Abstract
Protein kinase signal transduction pathways play critical roles in regulating nociception. Here we show that c-kit, a tyrosine kinase receptor, is expressed in lamina I and II layer of the dorsal horn. Moreover, the superficial c-kit+ fibers originate from the DRG, and c-kit in lamina II inner layer comes from the intrinsic expression of the spinal cord. KitW-v mice, which contain a hypomorphic mutation, exhibited normal acute pain in most pain behavior tests. In formalin test, the first phase was not affected, whereas the second phase pain response of KitW-v mice was significantly reduced relative to wild-type littermates. KitW-v mice also showed abnormal neuropathic pain, notably in the contralateral side of nerve injury. The expression and release of CGRP and substance P was not altered by the c-kit mutation. Together, these results implicate c-kit-mediated signaling transduction in the development of persistent pain.
Keywords: typrosine kinase receptor, c-kit, DRG, spinal cord, formalin test, CCI, persistent pain
Introduction
The dominant white-spotting (W) and steel (Sl) loci in mouse encode the proto-oncogene c-kit and its ligand, stem cell factor (SCF), respectively [5,9,32]. c-kit is a type III receptor protein-tyrosine kinase and binding of SCF to c-kit leads to activation of downstream signal transduction pathways such as Ras/mitogen-activated protein kinases, Src family kinases, phosphatidylinositol 3-kinase, phospholipase Cγ, and Janus kinase and Jak/STAT pathways [24]. SCF /c-kit signaling pathway has been implicated in myriad developmental and physiological processes including spermatogenesis, hematopoiesis, melanogenesis and oncogenesis [8,15,24,25]. Although both SCF and c-kit have been found to be expressed in glial and neuronal cells during development as well as in adult mice [30], their roles in the nervous system are not well elucidated. In dorsal root ganglion (DRG) neurons, c-kit appears to be expressed in small and/or medium size neurons, which project to the superficial lamina I-II in the dorsal spinal cord [10]. Recombinant murine SCF (rmSCF) induced the outgrowth of c-kit-positive neurites from DRGs isolated from mouse embryos, suggesting that the stimulus through the c-kit receptor tyrosine kinase has an important role in development of the peripheral nervous system [11]. This is supported by the finding that the number of C-fibers is reduced in Sl/Sld and W/Wv, implicating Steel-Kit interactions in neuronal development [16].
Recent studies have suggested an important role of c-kit in acute pain [18,29]. Nevertheless, the SCF/c-kit signaling pathway has not been implicated in the generation and/or maintenance of persistent pain. The present study was carried out to characterize the c-kit positive fibers in the dorsal spinal cord, and investigate the functional role of c-kit in altering acute nociception or pain behavior in chronic pain models by the use of KitW-v mutant mice, which contain a hypomorphic mutation of the c-kit gene. In these mice, the kinase activity of c-kit is reduced by 80% [22] .
2. Materials and methods
2.1 Materials
KitW-v mice (Jackson laboratory) and their wild-type littermates were used in the behavior experiments. The KitW-v mice on C57BL6 background carry a missense point mutation in the kinase domain of c-kit, which results in the reduction of the kinase activity [4,23]. Homozygotes are white in color and heterozygotes have various degrees of white spotting; wild-type mice from this colony are black. Homozygotes and wild-type of both male and female mice between 8 and 12 weeks old were acclimatized to the experimental room and were used for behavioral tests. No obvious difference between the sexes was found in the tests. All the experiments were performed in accordance with the guidelines of the National Institutes of Health and the International Association for the Study of Pain and were approved by the Animal Studies Committee at Washington University School of Medicine.
2.2 Pain behavioral experiments
Thermal sensitivity
Pain behavior tests were performed as described before [27,28]. Thermal sensitivity was determined using hot-plate (48, 52, 56 °C), paw-flick (method of Hargreaves) or water immersion tail-flick methods (48, 50, 52 °C). For the Hargreaves test, thermal sensitivity was measured using a Hargreaves-type apparatus (IITC Inc., Woodland Hills, CA). Baseline of the paw withdrawal latency was calculated as the mean of 3–5 different measurements taken at 15 min intervals. For the hot plate, the latency for the mouse to lick its hindpaw or jump was recorded. For water immersion tail-flick, mice were held gently in a towel and the tips of their tails (between 2 and 3 cm from the tip) were immersed into a temperature-controlled water baths (IITC Inc., Woodland Hills, CA). The flick response is defined as the removal of the tail from the water. A cutoff of 10 sec was applied to prevent damage to the tail.
Mechanical sensitivity
Mechanical sensitivity was assessed using a set of calibrated von Frey filaments (Touch-Test kit, Stoelting, Chicago, IL). Each filament was applied 5 consecutive times and the smallest filament that evoked reflexive withdrawal of the paw on at least 3 out of the 5 trials was taken as the paw withdrawal threshold.
Formalin test
5% Formalin (Sigma, 10 µl in saline) was injected into the plantar surface of one hindpaw and the total time spent licking or flinching of the injected paw was monitored for 60 min and recorded at 5-min intervals.
Chronic constriction injury (CCI)
Chronic constriction injury of the sciatic nerve was induced in mice under pentobarbital anesthesia (60 mg/kg, i.p.), as described [1], with minor modifications. A small incision was made just below the left hip bone, and the biceps femoris was bluntly dissected to reveal the underlying sciatic nerve. The left common sciatic nerve was isolated from surrounding tissues, and two loose ligatures (6-0 chromic gut, Ethicon 1816G) were placed around the nerve proximal to the trifurcation with 1 mm spacing. The ligatures were tied until they just slightly constricted the diameter of the nerve and a brief twitch was seen in the hindlimb. The incision was closed using veterinary tissue glue (Nexaband 5295-04-01). Mechanical sensitivity was tested using calibrated von Frey filaments as described above. On each testing day, five measurements were taken from each hindpaw, and the average was calculated. Mice were tested for baseline withdrawal thresholds prior to CCI surgery and at five-day intervals after surgery until day 30.
2.3 Dorsal rhizotomy
Dorsal rhizotomy was performed as described before [3]. Briefly, in deeply anaesthetized mice (sodium pentobarbital, 60 mg/kg), the lumbar spinal cord was exposed by laminectomy at the lumbar 4 region, and all the exposed left dorsal roots were transected. The animals were perfused 12 days later, and the spinal cord was used for immunocytochemistry.
2.4 Immunocytochemical staining and in situ hybridization
Immunocytochemical staining was performed as previously described [7]. Briefly, mice were anesthetized with sodium pentobarbital (60 mg/kg i.p.) and euthanized by transcardiac perfusion (saline wash, followed by 4% paraformaldehyde in 0.01 M phosphate buffer saline pH 7.4). The mouse spinal cord and DRG was removed and post-fixed for 4 hr in paraformaldehyde (4%), then stored in 0.01 M PBS containing 30% sucrose for at least 24 hr for cryoprotection. For immunocytochemistry, the sections were incubated with antibodies against c-kit (Armenian Hamster, kindly provided by Tatsumi Hirata), calcitonin gene related peptide (CGRP, rabbit, Chemicon), PKCγ (rabbit, Santa Cruz), substance P (SP, rabbit, Peninsula Lab). The secondary antibodies (Jackson ImmunoResearch, West Grove, PA) include: donkey anti-rabbit IgG coupled to FITC, goat anti-Armenian Hamster coupled to Cy3, donkey anti-rabbit IgG coupled to Cy3. For double staining, sections were incubated with c-kit antibody together with PKCγ (rabbit, Santa Cruz), CGRP (rabbit, Chemicon) antibodies or FITC-IB4 respectively. Secondary antibodies (Jackson ImmunoResearch, West Grove, PA) include: Goat anti-Armenian Hamster coupled to Cy3, goat anti-rabbit coupled to FITC. FITC-IB4 (Sigma) was use to visualize the IB4+ fiber. To visualize c-kit mRNA, in situ hybridization was performed as described [7].
