Abstract
Hippocampal-based behavioral memories and hippocampal-based forms of synaptic plasticity, such as long-term potentiation, are divisible into short- and long-term phases, with the long-term phase requiring the synthesis of new proteins and mRNA for its persistence. By contrast, it is less clear whether long-term depression (LTD) can be divisible into phases. We here describe that in stable hippocampal organotypic cultures, LTD also is not a unitary event but a multiphase process. A prolonged stimulus of 900 stimuli spaced at 1 Hz for 15 min induces a late phase of LTD, which is protein- and mRNA synthesis-dependent. By contrast, a short train of the same 900 stimuli massed at 5 Hz for 3 min produces only a short-lasting LTD. This short-lasting LTD is capable of capturing late-phase LTD. The 5-Hz stimulus or the prolonged 1-Hz stimulus in the presence of protein synthesis inhibitors each can be transformed into an enduring late phase of depression when the prolonged stimulus is applied to another input in the same population of neurons.
Bidirectional modifications in synaptic efficacy, exemplified by presynaptic facilitation and inhibition in Aplysia and long-term potentiation (LTP) and long-term depression (LTD) in the mammalian brain, are thought to contribute to certain types of information storage. The capacity for bidirectional change at the synapse and the ability to store not only increases but also decreases in synaptic weight allows for great precision, flexibility, and subtlety in the long-term encoding of patterns of synaptic activity.
LTP, an activity-dependent increase in synaptic strength, can be divided into an early phase that is short-lasting and a longer-lasting late phase that depends for its stabilization on gene transcription and new protein synthesis (1, 2). These early and late phases of LTP have a correlate in behavioral memory, which consists of short-term memory lasting minutes to hours and a more permanent long-term memory lasting days or longer, which requires gene transcription and protein synthesis (3, 4).
The finding that neurons use transcription and translation for long-term synaptic changes raises questions in the biology of memory. Is the unit for long-term change the nerve cell or the synapse? Can a neuron selectively change the synaptic input to any one of its hundreds of dendrites without changing others? This selectivity becomes stringent when new mRNA and proteins must be synthesized in the cell body and sent to the cell's dendritic branches that received the appropriate input. Can some of these dendritic branches become altered in a more enduring, protein synthesis-dependent manner and some not?
Studies of long-term facilitation in Aplysia and LTP in the hippocampus have led to the discovery of synaptic tagging or capture (4–6). During hippocampal LTP or long-term facilitation in Aplysia, potentiated synapses may become tagged by a protein synthesis-independent mark generated by the short-term early process. Under appropriate conditions, this mark then can sequester the relevant proteins necessary to modify the synapse in a protein synthesis-dependent manner. A single tetanic stimulus, which leads only to early facilitation or early LTP, can result in the protein synthesis-dependent late phase of facilitation or late LTP as long as repeated stimulation is applied to another input within a specific window of time (5–8). An activated synapse thereby can capture previously or subsequently made proteins to exhibit long-lasting alterations in synaptic efficacy.
So far, synaptic capture has been studied only in relation to facilitating synaptic actions. However, the extensive distribution of inhibiting processes in the brain raises questions regarding the generality of synaptic capture. Is it limited to the capture of facilitation-related proteins? Or is the capacity for bidirectional modification expansive enough to encompass the phenomenon of synaptic tagging and capture?
LTD, an activity-dependent weakening of synaptic strength, has been described in many parts of the brain, with various induction protocols. Can LTD also be divided into a short-lasting early phase and a more prolonged-lasting late phase that depends on gene transcription and protein synthesis?
Studies performed in a culture preparation of cerebellar Purkinje neurons have revealed an input-specific late phase, requiring metabotropic glutamate receptor (mGluR) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor activation for induction, that is blocked by translation inhibitors, transcription inhibitors, or somatic/nuclear removal (9). In addition, local postsynaptic mRNA translation has been found to be required for the induction of mGluR-dependent LTD in the hippocampus (10). Thus, there are examples of protein synthesis-dependent LTD. However, we were particularly interested in a prominent form of N-methyl-d-aspartate (NMDA) receptor-dependent LTD (11, 12), because only this form of LTD is capable of reversing LTP (13).
