Ty21a had smaller colonies. The plating efficiency on MacConkey agar for Ty21a was 0.6 compared with 1 for H901. Neither strain can be recommended unequivocally for teaching purposes; instead, the advantages and disadvantages of each must be considered. Both strains have been deposited in the American Type Culture Collection (Ty21a = ATCC 33459 = CDC 2861-79; H901 = ATCC 33458 = CDC 2862-79).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J., Hickman F. W., Farmer J. J., 3rd, Brenner D. J., Balows A., Feldman R. A. Salmonella typhi: the laboratory as a reservoir of infection. J Infect Dis. 1980 Dec;142(6):934–938. doi: 10.1093/infdis/142.6.934. [DOI] [PubMed] [Google Scholar]
- Dupont H. L., Hornick R. B., Snyder M. J., Dawkins A. T., Heiner G. G., Woodward T. E. Studies of immunity in typhoid fever. Protection induced by killed oral antigens or by primary infection. Bull World Health Organ. 1971;44(5):667–672. [PMC free article] [PubMed] [Google Scholar]
- FORMAL S. B., BARON L. S., SPILMAN W. Studies on the virulence of a naturally occurring mutant of Salmonella typhosa. J Bacteriol. 1954 Jul;68(1):117–121. doi: 10.1128/jb.68.1.117-121.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanier R., Füer E. Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis. 1975 May;131(5):553–558. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- Gilman R. H., Hornick R. B., Woodard W. E., DuPont H. L., Snyder M. J., Levine M. M., Libonati J. P. Evaluation of a UDP-glucose-4-epimeraseless mutant of Salmonella typhi as a liver oral vaccine. J Infect Dis. 1977 Dec;136(6):717–723. doi: 10.1093/infdis/136.6.717. [DOI] [PubMed] [Google Scholar]
- Hickman F. W., Farmer J. J., 3rd Salmonella typhi: identification, antibiograms, serology, and bacteriophage typing. Am J Med Technol. 1978 Dec;44(12):1149–1159. [PubMed] [Google Scholar]
- Hornick R. B., Greisman S. E., Woodward T. E., DuPont H. L., Dawkins A. T., Snyder M. J. Typhoid fever: pathogenesis and immunologic control. N Engl J Med. 1970 Sep 24;283(13):686–691. doi: 10.1056/NEJM197009242831306. [DOI] [PubMed] [Google Scholar]