2.5 Measurement of SP and CGRP release in the spinal cord
Capsaicin, peptidase inhibitors and other chemicals were purchased from Sigma Chemical Company (St. Louis, MO). A stock concentration of 10 mM capsaicin was prepared using 1-methyl, 2-pyrrolidinone (MPL) purchased from Aldrich Chemical Co. (Milwaukee, WI) and then diluted to 500 nM with HEPES buffer. SP and CGRP were purchased from Bachem (Belmont, CA).
Spinal cord slice preparation
Spinal cord slices were prepared as previously described with slight modifications [6]. Briefly, mice were decapitated and the entire spinal cord, was dissected and chopped first cross-sectionally and then parasagitally into 0.3 × 0.3 mm sections. The tissue was then weighed, placed into a cylindrical perfusion chamber with an internal volume of 0.5 ml, and perfused with HEPES buffer, consisting of HEPES 25 mM, NaCl 135 mM, KCl 3.5 mM, MgSO4 1 mM, CaCl2 2.5 mM, dextrose 3.3 mM, bovine serum albumin 1%, ascorbic acid 200 µM, and the peptidase inhibitors pheala 200 µM, p-chloromercuriphenyl sulfonic acid (PCMS) 50 µM and bacitracin 20 µM. The buffers were aerated with 95% O2 - 5% CO2, pH 7.4–7.5, and maintained at 36–37°C, throughout the experiment. The tissue was perfused at a flow rate of 0.5 ml/min for the first 20 min. Following this initial perfusion, the flow rate was decreased to 0.1 ml/min for collection of perfusate samples. One milliliter fractions were collected into test tubes containing 75 µl of 2-(N-morpholino)ethanesulfonic acid (MES) buffer (1 M, pH 6.7–6.9) every 10 min. Basal release was established by first perfusing the tissue with HEPES buffer for 30 min, after which the tissue was exposed to HEPES buffer containing 500 nM capsaicin for 20 min, to evoke immunoreactive CGRP (iCGRP) and immunoreactive SP (iSP) release. To demonstrate a return to basal release after stimulation, the tissue was perfused with HEPES buffer for another 40 min. At the end of the experiment, the tissue was collected from the chambers and homogenized in 3 ml of 0.01 M HCl, and centrifuged for 20 min at 2500 × g at 4°C. The supernatant was diluted with HEPES buffer and total peptide content remaining in the tissue and the perfusate samples was measured by radioimmunoassay. The amount of peptide released was expressed as per cent of the total peptide content/min.
Radioimmunoassay of iCGRP and iSP
Immunoreactive-SP and iCGRP were assayed from perfusates and tissue aliquots by radioimmunoassay as previously described [6]. Briefly 450 µl of each of the perfusate samples were assayed directly for SP. To each sample, 25 µl of a 1:160,000 dilution of rabbit anti-SP antiserum and 25 µl of 125I-[8Tyr]-SP containing 6000–8000 cpm was added to each sample. For radioimmunoassay of iCGRP, 300 µl aliquots of the perfusate were assayed. 25 µl of a 1: 1,000,000 dilution of CGRP antibody (from Michael J. Iadarola; NIH) and 25 µl of 125I-[0Tyr]-CGRP (28–37) containing 4000–6000 cpm were added to each sample and to standards. The samples were incubated for 16–20 h at 4°C. To separate the unbound labeled peptide from that bound to the antibody, 0.5 ml of a 0.1 M phosphate buffer (pH 7.4) containing 1% Norite charcoal, 50 mM NaCl, and 1 % bovine serum albumin was added to each sample and was centrifuged at 2500 × g for 20 min at 4°C, and the radioactivity in the supernatant was measured by gamma scintillation spectrometry. The amount of iSP and iCGRP in perfusate samples was estimated by comparing the percent bound radioactivity in samples to a standard curve using a 4 point non-linear least squares regression analysis. Using this method, the minimal detectable amount of iSP and iCGRP was 5 fmoles (95% confidence interval). Each chamber contained the spinal cord tissue obtained from one animal and is reported as one experimental sample. Results are expressed as percent of iCGRP or iSP of total tissue content/min, and as fmol/mg of tissue/min. Resting and stimulated release were calculated by taking the mean of the three collections in the presence of capsaicin (stimulated) and the mean of the three collections immediately preceding exposure to capsaicin exposure (basal).
2.6 Statistical analysis
In behavioral studies statistical comparisons were performed with two-way analysis of variance (ANOVA) or Student’s t-test. For peptide release basal, stimulated and evoked release were compared using Student’s t-test. All data were expressed as the mean ± standard error of the mean (s.e.m.) and error bars represent s.e.m. In all cases, P < 0.05 was considered statistically significant.
3. Results
3.1. Expression of c-kit in DRG and the dorsal spinal cord
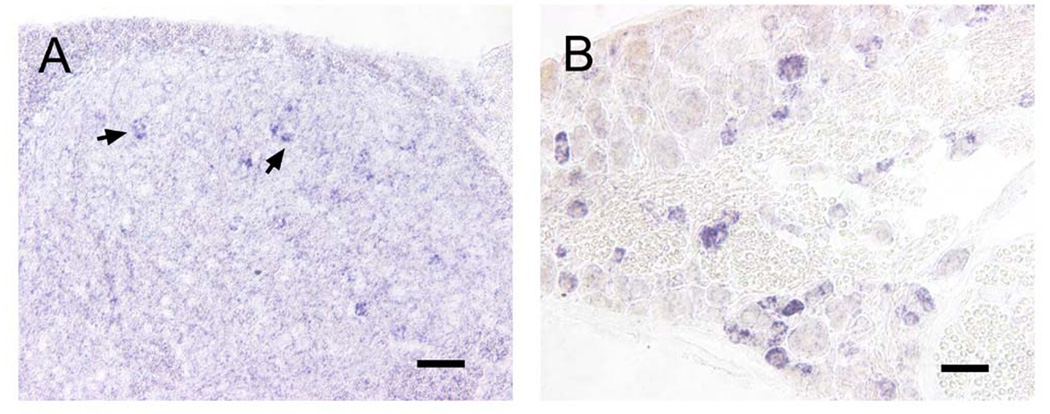
To determine the expression of c-kit in DRGs and the spinal cord of adult mice, we examined the distribution of c-kit mRNA by in situ hybridization. We found c-kit mRNA positive neurons in both DRGs and the spinal cord (Fig. 1). In the DRG of adult mice, c-kit was expressed in a subset of small and medium sized neurons (Fig.1B), which is consistent with previous studies [10,30]. In the spinal cord, c-kit mRNA positive neurons located in the superficial dorsal horn, and few of them were in lamina X, but not in the ventral horn (Fig.1A, and data not shown).