In our initial set of experiments (E.R.K., unpublished data), NMDA-dependent LTD in acute hippocampal slices did not reveal a protein synthesis-dependent phase of expression (see also ref. 10). We then examined LTD in an optimally stable preparation, hippocampal slice cultures. Because these cultures steady their cytoarchitecture and processes over the course of a week in culture, their stability is greater than that of acute slices (14). For example, the cut processes of acute slices have been found to release stimulatory, inhibitory, and even toxic substances and display important differences in spine and synapse number relative to in vivo states (15). Using the organotypic culture preparation, we here describe evidence for phases of expression in an NMDA-dependent form of LTD in the hippocampus, show that this LTD manifests a difference between massed and spaced training, and provide evidence of tagging and synaptic capture in LTD.
Materials and Methods
Organotypic slice cultures from 8- to 9-day-old Sprague–Dawley rats were prepared in accordance with previously described methods (14). After 8–12 days in culture, slices were tested in a submerged chamber and perfused with artificial cerebrospinal fluid containing 119 mM NaCl, 2.5 mM KCl, 1.3 mM MgSO4-7H2O, 2.5 mM CaCl2 solution, 26.2 mM NaHCO3, 1 mM NaH2PO4-H2O, and 11 mM glucose (equilibrated with 95% O2 and 5% CO2) at a flow rate of 1 ml min−1.
Monopolar glass electrodes filled with artificial cerebrospinal fluid, with a resistance of 1–4 MΩ, were positioned in the CA3 cell body layer for focal stimulation. Glass recording electrodes, with a resistance of 1–4 MΩ, were filled with 3 M NaCl and placed in the stratum radiatum of the CA1 region. All experiments were done at room temperature and began after perfusing the slices for 1–3 h. In each experiment, a full input-output curve was determined. A stimulus strength that yielded between one-half and two-thirds of the maximal response was selected for baseline measurements. Test stimuli were applied at a frequency of 1 per min, with duration of 0.2 ms. The excitatory postsynaptic potential (EPSP) slope (mV/ms) was measured from the average waveform recorded from five consecutive stimuli. EPSP slopes were collected and analyzed with custom software written in the labview programming environment.
The late phase of LTD (L-LTD) was elicited by using 900 pulses delivered at 1 Hz. The conditioning stimulation for the early phase of LTD (E-LTD) was 900 pulses delivered at 5 Hz. Theta burst stimulation consisted of 10 stimulus trains delivered at 5 Hz, with each train consisting of four pulses at 100 Hz. Experiments using control artificial cerebrospinal fluid were interleaved with those using inhibitors. Capture experiments were interleaved with single path experiments. Actinomycin D was prepared as a stock solution (0.1% DMSO) and then diluted to the specified final concentration. Application of DMSO (0.1%) alone had no effect on LTD. All other drugs were dissolved in artificial cerebrospinal fluid immediately before application.
As the cultures were found to be more susceptible to baseline drift than acute slices, baseline responses were monitored on-line. Experiments were begun on cultures where baseline drift was no more than approximately 5% during a 30-min baseline period. Experiments were discontinued if drug application affected baseline responses in excess of this percentage. Statistics were calculated by measuring EPSP slopes before and after the depression protocol. Because in some cases drug application did lead to some baseline drift (although not more than the drift permitted during the overall course of 30 min, as discussed above), the representative before/after comparison was made by taking the average of baseline responses during the last 10 min of the baseline and comparing it to the average of a 10-min period 1 h after inducing LTD. Statistical evaluation was done by using the two-tailed Mann–Whitney U test.
