Abstract
Schizophrenia has been proposed to have a neurodevelopmental aetiology. Neural Cell Adhesion Molecule 1 (NCAM1) is involved in several neurodevelopmental processes and abnormal expression of this gene has been associated in the pathology of schizophrenia and, thus, altered NCAM1 expression may be characteristic of the early stages of the illness. Alternative splicing of the NCAM1 transcript produces 3 major isoforms. Using qPCR we analysed mRNA expression of one of these isoforms; the 180 kDa isoform of NCAM1 (NCAM-180), in Brodmann Area (BA) 46, BA10 and BA17, postmortem, from 15 subjects with a short duration of illness of schizophrenia (<7 years) and 15 control subjects. NCAM-180 mRNA expression was increased in BA46 from subjects with schizophrenia compared to controls (P=0.013). By contrast, there were no significant differences in the expression of NCAM-180 mRNA in BA10 (P=0.575) or BA17 (P=0.772). We then analysed NCAM-180 mRNA expression in BA46 from 15 subjects with a longer duration of illness of schizophrenia (>22 years) and 15 controls. There was no significant difference in NCAM-180 mRNA expression in this second cohort. This data suggests NCAM-180 mRNA expression is altered in a regionally-specific manner in schizophrenia and these changes are associated with the early period following diagnosis.
Keywords: Schizophrenia, NCAM1, Prefrontal Cortex, Brodmann's Area 46, mRNA
1 Introduction
The cell recognition molecule Neural Cell Adhesion Molecule (NCAM1) regulates a broad range of neural processes throughout development and into adulthood, including axon and dendrite development (Cremer et al., 1997), synaptogenesis (Uryu et al., 1999) and synaptic plasticity (Theodosis et al., 1999). Transcription of the NCAM1 gene produces 3 major isoforms; NCAM-120, NCAM-140 and NCAM-180, although mouse studies suggest these isoforms represent classes of several alternatively spliced transcripts of similar size (Santoni et al., 1989). NCAM-180, which is primarily restricted to the postsynaptic densities of neurons, and NCAM-140, which is expressed in both neurons and glia, are transmembrane glycoproteins with large carboxy-terminal intracellular domains. Contrasting NCAM-140, NCAM-180 contains an intracellular domain encoded by exon 18 (Barbas et al., 1988) which facilitates high affinity binding of NCAM1 to the cytoskeletal protein spectrin (Pollerberg et al., 1987; Pollerberg et al., 1986). The glial expressed NCAM-120 isoform lacks transmembrane and intracellular domains (Barbas et al., 1988) and is anchored to the cell membrane via glycophosphatidyl inositol (He et al., 1986). Post-translational modification of these NCAM1 isoforms leads to the generation of several smaller fragments and polysialylated proteins (Frost et al., 1991; Nybroe et al., 1989).
Altered of NCAM1 expression (Poltorak et al., 1995, Tanaka et al., 2007) and disturbances in post-translational modification of the protein (Barbeau et al., 1995, Arai et al., 2006) have been reported in schizophrenia. Within the cerebrospinal fluid (CSF), increased NCAM-120 protein expression has been observed in individuals with schizophrenia (Poltorak et al., 1995, van Kammen et al., 1998, Tanaka et al., 2007). However, while modest increases in NCAM-140 and NCAM-180 protein expression associated with schizophrenia have been reported (Tanaka et al., 2007), contrasting data shows no evidence of altered expression of NCAM1 protein isoforms between 180kDa to 200kDa (Poltorak et al., 1995). Changes in the post-translational cleavage products of the NCAM1 protein have also been reported, such that increased levels of a 68kDa isoform of NCAM1 and decreased levels of a 73–75 kDa isoform and a 52 kDa isoform have been reported in the CSF of subjects with schizophrenia (Tanaka et al., 2007). Within the post-mortem brain, increased NCAM1 protein expression has been found in the cingulate cortex from subjects with schizophrenia (Honer et al., 1997). However, other studies have shown no change in the expression of NCAM-120, NCAM-140 or NCAM-180 protein in the dorsolateral prefrontal and anterior frontal cortices or the hippocampus, instead reporting increased levels of polysialylated NCAM1 within the hippocampus and a 105–115kDa NCAM1 isoform, a cleavage product of NCAM-180, in the hippocampus and dorsolateral prefrontal cortex (Barbeau et al., 1995, Vawter et al., 2001, Vawter et al., 1998).