Fig. 1. c-kit mRNA positive neurons were detected in both DRGs and the dorsal spinal cord.
A, c-kit mRNA positive neurons were detected in the dorsal spinal cord (Arrows). B, In situ hybridization showed that c-kit mRNA positive neurons in the DRG were small to medium sized neurons. Scale bars: A, 100 µm; B, 50 µm.
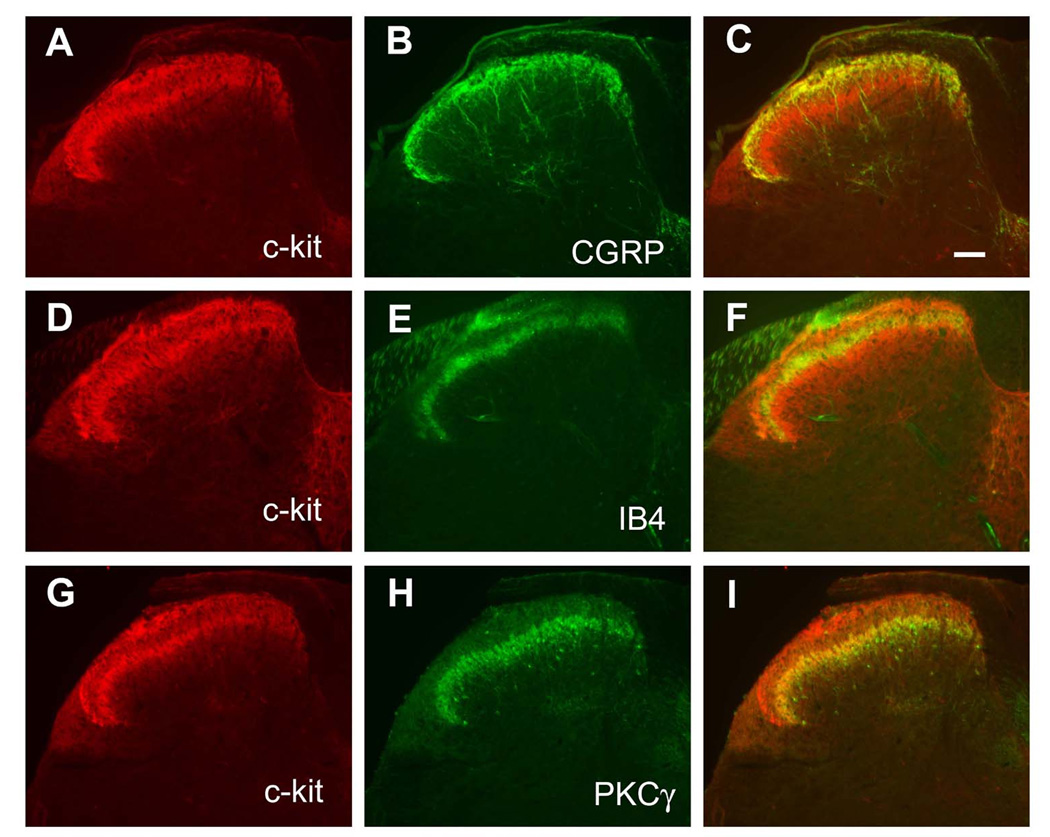
c-kit+ fibers detected by immnohistochemistry exhibited a double-layer pattern in the spinal cord (Fig. 2A). Double immunostaining showed that the top layer of c-kit+ fibers was colocalized with CGRP, which marks the lamina I and lamina II outer layer (IIo, Fig.2A–C), whereas the bottom layer of c-kit staining was colocalized with PKCγ, which marks the lamina II inner layer (IIi, Fig.2G–I). IB4+fibers were not colocalized with c-kit staining (Fig.2D–F), consistent with previous results showing that IB4 positive fibers locate in between CGRP and PKCγ labeled layers [21,33].
Fig. 2. Characterization of c-kit positive fibers in the dorsal spinal cord.
c-kit+ fibers detected by immnohistochemistry showed a double-layer pattern in the dorsal spinal cord. A–C, the first layer of c-kit was colocalized with CGRP. D-F, IB4 binding axons terminated in the middle of two c-kit+ layers, and did not overlap with c-kit. G–I, The second layer of c-kit was colocalized with PKCγ+ staining. Scale bar: C, 100 µm (A–I).
3.2. Part of the c-kit+ fibers originate from DRGs
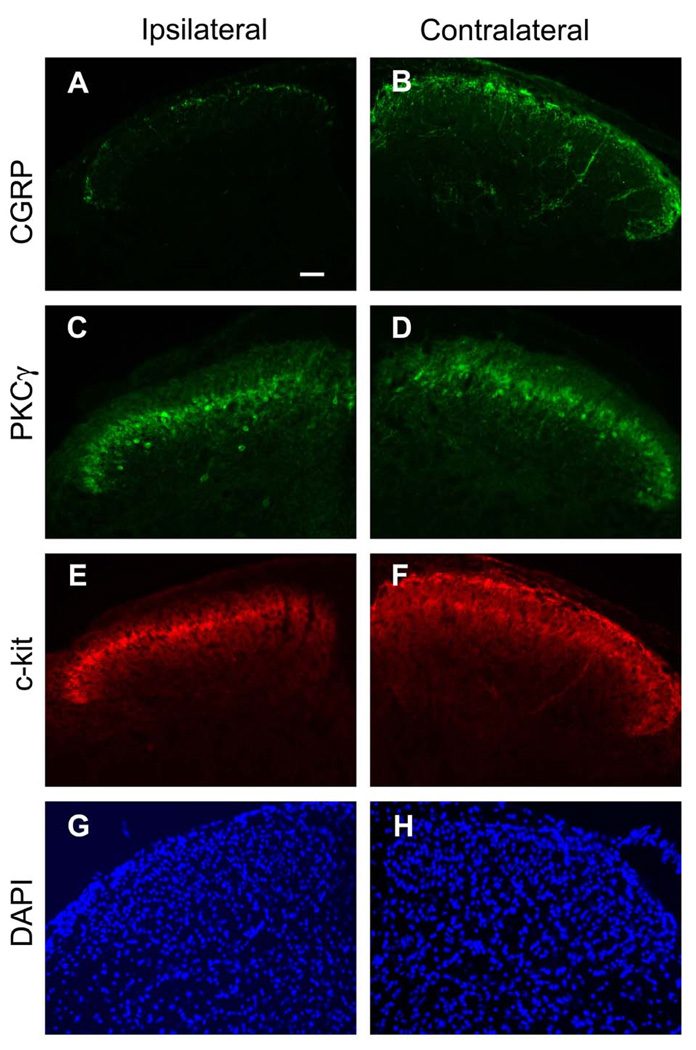
To confirm whether the c-kit+ fibers detected in the dorsal spinal cord originate from the primary afferent fibers or intrinsic expression in the spinal cord, we conducted the dorsal rhizotomy. As shown in Fig. 3E, the top layer of the c-kit+ fibers in the ipsilateral dorsal horn was diminished after dorsal rhizotomy, indicating that c-kit+ fibers in the top layer arise from primary afferent fibers. In contrast, the bottom layer of the c-kit staining remained the same as the contralateral side (Fig. 3F), suggesting that c-kit of this layer may come from the intrinsic expression in the spinal cord. As expected, the CGRP+ fibers were reduced in the ipsilateral dorsal spinal cord after dorsal rhizotomy (Fig.3A, B), but the PKCγ staining remained the same (Fig. 3C, D). And there was no morphological change as indicated by nucleus staining by DAPI (Fig.3G, H), indicating that the loss of primary afferent fiber staining is not due to the damage of the spinal cord.