Results
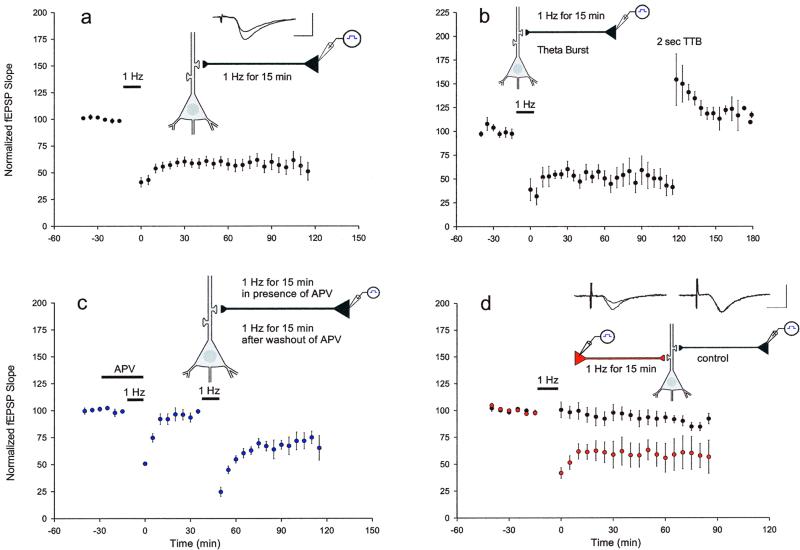
Extracellular field potentials were recorded at the CA3–CA1 synapse mediated by the Schaffer collaterals. The cell bodies, of the CA3 pyramidal cell neurons that give rise to the Schaffer collateral axons, were stimulated directly to insure that the Schaffer collateral axons were selectively stimulated. This type of stimulation produced larger and more reliable field EPSP responses as compared with stimulation of the Schaffer collateral axons themselves. In addition, this stimulation led to less drift in baseline responses, which were continuously monitored on-line in accordance with parameters described above. We elicited LTD with an induction protocol of 1 Hz for 15 min, which gave rise to long-lasting depression, with an average decrease in the EPSP after 1 h to 61 ± 5% of pretetanus baseline values. The decrease in the EPSP persisted for the duration of the experiment, typically 2 h (Fig. 1a). This prolonged depression was a manifestation of synaptic plasticity and not the result of fatigue or damage to the synapses. Indeed, theta burst stimulation of 2 s completely reversed the previously established LTD (Fig. 1b) (16).
Figure 1.
Characteristics of LTD evoked with a stimulus of 1 Hz for 15 min at the Schaffer collateral–CA1 synapses. Shown are the time courses of % changes in field EPSP slopes. (a) Prolonged 1-Hz stimulation for 15 min resulted in a significant long-lasting depression (n = 12). Labeled 1-Hz bar indicates interval of stimulation. (Inset) Representative EPSP traces recorded before and 1 h subsequent to the depression protocol. (The same succession of traces is shown for each experiment. Calibration bars for each experiment are 0.5 mV, 5 ms.) (b) LTD was elicited with 1-Hz stimulation. After 2 h of depression, theta burst stimulation (TTB) was applied. LTD was completely reversed and LTP was induced (n = 4). (c) Application of the NMDA antagonist APV (50 μM) (labeled horizontal bar) blocked induction of LTD. After washout of APV, LTD was elicited with 1-Hz stimulation (n = 5). (d) Two independent inputs to the same population of postsynaptic cells were stimulated in an alternating manner. Only one pathway received the depression protocol. LTD was induced only in the synapses that received the depression protocol (n = 7).
Two forms of LTD have been reported to coexist in the CA1 region of the hippocampus (13, 17). One form is not blocked by the NMDA receptor antagonist dl-2-amino-5-phosphonovaleric acid (APV) (10, 18, 19), whereas the other form depends on the activation of NMDA receptors (11, 12). We found that the LTD induced by stimulation of 1 Hz for 15 min was completely and reversibly blocked by APV (50 μm), suggesting it is mediated by the NMDA receptor (Fig. 1c).
Another characteristic of LTD is synapse specificity, which has been demonstrated in acute slices (11, 12, 17). To test whether the LTD was synapse-specific, we stimulated two independent groups of CA3 cells synapsing on the same population of CA1 cells, at a rate of 1 stimulus per min for each pathway. To one of these pathways we applied an LTD protocol of 1-Hz stimulation for 15 min, whereas the other pathway served as a control pathway and continued to receive test stimuli at a frequency of 1 per min. LTD was induced only in the synapses that received stimulation of 1 Hz for 15 min, indicating that the LTD was homosynaptic and synapse-specific (Fig. 1d).
Taken together, the results described in Fig. 1 reveal an LTD that is more profound than that observed in acute slices, but that displays characteristics similar in other respects to those seen in acute slices. The LTD is persistent and synapse-specific, can be reversed by LTP, and is NMDA-dependent. Indeed, these characteristics are shared by LTP. Several lines of evidence suggest that these NMDA-dependent forms of LTP and LTD in the Schaffer collateral pathway may occur by reversible modifications of common mechanisms of induction or expression (refs. 20 and 21; reviewed by ref. 22). It is well established that LTP has phases that can be distinguished by inhibitors of protein and RNA synthesis (1, 23, 24). We next explored the possibility that LTD recorded in organotypic slices also may be divisible into temporal phases.