Recent genetic analyses suggest an association between NCAM1 and cognitive deficits associated with schizophrenia (Sullivan et al., 2007). Such associations are supported by the NCAM1−/− mouse model, which displays impaired spatial learning (Cremer et al., 1994) and reduced prepulse inhibition, a behavioural model of schizophrenia (Wood et al., 1998). Furthermore, increased serum levels of NCAM1 positively correlate with the severity of negative symptoms in schizophrenia (Lyons et al., 1988). Together these data suggest that changes in NCAM1 could be associated with both the negative and cognitive symptoms of schizophrenia, both of which are resistant to current therapeutics (Keefe et al., 2007).
Schizophrenia is hypothesised to have a neurodevelopmental aetiology. A recent microarray study highlighted that different genes are associated with the early and late stages of schizophrenia (Narayan et al., 2008). NCAM1 is involved with several developmental processes within the nervous system and has been suggested to play a role in the negative and cognitive symptoms of schizophrenia (Lyons et al., 1988; Sullivan et al., 2007), which are known to present early in the pathology of the illness (Parnas, 1999). Thus, changes in NCAM1 expression associated with schizophrenia may be associated with the early stages of the illness. We used qPCR to examine NCAM mRNA expression in the cortex of post-mortem subjects with schizophrenia who had a relatively short duration of illness (short DOI). Expression was examined in Brodmann’s Area (BA)10, BA46 and BA17 representing regions that have been shown to be involved with various aspects of the pathology of schizophrenia (MacDonald et al., 2005; Dorph-Petersen et al., 2007; Yoon et al., 2008). In regions where altered NCAM-180 mRNA expression was observed, we subsequently analysed expression in a separate cohort of subjects with a comparatively longer duration of illness (long DOI) to determine whether these changes in mRNA expression are maintained throughout the progression of the illness. As, previous studies have reported differentially altered expression of the NCAM1 isoforms associated with schizophrenia, we examined the isoform specific NCAM1 expression. Efficient oligonucleotide primers that were specific to the NCAM-120 and NCAM-140 mRNA sequences could not be designed. Thus, we focused our study on the NCAM-180 isoform.
2 Methods
2.1 Tissue Collection
All tissue was obtained from the Victorian Brain Bank Network at the Mental Health Research Institute of Victoria. Approval was obtained from the Ethics Committee of the Victorian Institute of Forensic Medicine and the Mental Health Research and Ethics Committee of Melbourne Health. Left hemisphere tissue from BA10, BA46 and BA17 was obtained post-mortem from 15 subjects diagnosed with schizophrenia <7 years prior to death (short DOI) and 15 subjects with no history of psychiatric illness (controls). Subsequent analysis of the effect of duration of illness on schizophrenia associated changes in mRNA levels were carried out in a second cohort consisting of 15 subjects diagnosed with schizophrenia >22 years prior to death (long DOI) and 15 controls. The two cohorts were expanded from the short and long duration of illness cohorts published in Narayan et al. (2008). The duration of illness limits imposed upon the two cohorts were based upon the availability of subjects with short and long DOI schizophrenia. BA10 was taken as the most rostral portions of the superior frontal gyrus and middle frontal gyrus, bounded ventrally by the superior rostral sulcus. BA46 was taken as the lateral surface of the frontal lobe and includes approximately the middle third of the middle frontal gyrus and the most rostral portion of the inferior frontal gyrus. BA17 was taken as the part of the occipital cortex that is defined by the presence of the band of Gennari.
For each subject, consensus diagnosis was reached between a psychologist and a psychiatrist using the Diagnostic Instrument for Brain Studies (Keks et al., 1999); a semi-structured protocol for post-mortem assessment allowing psychiatric diagnosis according to DSM-IV criteria (American Psychiatric Association, 1994). Demographic factors and tissue quality markers, including gender, age, incidence of suicide, duration of illness, post-mortem interval (PMI), CNS pH and RIN were used to assess the impact of subject variability on the experiment (table 1). The effect of antipsychotic medication was assessed using the chlorpromazine equivalence of the neuroleptic dosage recorded in the subject’s clinical case history. The cause of death and medication history for the subjects used in this study are outlined in table 2.