Fig. 3. Part of c-kit positive fibers diminished after dorsal rhizotomy.
Immunofluorescence micrographs of cross sections of the lumbar dorsal horn of the spinal cord of mice after unilateral dorsal rhizotomy incubated with CGRP, PKCγ, c-kit antibody respectively. A,B, CGRP+ fibers were diminished in the ipsilateral side (A) after dorsal rhizotomy. C,D, PKCγ staining remained the same. E,F, the first layer of c-kit+ fibers was diminished in the ipsilateral side (E) after unilateral dorsal rhizotomy. G,H, the morphology of spinal cord was not changed as indicated by DAPI staining. Scale bar: A, 100 µm (A–H).
3.3 Acute pain in KitW-v mice
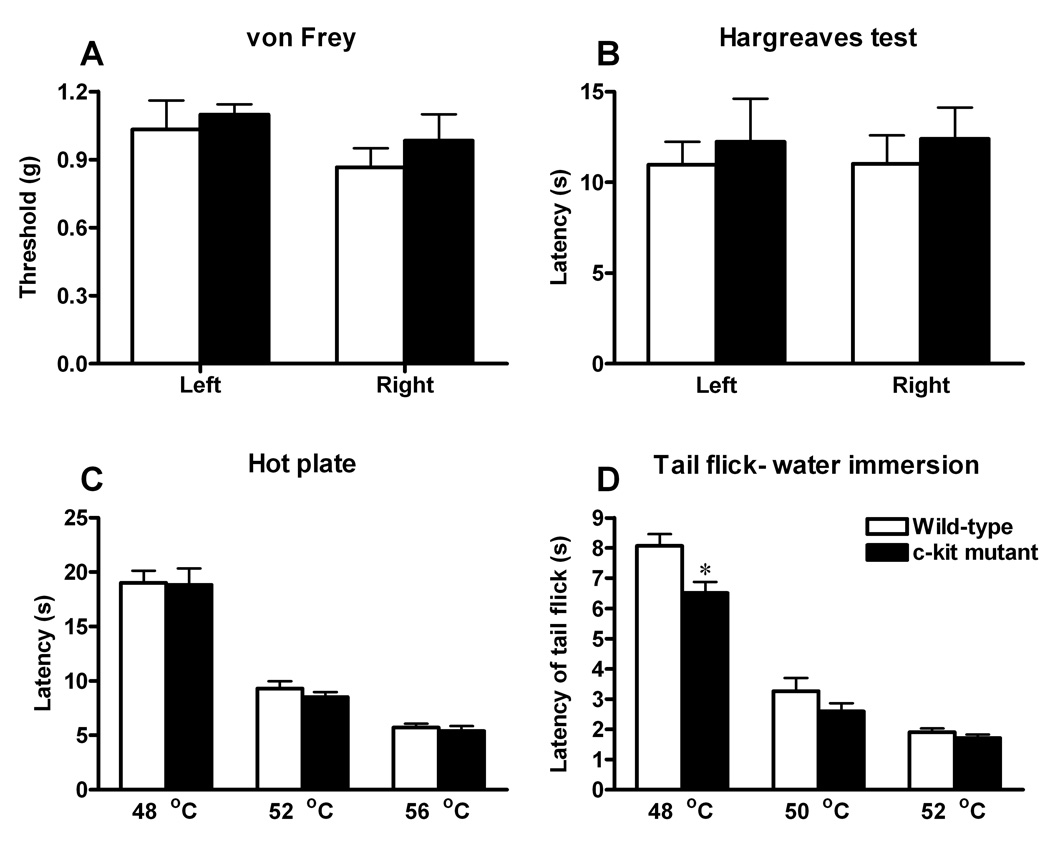
Because of the expression of c-kit in the nociceptive pathway, we next sought to assess whether c-kit may contribute to acute pain sensation. We examined thermal, mechanical responses of KitW-v mice, in which the kinase activity of c-kit is largely reduced [23]. Mechanical pain sensitivity was measured using graded von Frey filaments. The mechanical threshold in c-kit mutant mice was similar to the wild-type mice (Fig. 4A). KitW-v mice and wild-type mice did not differ in the Hargreave’s paw withdrawal test of thermal nociception (Fig. 4B). And in the hot plate and tail-flick tests, c-kit mutant mice showed thermal sensitivity comparable to the wild-type control, across a range of temperature (48°C, 52°C and 56°C for hot plate, and 50°C and 52°C for tail-flick), except that KitW-v mice showed slightly, but significantly, reduced response in tail-flick (48°C, Fig. 4C, D).
Fig. 4. Effects of the c-kit mutation on acute pain.
A, Sensitivity to mechanical stimuli of c-kit mutant mice (n=6; black bars) as measured by paw withdrawal threshold upon exposure to von Frey filaments was comparable to wild-type mice (n=6; white bars). B–D, Responses to noxious thermal stimulation were measured by the paw withdrawal latency (Hargreaves test, B), hotplate (C) and the water immersion tail-flick latency (D). There were no significant differences in thermal pain responses between wild-type (n=6; white bars) and c-kit mutant mice (n=6; black bars), except that c-kit mutant mice showed significant decreased response in the tail-flick test (48°C). * P<0.05. Student’s t-test. All data are presented as means ±s.e.m.