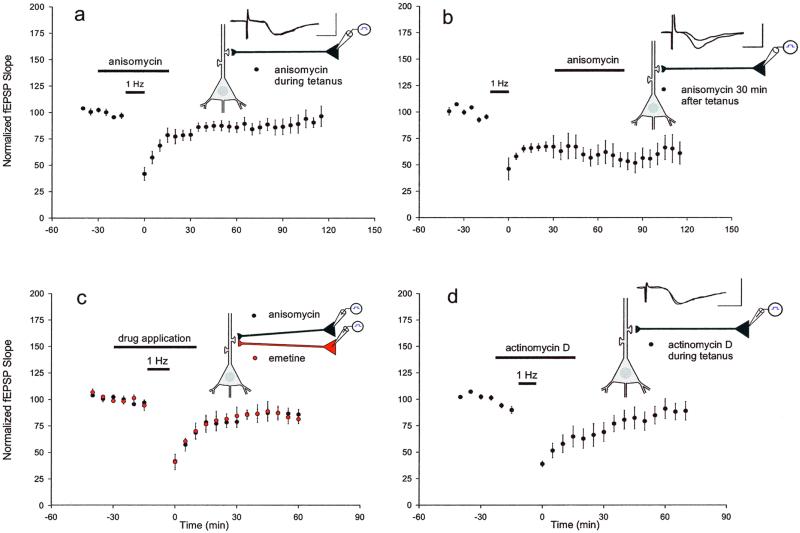
We first applied anisomycin (30 μM), an inhibitor of protein synthesis, 15 min before initiating the 1-Hz depression protocol and continued to perfuse with inhibitor until 15 min afterward (for a total of 45 min) and found that this inhibitor blocked the induction of L-LTD for all time points later than 40 min. The mean EPSP slope 1 h after the tetanus was 91 ± 6% of baseline (Fig. 2a), significantly different from control slices (P < 0.01). However, as in LTP and behavioral memory, the effectiveness of the drugs occurs only during a specific window of vulnerability. Protein synthesis inhibitors interfere with the consolidation of information storage only during a critical time interval. When the application of anisomycin was delayed until 30 min after the tetanus, there was no blockade of L-LTD. The mean EPSP slope 1 h after the depression protocol was 62 ± 7.8% of baseline, not significantly different from control slices (Fig. 2b) (P > 0.5).
Figure 2.
Induction of L-LTD with 1-Hz stimulation was blocked by inhibitors of translation and transcription. (a) Application of the protein synthesis inhibitor anisomycin (30 μM) (beginning 15 min before the 1-Hz depression protocol until 15 min afterward, horizontal bar) blocked L-LTD for time points subsequent to 40 min (n = 9). (b) When anisomycin application was delayed until 30 min after the 1-Hz depression protocol, there was no effect on L-LTD maintenance (n = 3). (c) Similarly, when the protein synthesis inhibitor emetine (20 μM) was applied (beginning 15 min before the 1-Hz stimulus until 15 min afterward), the induction of L-LTD was prevented for time points later than 40 min (n = 4). Field EPSPs after anisomycin or emetine application were both significantly different from control EPSPs (P < 0.01, measured 1 h after the 1-Hz stimulus). (d) Application of the transcriptional inhibitor actinomycin D (20 μM) (beginning 15 min before the 1-Hz stimulus and continuing until 15 min afterward) blocked L-LTD for time points subsequent to 60 min (n = 6; P < 0.05, as compared with control EPSPs, measured 1 h after the 1-Hz stimulus).
Inhibition was not limited to anisomycin. We obtained similar results with another protein synthesis inhibitor, emetine. When emetine (20 μM) was added to the bath medium for 15 min before, during, and 15 min subsequent to the depression protocol, it also prevented the induction of L-LTD. The mean field EPSP slope 1 h after the stimulation was 87 ± 4.4% of baseline (Fig. 2c). As was the case with anisomycin, mean field EPSPs after emetine application were significantly different from EPSPs measured in control slices (P < 0.01).