Table 1.
A summary of the demographic data for the subjects used in the study. Gender data is provided as the ratio of males (M) to females (F). Incidence of suicide data is provided as the number of subjects who committed suicide per sample size. All other data is provided as means ± the standard deviation. Antipsychotic mediation is listed as final prescribed dose of neuroleptic medication expressed as chlorpromazine equivalence per day.
| Short Duration of Illness | Long Duration of Illness | |||
|---|---|---|---|---|
| Control | Schizophrenia | Control | Schizophrenia | |
| Gender | 12 M : 3 F | 12 M : 3 F | 12 M : 3 F | 12 M : 3 F |
| Age | 29.9±12.1 yr | 29.4±12.5 yr | 58.9±10.7 yr | 58.2±11.0 yr |
| Post-mortem interval | 47.3±11.8 hr | 47.5±13.2 hr | 37.1±16.9 hr | 38.4 hr |
| CNS pH | 6.30±0.21 | 6.29±0.15 | 6.35±0.18 | 6.33±0.17 |
| RIN | 8.0±1.0 | 7.8±1.4 | 7.26±1.7 | 7.7±1.0 |
| Duration of illness | 4.1±1.8 yr | 33.7±9.3 yr | ||
| Incidence of suicide | 0/15 | 11/15 | 0/15 | 1/15 |
| Antipsychotic medication | 732.85±780.74 mg | 494.03±355.86mg | ||
Table 2.
The cause of death and medication history of the subjects with a short duration of illness following diagnosis with schizophrenia prior to death, a long duration of illness following diagnosis with schizophrenia prior to death and their matched control subjects. Drugs that were detected following toxicological screening that were not recorded in the subject’s prescribed drug history are noted in brackets.
| Control | Short Duration of Illness Schizophrenia | |||||||
|---|---|---|---|---|---|---|---|---|
| ID# | Cause of Death | ID# | Cause of death | Neuroleptics | Anti-depressants | Benzodiapines | Anti-cholinergics | Mood stabilisers |
| 1 | Trauma/asphyxia | 1 | Meningo-encephalitis | Haloperidol | Fluoxetine | Benztropine | ||
| Chlopromazine | ||||||||
| Clozapine | ||||||||
| 2 | Coronary artery atheroma | 2 | CO poisoning | Haloperidol | Oxazepam | |||
| Nitrazepam | ||||||||
| 3 | Electrocution | 3 | CO poisoning | Haloperidol | Desipramine | Clonazepam | Benztropine | |
| 4 | Electrocution | 4 | Multiple injuries | Haloperidol | Sertraline | (Diazapam) | Benztropine | |
| 5 | Acute epiglottitis | 5 | Unascertained | Haloperidol | Diazapam | Benztropine | ||
| Risperidone | ||||||||
| Chlorpromazine | ||||||||
| 6 | Dilated cardiomyopathy | 6 | Coronary artery thrombosis | Haloperidol | Benztropine | |||
| 7 | Iatrogenic haemorrhage | 7 | Combined drug toxicity | Pimozide | Sertraline (Moclobemide) | |||
| 8 | Ischemic heart disease | 8 | Hanging | Haloperidol | Lithium | |||
| 9 | Exsanguination | 9 | Pericarditis | Trifluoperazine | (Diazapam) | |||
| Flupenthixol | ||||||||
| 10 | Exsanguination | 10 | Asphyxia plastic bag | Fluphenazine | Temazapam | Carbamazep ine | ||
| Chlorpromazine | ||||||||
| 11 | Accidental drowning | 11 | Hanging | Fluphenazine | ||||
| 12 | Ventricular hypertrophy | 12 | Combined drug toxicity | Trifluoperazine | Imipramine (Desipramine) | |||
| 13 | Myocarditis | 13 | CO poisoning | Haloperidol | Doxepin | Benztropine | ||
| 14 | Asthma attack | 14 | Hanging | Haloperidol | Benztropine | |||
| 15 | Ischemic heart disease | 15 | CO poisoning | Haloperidol | Dosulepin | Flunitrazepam | Benztropine | |