3.4. Inflammatory pain in KitW-v mice
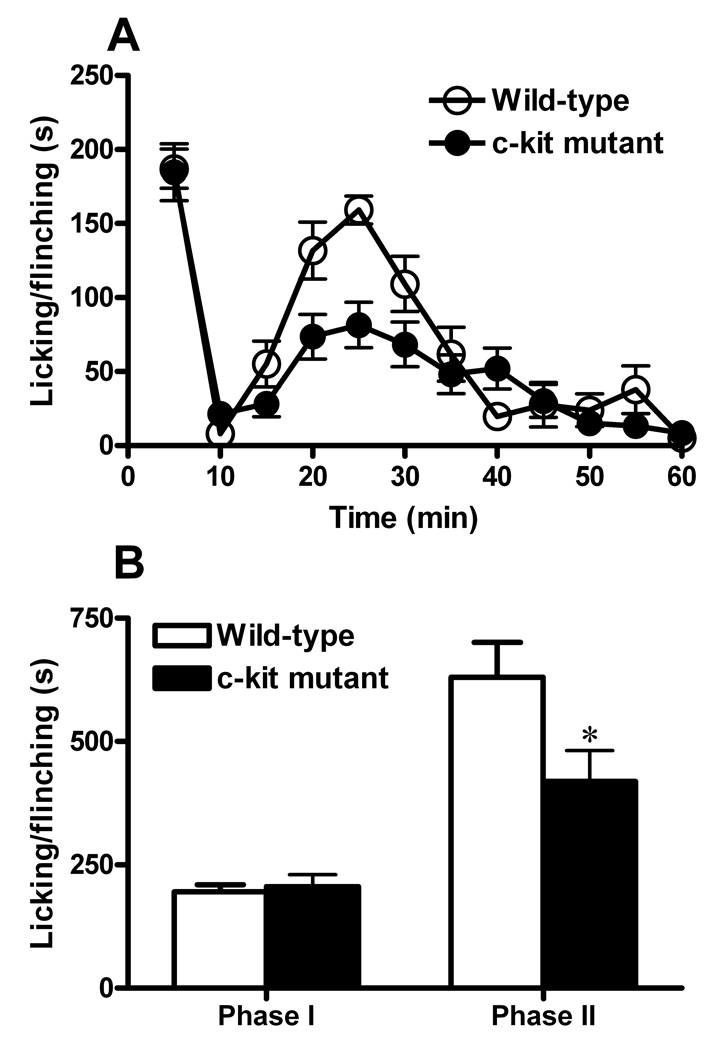
To measure the response of KitW-v mice to inflammatory pain, flinching and licking behaviors of KitW-v mice were compared with their control littermates after injection of 5% formalin (10 µl) into the right hindpaw of the animals. No statistically significant differences were found in the first phase (0–10 min) between the mutant and wild-type mice (Fig. 5A, B). In contrast, the second phase (10–60 min) was significantly decreased in the c-kit mutant mice compared to the wild-type mice (Fig. 5A, B). Since the second phase is believed to largely reflect the central sensitization in the spinal cord, c-kit may play an important role in the development of central sensitization after formalin injection.
Fig. 5. Effects of the c-kit mutation on inflammatory pain.
A, Spontaneous pain responses in first (0–10 min) of formalin test were comparable between wild-type (n=12; open circles) and c-kit mutant mice (n=13; filled circles). The second phase (10–60 min) was significantly decreased in c-kit mutant mice. Data was plotted in 5 min intervals, *P<0.05, repeated two-way ANOVA. B, Data from A were grouped into 2 phases, *P < 0.05, Student’s t-test. All data are presented as means ±s.e.m.
3.5. Neuropathic pain in KitW-v mice
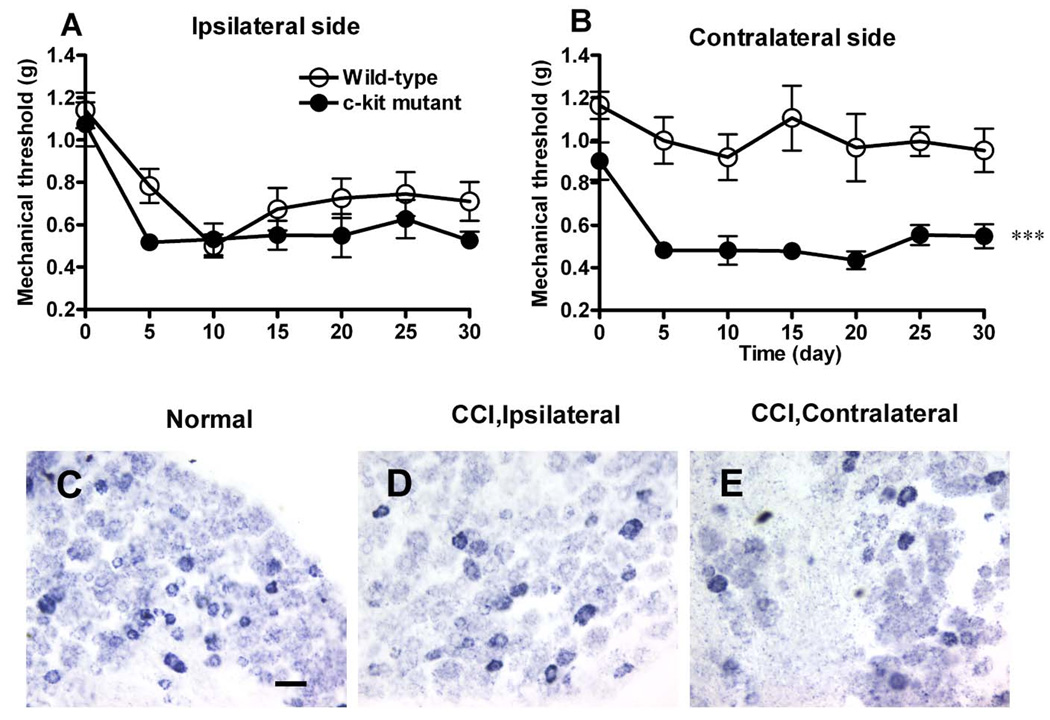
To determine the role of c-kit in neuropathic pain, we used the CCI model [1]. We found that after induction of CCI in the left sciatic nerve, the wild-type mice showed mechanical allodynia in the ipsilateral hindpaw, with the withdrawal threshold decreasing to the lowest point on day 10, and recovering slightly by day 30 (Fig. 6A). There was no significant difference in mechanical allodynia in the ipsilateral hindpaw between the c-kit mutant and wild-type mice (Fig. 6A). The withdrawal threshold of the contralateral hindpaw of wild-type mice did not differ from baseline (Fig. 6B). In contrast, KitW-v mice developed mechanical allodynia in the contralateral hindpaw (Fig. 6B), and the withdrawal thresholds decreased more rapidly and did not recover during the testing period. In wild-type mice, no obvious change of the expression of c-kit mRNA in either ipsilateral (Fig. 6D) or contralateral DRG (Fig. 6E) was observed on the 14th day after ligation of the sciatic nerve when compared with the control (Fig. 6C).
Fig. 6. Effects of the c-kit mutation on neuropathic pain.
A, Hyperalgesia developed in ipsilateral side was comparable between wild-type (open circles) and c-kit mutant mice (filled circles). B, Mechanical threshold in the contralateral side of wild-type mice was not affected, while c-kit mutant mice (filled circles) developed significant mechanical hyeralgesia compared with the wild-type mice (open circles). n=7–8 for each group. *** P<0.001, repeated two-way ANOVA. All data are presented as means ±s.e.m. C-E, c-kit mRNA+ neurons in the DRG were detected by in situ hybridization. No obvious changes were observed in ipsilater or contralateral DRG of mice 14 days after ligation of the sciatic nerve. Scale bar: C, 50 µm (C–E).