A requirement for protein synthesis also could involve the transcription of genes. In fact, both long-term memory in the intact animal and the late phase of LTP exhibit a significant requirement for gene transcription as well as mRNA translation (2). To test for a concomitant requirement in L-LTD, we applied actinomycin D (20 μM, 0.1% DMSO), which inhibits transcription, for 15 min before, during, and for 15 min subsequent to the 1-Hz depression protocol. Actinomycin D blocked L-LTD for all time points later than 60 min. The mean field EPSP slope 1 h after the stimulation was 92 ± 10% of baseline, significantly different from EPSPs measured in control slices at this time (P < 0.05) (Fig. 2d). The blockade of L-LTD by actinomycin D took longer to develop than the blockade resulting from the application of anisomycin or emetine. The inhibition of transcription by actinomycin D suggests that the induction of L-LTD requires gene expression in addition to translation of existing mRNAs.
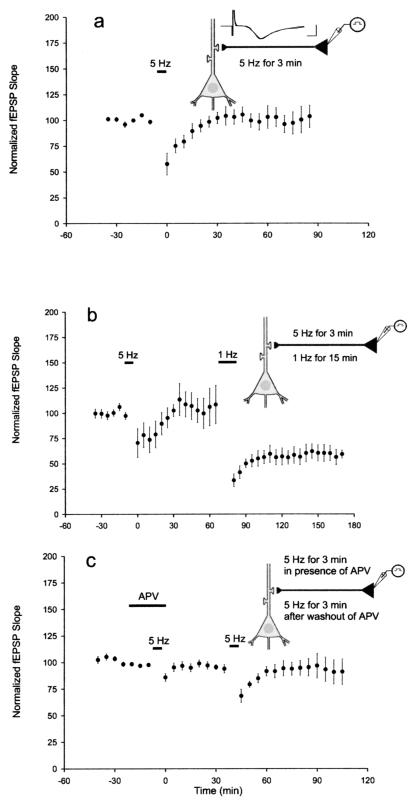
An E-LTD was unmasked by the application of inhibitors of translation and transcription. We next explored the possibility of inducing E-LTD by itself, in the absence of inhibitors, by varying the pattern of electrical stimulation from spaced to massed. We found that delivering the same 900 pulses at 5 Hz for 3 min led to a shorter, more attenuated LTD that approached baseline by 30 min (Fig. 3a). The slices did not exhibit L-LTD and showed a time course that was similar to the anisomycin-treated slices and the emetine-treated slices. The mean field EPSP slope was 98 ± 7.6% of baseline after 1 h. To confirm that synapses that received 5-Hz stimulation were capable of exhibiting L-LTD, we also carried out a set of experiments inducing LTD with first 5-Hz stimulation and then 1-Hz stimulation at the same synapses. As before, 5-Hz stimulation produced E-LTD. However, 1-Hz stimulation at the same synapses led to a persistent and enduring L-LTD with a mean field EPSP slope of 57 ± 7.7% of baseline after 1 h (Fig. 3b).
Figure 3.
Characteristics of LTD evoked with a stimulus of 5 Hz for 3 min. (a) A weak stimulation protocol of 5 Hz for 3 min elicited a more attenuated short-term depression (E-LTD) that returned to baseline for time points later than 30 min (n = 10). (b) E-LTD was elicited with 5-Hz stimulation. Subsequent delivery of 1-Hz stimulation to the same synapses led to an enduring L-LTD (n = 4). (c) The NMDA antagonist APV was applied (labeled horizontal bar) and blocked the induction of LTD. After washout of APV, LTD was elicited with 5-Hz stimulation (n = 7).
The E-LTD induced by the massed 5-Hz stimulus depended on NMDA activation; APV (50 μM) completely and reversibly blocked LTD. This suggests that the massed protocol, like the spaced protocol, is mediated by the NMDA receptor (Fig. 3c).
Studies in LTP have revealed at least two major phases elicited in turn by one train (for 1 s at 100 Hz) or three or more trains (for 1 s at 100 Hz) (1, 25). We now propose that LTD also is not a unitary phenomenon, but may be divisible into phases. A massed stimulus of 5 Hz elicits E-LTD (comparable in time course to LTD elicited in the presence of translation inhibitors), whereas a spaced, prolonged low-frequency stimulation of 1 Hz induces L-LTD that requires protein synthesis for its consolidation.