3.6. Primary afferent fibers are normal in KitW-v mice
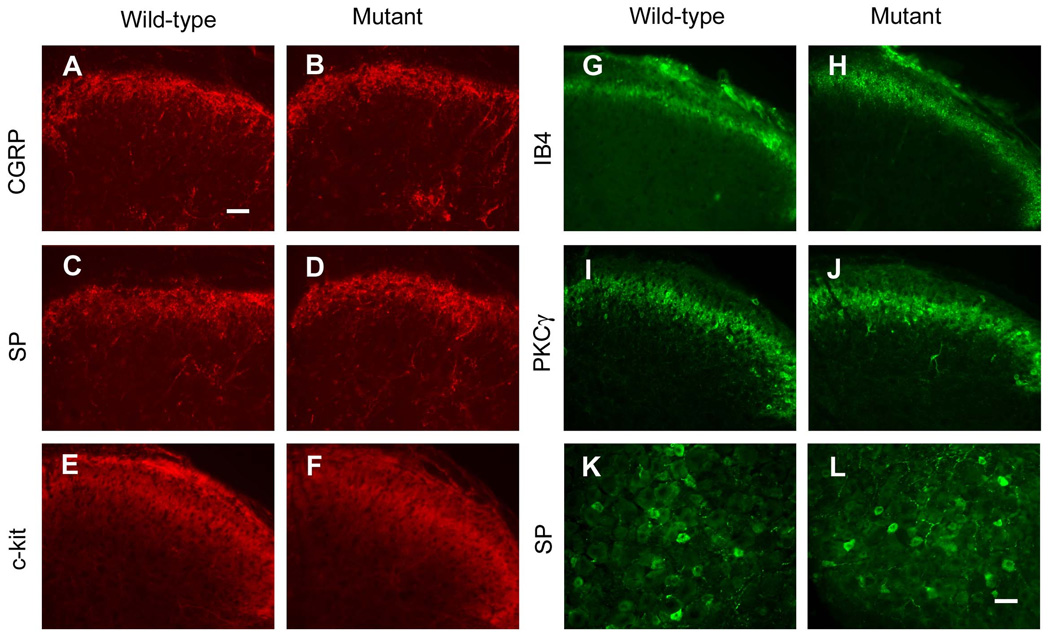
To determine whether there was any developmental defect of the primary afferents fibers, we examined the pattern of CGRP (Fig. 7A,B), SP (Fig. 7C,D), and IB4 (Fig. 7G,H) staining in the spinal cord. We did not find any distinguishable difference between wild-type and KitW-v mice. The SP+ neurons in the DRG were also comparable between wild-type and mutant mice (Fig.7 K, L). PKCγ staining, which labels the lamina II inner layer, remained the same between groups as well (Fig. 7I,J). As expected, the expression level of c-kit in the superficial spinal cord was reduced in KitW-v mice (Fig. 7E,F), which was more notable in the lamina I.
Fig. 7. The expression of c-kit was reduced in c-kit mutant mice.
Primary afferent fibers labeled by CGRP (A,B), SP (C,D), IB4 (G,H) are comparable between wild-type and c-kit mutant mice. c-kit expression in the spinal cord of c-kit mutant mice (F) was obviously reduced compared with wild-type (E). PKCγ (I,J), which labels the lamina II inner layer of the dorsal spinal cord, remained the same. (K,L), Peptidergic neurons labeled by SP did not show obvious difference between wild-type and c-kit mutant mice. Scale bars: A, 100 µm (A–J); K, 50 µm (K,L).
3.7. Release of CGRP and SP is not affected by the c-kit mutation
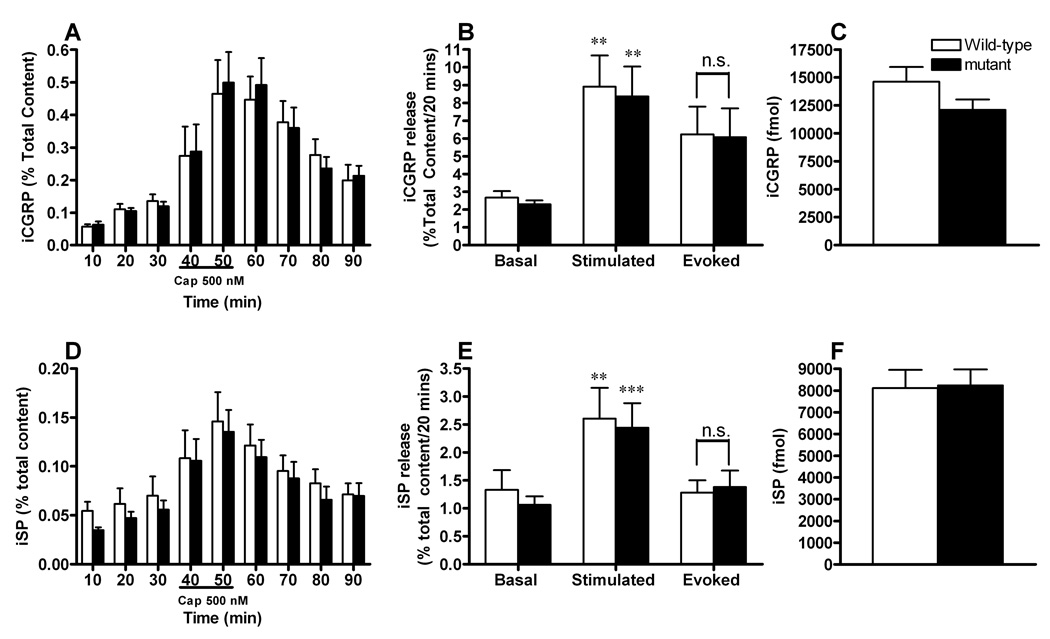
To further determine whether there was a defect of the primary afferent fibers from DRG neurons, release of SP and of CGRP from terminals of sensory neurons in the spinal cord were compared in c-kit mutant and wild-type mice. Spinal cord tissues were perfused with HEPES buffer in the absence or presence of 500 nM capsaicin and the amount of iCGRP and of iSP released, was determined by radioimmunoassay. At the end of the release protocol, the content of neuropeptides was determined in the tissue and the amount of peptide released calculated as % of total content. As shown in Fig. 8 C,F, there was no significant difference in the content of iSP or iCGRP in spinal cords from wild-type and c-kit mutant mice. When tissues were perfused with HEPES buffer in the absence of capsaicin, there was no significant difference in the resting release of iSP (Fig. 8D,E) or iCGRP (Fig. 8A,B) between the genotypes. In a similar manner, capsaicin evoked release was not different between the wild type and the mutant mice (Fig. 8B,E). These data suggest that the sensitivity of sensory neurons to capsaicin is not altered by a hypomorphic c-kit mutation, and that release of neuropeptides implicated in nociception is also the same as these mutant mice.
Fig. 8. Effect of the c-kit mutation on the capsaicin-induced release of iSP and iCGRP from mouse spinal cord slices.
A,D, Each column represents iCGRP (A) or iSP (D) levels in each 10-min perfusate sample in both wild-type(white bars) and c-kit mutant mice (black bars), expressed as percent of total peptide content in the tissues. B,E, Each column represents peptide release from spinal cord slices from wild type and c-kit mutant mice. The evoked release was calculated by subtracting the resting release obtained for 20 min immediately before exposure to capsaicin (basal), from the amount released during the 20-min exposure to capsaicin (stimulated). Capsaicin treatment induced significantly elevated release of iCGRP (B) or iSP (E) in both wild-type (white bars) and c-kit mutant mice (black bars). Evoked release of iCGRP (B) or iSP (E) was not significantly affected by c-kit mutation. C,F, Total content of iCGRP(C) and iSP (F) was comparable between wild-type(white bars) and c-kit mutant mice (black bars). n=9–10 for each group. Student’s t-test; **P<0.01, ***P<0.001. n.s.: not significant.
4. Discussion
In the present study, we have characterized expression of c-kit in the dorsal spinal cord. We found that c-kit is expressed in the small and medium sized neurons in the DRG, and in the superficial dorsal spinal cord. By studying the behavioral response of KitW-v mice, we found that the c-kit mutant mice showed mostly normal acute pain response, but abnormal persistent pain behaviors. Our study suggests that c-kit is important for the development of persistent pain.