If L-LTD requires transcription and translation, then the issue arises as to how the depressed synapses of a single neuron become selectively modified by the newly synthesized proteins without affecting the unmodified sites within the same neuron. The synaptic tag or synaptic capture hypothesis (4–6) suggests that only when an activated synapse is tagged is it able to sequester or capture plasticity-related proteins that were synthesized (in the soma or dendrites) and widely distributed throughout the dendritic tree.
We tested whether capture might occur during the protein synthesis-dependent phase of LTD by examining the interaction between two independent populations of CA3 neurons synapsing on the same postsynaptic CA1 neurons. To test for independence, we used a paired-pulse paradigm, in which we first stimulated one input and then 30 ms later stimulated the second input (and vice versa), because if the pathways were independent there would be no paired-pulse effect. We then positioned the stimulating electrodes to ensure that we were stimulating two separate pathways. As illustrated in Fig. 1d, 1-Hz stimulation of one pathway had minimal effect on the second input that did not receive the stimulation. Synaptic efficacy in the test pathway after 1 h was 62 ± 10.6% of baseline whereas the control pathway was 93 ± 6.2% of baseline (P < 0.01).
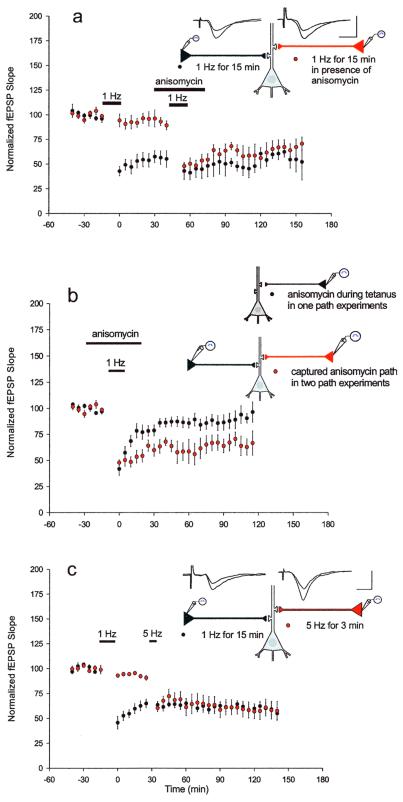
In the first set of experiments testing the synaptic capture hypothesis in LTD, we induced LTD in one pathway with 1-Hz stimulation (Fig. 4a) and added anisomycin to the bath medium 30 min later when, as observed above, the protein synthesis inhibitor did not have an effect on previously established LTD. After 15 min, we stimulated the second pathway with 1-Hz stimulation. Anisomycin remained in the bath during the stimulus and for 15 subsequent min, for a total of 45 min. We found that the second pathway underwent protein synthesis-dependent LTD, even though protein synthesis was inhibited by anisomycin application. The mean EPSP slopes 1 h subsequent to the stimulation of the second pathway were 57 ± 6.2% of baseline for path 1 and 61 ± 8.6% of baseline for path 2, indicating that L-LTD in the second pathway was established (Fig. 4a). The L-LTD exhibited by this pathway was in marked contrast to the E-LTD elicited during the inhibition of protein synthesis in the more simplified one-pathway experiments (Fig. 4b) (P < 0.05).
Figure 4.
The late protein synthesis component of LTD can be captured by another input. (a) L−LTD was induced in one pathway with 1-Hz stimulation. After 30 min, anisomycin was applied (for a total of 45 min) and the second pathway received the depression protocol in the presence of anisomycin. Despite the inhibition of protein synthesis, the second pathway exhibited L-LTD (n = 5). (b) The L-LTD exhibited by the second pathway in a was significantly different from the LTD observed during the inhibition of protein synthesis in the one-pathway experiments described in Fig. 2a (P < 0.05, measured 1 h after the stimulus delivered in the presence of anisomycin). (c) L-LTD was elicited in one pathway with 1-Hz stimulation. After 30 min, E-LTD was elicited in the second pathway with the weaker 5-Hz stimulation. E-LTD in the second pathway was transformed into prolonged L-LTD (n = 7; P < 0.001, as compared with 5-Hz stimulation alone).
Next, we investigated whether the late protein synthesis component of LTD could be captured by a weak input produced by massed stimulation of 5 Hz. Could this also be transformed into L-LTD by the induction of L-LTD at another input?
We induced LTD in one pathway with 1-Hz stimulation (Fig. 4c). After 30 min, we elicited LTD in the second pathway with the weaker 5-Hz stimulus that normally led to short-lasting LTD. Now, however, LTD in the second pathway was transformed into L-LTD, with a mean EPSP slope of 61 ± 8% of baseline 1 h after LTD was induced. LTD in the first pathway had an EPSP slope of 63 ± 5.3% of baseline at this time (Fig. 4c). The depression in the weakly stimulated pathway persisted as prolonged, stable LTD for the duration of the experiment. This result is predicted by the capture hypothesis because proteins generated by LTD in the first pathway can be sequestered by the weakly activated synapses of the second pathway (P < 0.001, as compared with 5-Hz stimulation alone).
Discussion
Our results indicate that NMDA-dependent LTD has phases much like LTP. In addition to a transient phase of LTD (E-LTD) that is mediated by covalent modification of preexisting proteins, there is also a persistent late form of LTD (L-LTD) that requires transcription and translation.
A recent study focused on metabotropic glutamate receptor-dependent LTD in acute hippocampal slices and found a requirement for local protein synthesis in the expression of LTD (10). The study also looked at NMDA-dependent LTD in acute slices and, using the translation inhibitor cycloheximide, found that it did not require protein synthesis. The NMDA-dependent LTD that was observed under control conditions was approximately 85% of baseline after 1 h, whereas the LTD under cycloheximide conditions was approximately 87% of baseline after 1 h. We also observed a similarly small decrease in field EPSPs in acute slices and had a similar difficulty in observing a requirement for protein synthesis. However, we found that organotypic slice cultures offered a more stable system in which to study LTD as the cultures recover from the trauma to their cut processes and show more profound LTD than acute slices. The cultures displayed a substantial and stable LTD of approximately 60% of baseline. We observed LTD under emetine conditions that was the same 87% (after 1 h) observed in the above study. However, we were also able to examine emetine's (and other inhibitors') effects on the more pronounced E-LTD, observed in culture. Studies of profound and protein synthesis-dependent LTD in other parts of the brain also have benefited from culture preparations (9, 26).
The L-LTD that we observed was synapse-specific but could be captured. E−LTD elicited by 5-Hz stimulation was transformed into L-LTD by the induction of L-LTD at the other input. In addition, L-LTD was elicited in the presence of protein synthesis inhibitors when there was a prior induction of L-LTD in the other pathway. Thus, decreases in synaptic strength can have more far-reaching decremental effects on other synapses of the cell, depending on the hippocampal neuron's history of synaptic activity. Such history may now be considered to encompass depression-inducing as well as potentiation-inducing processes. The long-term modification of these processes may likely involve nuclear-mediated integration.
Furthermore, the phenomenon of capture is important not only for the long-term modifications resulting from newly synthesized proteins but also because the occurrence of capture underscores the importance of the early phase. The early phase should no longer be looked on just for its role in leading to covalent modifications but also should be recognized for the part it plays in preparing or biasing a synapse to achieve late-phase modifications.
Only within the last decade have consistent protocols for inducing LTD in vitro been demonstrated (11, 12). Although the physiological correspondence of these protocols to in vivo mechanisms remains to be worked out, these reliable procedures have led to LTD being found in many parts of the brain, including the neocortex, cerebellum, striatum, nucleus accumbens, and hippocampus, and have allowed for a more systematic examination of its characteristics and mechanisms. The behavioral function of hippocampal LTD has remained difficult, however, to ascertain and has been a matter of much speculation in neural network theory (see for example ref. 27) and among physiologists. Among physiologists, one role that has been conceived for LTD has been as part of a learning and memory mechanism that is complementary to the role of LTP. When experiences are recorded, synaptic weights are altered, with some synapses increasing in synaptic weight and others decreasing. Modifying the strength of synaptic connections in both directions provides a straightforward method for improving the flexibility, accuracy, and capacity of information storage (for review, see ref. 22). LTD has been thought, however, to be less robust than LTP in the hippocampus of awake adult animals during behavioral learning tasks. A recent study resolved some questions with regard to LTD's robustness in vivo by finding strain differences in learning between rats. In this study, inducing homosynaptic LTD at 1 Hz for 15 min led to a long-lasting depression in the rat strain that learned to recognize a novel, unstressful environment (28). As suggested by this study, LTD-like stimulations may underlie learning during novel experiences. Therefore, during certain forms of learning, LTD may itself be a memory mechanism, without LTP. However, if a mechanism for decreasing synaptic strength proves to be subtler than a mechanism for increasing synaptic strength, then it may be that LTD provides fine-tuning, adding nuance to its more overt counterpart LTP, as they together shape a dynamic neural landscape.