4.1. Restricted intrinsic expression of c-kit in lamina II inner layer of the dorsal horn
It has been suggested that all the c-kit+ fibers in the spinal cord derive from DRG afferents and c-kit is not expressed in cells of the spinal cord [29,30]. However, in this study our immunocytochemical staining revealed two distinct layers of the c-kit staining in the dorsal horn and two observations indicated that only the lower layer of the c-kit staining represents expression of c-kit in the cells of the dorsal spinal cord. First, after dorsal rhizotomy this layer of staining remained unaltered, whereas the upper one diminished, suggestive of its DRG origin; Second, c-kit mRNA was found in lamina IIi layer of the dorsal horn neurons. Interestingly, while c-kit and PKCγ are colocalized, IB4+ afferents do not overlap with c-kit in the spinal cord. It was commonly accepted that the superficial dorsal horn can be divided into lamina I, lamina II outer and II inner layers with the topographic organization of CGRP/SP+ fibers projecting to lamina I and IIo and IB4+/c-ret to lamina IIi, which is labeled by PKCγ, respectively [26]. This seems not true in mice, and our results are consistent with a recent study [21]. Indeed, our result is also in line with the study showing that Mas-related G protein-coupled receptors (Mrgprd+) afferents overlap with IB4+ afferents that terminate at a distinct region called lamina IIm between lamina I and II outer layer innervated by CGPR/SP+ afferent and lamina II inner layer [33]. Thus, c-kit represents the second known gene in addition to PKCγ whose mRNA is restricted to lamina IIi in the dorsal horn of the spinal cord [17], raising the possibility that c-kit may be involved in persistent pain.
4.2. Involvement of c-kit in persistent pain transmission
The c-kit gene is widely expressed in the developing nervous system and W mutants have pleiotropic effects on a variety of developmental processes, and a null mutation results in early embryonic lethality, precluding the studies of role of c-kit in pain behaviors [20]. Moreover, mice with different W mutations exhibit a wide spectrum of phenotypes, which may complicate the interpretation of pain behaviors of these animals. On the other hand, the availability of a series of hypomorphic alleles in W locus provides a unique tool to study how c-kit expression may modulate pain sensitivity in a dose-related manner. In this study, we took advantage of the KitW-v mutant mice that carry a hypomorphic allele and retain only 20% of c-kit tyrosine kinase activity to explore the role of c-kit in pain behaviors. The KitW-v mutant mice are of particular use because they survive to maturity and exhibited relatively mild phenotype compared to other severe W alleles [20].
The KitW-v mutant mice showed comparable response to thermal and mechanical stimuli in most of the acute pain behavior tests. This phenotype is different from that of KitW/W mutants which carry a null mutation and displayed profound thermal hypoalgesia [18], suggesting that a full expression of c-kit is not obligatory for normal transmission of acute noxious information, with the possibility that the KitW-v mice may express enough c-kit for acute pain signaling. In contrast to acute pain, the second phase of formalin test was significantly decreased in KitW-v mutant mice as compared with wild-type mice. Previous work indicated that there was a reduction of a subpopulation of DRG neurons and reduced staining of CGRP+ fibers in the dorsal horn of the c-kit mutant mice [16]. Could the abnormal pain response be attributable to the loss of DRG neurons? This is less likely because in the present study we did not find any significant difference either in the staining of afferent fibers or the release of SP and CGRP in the dorsal horn between the mutant mice and wild-type controls.
What is the underlying mechanism by which c-kit signaling mediates pain transmission? The findings that c-kit is expressed in primary afferents and lamina IIi in the spinal cord suggest that c-kit may act either at presynaptic or postsynaptic sites or both. Many presynaptically localized receptors have been shown to be important in controlling synaptic strength by regulating neurotransmitter release [19]. For example, brain-derived neurotrophic factor (BDNF) stimulates neurotransmitter release by activating a presynaptic tyrosine kinase receptor [13]. Opioids may also act via presynaptic opioid receptors on the central C-fiber terminals [2]. c-kit has been implicated in modulating neuronal activity, thereby stimulating pituitary-adrenal axis and prolactin secretion in rats [14]. SCF has been shown to be expressed in the spinal cord neurons [30], which upon release can activate the presynaptic c-kit receptors. It is conceivable that the SCF/c-kit signaling pathway may invoke a similar mechanism in pain regulation. Although we did not detect significant changes in the release of CGRP and substance P in the c-kit mutant mice, we could not exclude the possibility that c-kit might modulate release of other neurotransmitters or neuropeptides. At the postsynaptic level, it is interesting to note that c-kit is coexpressed with PKCγ in lamina IIi, which has been suggested to be important for maintenance of persistent pain [17]. The expression of c-kit in lamina IIi raises the intriguing possibility that c-kit signaling pathway may interact with PKCγ in modulating persistent pain.
4.3. Involvement of c-kit in neuropathic pain
Our results that hyperalgesia developed in the contralateral side of the c-kit mutant mice indicate that c-kit is a negative regulator of contralateral sensitiziation. Allodynia that arises from the healthy body region contralateral to the actual site of injury has been observed in several animal models as well as in patients with chronic pain [31]. The molecular mechanisms underlying these observations are poorly understood, and c-kit may be an important gene involved in the process. This is probably not mediated by c-kit in the DRG, because we have observed no change of c-kit mRNA after the ligation of the sciatic nerve, although a recent study showed that c-kit was down-regulated after transection of L5 spinal nerve [29]. Such a discrepancy could be due to different neuropathic models used, since underlying mechanisms may differ between chronic pain models. It is tempting to speculate that several c-kit-expressing brain regions including rostral ventromedial medulla, which have been suggested to exert extensive bilateral control over nociceptive processing in the spinal cord, may be involved [12]. In this regard, the c-kit mutant mice may provide a valuable genetic model for investigating the mechanism of contralateral sensitiziation.
One seemly paradoxical observation in the present study is that while the c-kit mutant mice showed a reduced pain response in formalin test, enhanced sensitivity was manifested only in the contralateral side in CCI model. Given that c-kit is widely expressed in the brain [30], one of the possible reasons could be that c-kit expression in different brain regions might be differentially involved in these two forms of pain. Regional deletion of c-kit may help to elucidate the c-kit-mediated nociceptive transmission in different persistent pain models.
In summary, several conclusions can be drawn from the present study. First, at the spinal cord level, c-kit is expressed in the pain pathway including the DRG neurons and lamina IIi of the dorsal spinal cord. To our best knowledge, c-kit represents the second known molecule marking PKCγ+ layer, and may be a useful marker for future molecular analysis of this lamina. Second, we showed that c-kit is important for the development of persistent pain. Finally, in contrast to previous study showing that c-kit is important for acute pain, our study suggests that a residual activity of c-kit is sufficient for the function of most acute pain transmission.