Acknowledgments
We thank S. Siegelbaum, D. Winder, T. O'Dell, and R. Hawkins for their helpful comments on the manuscript, C. Lam for assistance with the artwork, and H. Ayers and M. Pellan for typing the manuscript. We also thank P. Pavlidis for his elegant software.
Abbreviations
- LTP
long-term potentiation
- LTD
long-term depression
- E-LTD
early-phase LTD
- L-LTD
late-phase LTD
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- NMDA
N-methyl-d-aspartate
- EPSP
excitatory postsynaptic potential
- APV
dl-2-amino-5-phosphonovaleric acid
References
- 1.Huang Y Y, Kandel E R. Learn Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- 2.Nguyen P V, Abel T, Kandel E R. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- 3.Davis H P, Squire L R. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- 4.Goelet P, Castellucci V F, Schacher S, Kandel E R. Nature (London) 1986;322:419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- 5.Frey U, Morris R G. Nature (London) 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 6.Martin K C, Casadio A, Zhu H, Yaping E, Rose J C, Chen M, Bailey C H, Kandel E R. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- 7.Frey U, Morris R G. Neuropharmacology. 1998;37:545–552. doi: 10.1016/s0028-3908(98)00040-9. [DOI] [PubMed] [Google Scholar]
- 8.Casadio A, Martin K C, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey C H, Kandel E R. Cell. 1999;99:221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- 9.Linden D J. Neuron. 1996;17:483–490. doi: 10.1016/s0896-6273(00)80180-4. [DOI] [PubMed] [Google Scholar]
- 10.Huber K M, Kayser M S, Bear M F. Science. 2000;288:1254–1256. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 11.Dudek S M, Bear M F. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulkey R M, Malenka R C. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- 13.Nicoll R A, Oliet S H, Malenka R C. Neurobiol Learn Mem. 1998;70:62–72. doi: 10.1006/nlme.1998.3838. [DOI] [PubMed] [Google Scholar]
- 14.Stoppini L, Buchs P A, Muller D. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 15.Kirov S A, Sorra K E, Harris K M. J Neurosci. 1999;19:2876–2886. doi: 10.1523/JNEUROSCI.19-08-02876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudek S M, Bear M F. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliet S H, Malenka R C, Nicoll R A. Neuron. 1997;18:969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- 18.Bolshakov V Y, Siegelbaum S A. Science. 1994;264:1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- 19.Stanton P K, Chattarji S, Sejnowski T J. Neurosci Lett. 1991;127:61–66. doi: 10.1016/0304-3940(91)90895-z. [DOI] [PubMed] [Google Scholar]
- 20.Luscher C, Xia H, Beattie E C, Carroll R C, von Zastrow M, Malenka R C, Nicoll R A. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- 21.Kameyama K, Lee H K, Bear M F, Huganir R L. Neuron. 1998;21:1163–1175. doi: 10.1016/s0896-6273(00)80633-9. [DOI] [PubMed] [Google Scholar]
- 22.Bear M F, Abraham W C. Annu Rev Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- 23.Krug M, Lossner B, Ott T. Brain Res Bull. 1984;13:39–42. doi: 10.1016/0361-9230(84)90005-4. [DOI] [PubMed] [Google Scholar]
- 24.Stanton P K, Sarvey J M. J Neurosci. 1984;4:3080–3088. doi: 10.1523/JNEUROSCI.04-12-03080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y Y, Kandel E R. Neuron. 1996;16:611–617. doi: 10.1016/s0896-6273(00)80080-x. [DOI] [PubMed] [Google Scholar]
- 26.Ahn S, Ginty D D, Linden D J. Neuron. 1999;23:559–568. doi: 10.1016/s0896-6273(00)80808-9. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Dayan P. Network. 1999;10:59–77. [PubMed] [Google Scholar]
- 28.Manahan-Vaughan D, Braunewell K H. Proc Natl Acad Sci USA. 1999;96:8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]