ACKNOWLEDGEMENTS
We thank Tatsumi Hirata for providing the c-kit antibody, and Michael J. Iadarola for providing the CGRP antiserum. The work was supported by NIH grants to M.V;. R.G; and Z.F.C. This work was also supported by the Washington University Pain Center Animal Behavior Core and NIH Neuroscience Blueprint Interdisciplinary Center Core Grant P30 NS057105 to Washington University. The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 2.Besson JM. The neurobiology of pain. Lancet. 1999;353:1610–1615. doi: 10.1016/s0140-6736(99)01313-6. [DOI] [PubMed] [Google Scholar]
- 3.Brumovsky P, Hygge-Blakeman K, Villar MJ, Watanabe M, Wiesenfeld-Hallin Z, Hokfelt T. Phenotyping of sensory and sympathetic ganglion neurons of a galanin-overexpressing mouse--possible implications for pain processing. J Chem Neuroanat. 2006;31:243–262. doi: 10.1016/j.jchemneu.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Cable J, Jackson IJ, Steel KP. Mutations at the W locus affect survival of neural crest-derived melanocytes in the mouse. Mech Dev. 1995;50:139–150. doi: 10.1016/0925-4773(94)00331-g. [DOI] [PubMed] [Google Scholar]
- 5.Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 6.Chen JJ, Barber LA, Dymshitz J, Vasko MR. Peptidase inhibitors improve recovery of substance P and calcitonin gene-related peptide release from rat spinal cord slices. Peptides. 1996;17:31–37. doi: 10.1016/0196-9781(95)02091-8. [DOI] [PubMed] [Google Scholar]
- 7.Ding YQ, Marklund U, Yuan W, Yin J, Wegman L, Ericson J, Deneris E, Johnson RL, Chen ZF. Lmx1b is essential for the development of serotonergic neurons. Nat Neurosci. 2003;6:933–938. doi: 10.1038/nn1104. [DOI] [PubMed] [Google Scholar]
- 8.Fleischman RA. From white spots to stem cells: the role of the Kit receptor in mammalian development. Trends Genet. 1993;9:285–290. doi: 10.1016/0168-9525(93)90015-a. [DOI] [PubMed] [Google Scholar]
- 9.Geissler EN, Ryan MA, Housman DE. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 10.Hirata T, Kasugai T, Morii E, Hirota S, Nomura S, Fujisawa H, Kitamura Y. Characterization of c-kit-positive neurons in the dorsal root ganglion of mouse. Brain Res Dev Brain Res. 1995;85:201–211. doi: 10.1016/0165-3806(94)00205-e. [DOI] [PubMed] [Google Scholar]
- 11.Hirata T, Morii E, Morimoto M, Kasugai T, Tsujimura T, Hirota S, Kanakura Y, Nomura S, Kitamura Y. Stem cell factor induces outgrowth of c-kit- positive neurites and supports the survival of c-kit-positive neurons in dorsal root ganglia of mouse embryos. Development. 1993;119:49–56. doi: 10.1242/dev.119.1.49. [DOI] [PubMed] [Google Scholar]
- 12.Jones SL, Gebhart GF. Spinal pathways mediating tonic, coeruleospinal, and raphe-spinal descending inhibition in the rat. J Neurophysiol. 1987;58:138–159. doi: 10.1152/jn.1987.58.1.138. [DOI] [PubMed] [Google Scholar]
- 13.Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- 14.Kovacs KJ, Foldes A, Vizi ES. C-kit ligand (Stem Cell Factor) affects neuronal activity, stimulates pituitary-adrenal axis and prolactin secretion in rats. J Neuroimmunol. 1996;65:133–141. doi: 10.1016/0165-5728(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 15.Lev S, Blechman JM, Givol D, Yarden Y. Steel factor and c-kit protooncogene: genetic lessons in signal transduction. Crit Rev Oncog. 1994;5:141–168. doi: 10.1615/critrevoncog.v5.i2-3.30. [DOI] [PubMed] [Google Scholar]
- 16.Lourenssen S, Motro B, Bernstein A, Diamond J. Defects in sensory nerve numbers and growth in mutant Kit and Steel mice. Neuroreport. 2000;11:1159–1165. doi: 10.1097/00001756-200004270-00004. [DOI] [PubMed] [Google Scholar]
- 17.Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- 18.Milenkovic N, Frahm C, Gassmann M, Griffel C, Erdmann B, Birchmeier C, Lewin GR, Garratt AN. Nociceptive tuning by stem cell factor/c-Kit signaling. Neuron. 2007;56:893–906. doi: 10.1016/j.neuron.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 19.Miller RJ. Presynaptic receptors. Annu Rev Pharmacol Toxicol. 1998;38:201–227. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- 20.Motro B, van der KD, Rossant J, Reith A, Bernstein A. Contiguous patterns of c-kit and steel expression: analysis of mutations at the W and Sl loci. Development. 1991;113:1207–1221. doi: 10.1242/dev.113.4.1207. [DOI] [PubMed] [Google Scholar]
- 21.Neumann S, Braz JM, Skinner K, Llewellyn-Smith IJ, Basbaum AI. Innocuous, not noxious, input activates PKCgamma interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci. 2008;28:7936–7944. doi: 10.1523/JNEUROSCI.1259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nocka K, Tan JC, Chiu E, Chu TY, Ray P, Traktman P, Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J. 1990;9:1805–1813. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reith AD, Rottapel R, Giddens E, Brady C, Forrester L, Bernstein A. W mutant mice with mild or severe developmental defects contain distinct point mutations in the kinase domain of the c-kit receptor. Genes Dev. 1990;4:390–400. doi: 10.1101/gad.4.3.390. [DOI] [PubMed] [Google Scholar]
- 24.Roskoski R., Jr Structure and regulation of Kit protein-tyrosine kinase--the stem cell factor receptor. Biochem Biophys Res Commun. 2005;338:1307–1315. doi: 10.1016/j.bbrc.2005.09.150. [DOI] [PubMed] [Google Scholar]
- 25.Rossi P, Sette C, Dolci S, Geremia R. Role of c-kit in mammalian spermatogenesis. J Endocrinol Invest. 2000;23:609–615. doi: 10.1007/BF03343784. [DOI] [PubMed] [Google Scholar]
- 26.Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 27.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 28.Sun YG, Gao YJ, Zhao ZQ, Huang B, Yin J, Taylor GA, Chen ZF. Involvement of P311 in the affective, but not in the sensory component of pain. Mol Pain. 2008;4:23. doi: 10.1186/1744-8069-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takagi K, Okuda-Ashitaka E, Mabuchi T, Katano T, Ohnishi T, Matsumura S, Ohnaka M, Kaneko S, Abe T, Hirata T, Fujiwara S, Minami T, Ito S. Involvement of stem cell factor and its receptor tyrosine kinase c-kit in pain regulation. Neuroscience. 2008;153:1278–1288. doi: 10.1016/j.neuroscience.2008.02.073. [DOI] [PubMed] [Google Scholar]
- 30.Zhang SC, Fedoroff S. Cellular localization of stem cell factor and c-kit receptor in the mouse nervous system. J Neurosci Res. 1997;47:1–15. [PubMed] [Google Scholar]
- 31.Zhao M, Wang JY, Jia H, Tang JS. Roles of different subtypes of opioid receptors in mediating the ventrolateral orbital cortex opioid-induced inhibition of mirror-neuropathic pain in the rat. Neuroscience. 2007;144:1486–1494. doi: 10.1016/j.neuroscience.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, Hsu RY, Birkett NC, Okino KH, Murdock DC. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- 33.Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]