| Control | Long Duration of Illness Schizophrenia | |||||||
| ID# | Cause of Death | ID# | Cause of death | Neuroleptics | Anti-depressants | Benzodiapines | Anti-cholinergics | Mood stabilisers |
| 16 | Congestive cardiac failure | 16 | Pulmonary embolism | Fluphenazine | Lithium | |||
| Chlorpromazine | ||||||||
| 17 | Ischemic heart disease | 17 | Coronary artery atheroma | Fluphenazine | ||||
| 18 | Aortic stenotis | 18 | Ischemic heart disease | Fluphenazine | ||||
| 19 | Pulmonary throboembolism | 19 | Broncho pneumonia | Flupenthixol | ||||
| Thioridazine | Clonazepam Nitrazepam | |||||||
| Chlorpromazine | ||||||||
| 20 | Coronary artery atheroma | 20 | Coronary artery atheroma | Fluphenazine | Benztropine | |||
| 21 | Acute myocardial infarction | 21 | Coronary artery atheroma | Trifluoperazine | Benzhexol | |||
| Haloperidol | ||||||||
| 22 | Ischemic heart disease | 22 | Coronary artery atheroma | Thioridazine | Dosulepin | Temazepam | Benztropine | |
| 23 | Pulmonary embolism | 23 | Ischemic heart disease | Fluphenazine | Procyclidine | |||
| 24 | Coronary artery atheroma | 24 | Aspiration/food | Thioridazine | Diazepam | Benztropine | ||
| 25 | Ischemic heart disease | 25 | Aspiration/food | Trifluoperazine (Thioridazine) | Temazepam | |||
| Lorazepam | ||||||||
| 26 | Ruptured aneurysm | 26 | Ischemic heart disease | Thiordazine | ||||
| 27 | Ischemic heart disease | 27 | Pneumonia | Fluphenazine | Amitriptyline | Benztropine | ||
| 28 | Ischemic heart disease | 28 | Hanging | Trifluoperazine | Benztropine | |||
| Chlorpromazine | ||||||||
| 29 | Acute asthma | 29 | Congestive cardiac failure | Fluphenazine | Benztropine | |||
| Chlorpromazine (Thioridazine) | ||||||||
| 30 | Acute myocardial infarction | 30 | Ischemic heart disease | Fluphenazine | ||||
| Thioridazine | ||||||||
Cadavers were refrigerated within 5 hr and frozen to −70°C within 30 min of autopsy. Where death was witnessed, the time between death and autopsy was taken as the PMI. Where death was not witnessed, tissue was only collected from subjects who had been seen alive up to 5 hr prior to being found dead and the PMI was measured from the midpoint between the subject being found and being last seen alive. The CNS pH was measured as described previously (Kingsbury et al., 1995).
2.2 RNA Extraction and Reverse Transcription
Total RNA from was extracted from human post-mortem prefrontal cortex using a modification of the guanidine isothiocyanate–phenol method (Chomczynski and Sacchi, 1987) using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Extracts were treated with RNase-free DNase 1 (Ambion, Austin, Tx, USA) at 37°C for 30 minutes to remove residual genomic DNA. The concentration, purity and integrity of the samples were determined using both spectrophotometry (Biospec Mini, Shimadzu Corporation, Kyoto, Japan) and a bioanalyser (Agilent Technologies, Santa Clara, CA, USA). 2µg of each RNA extract was reverse transcribed with 100U MMLV (Ambion, Austin, Tx, USA) in 500mM Tris-HCl, ph 8.3, 750mM KCl, 30mM MgCl2 50mM DTT, 0.5mM dNTP’s, 2.5µM oligo dT primer, 2.5µM random decamers and 1U/µl SUPERase In RNase inhibitor (Ambion, Austin, Tx, USA) at 37°C for 1 hr followed by heat inactivation of the enzyme.
2.3 Quantitative Real-Time PCR
The iQ5 real-time PCR detection system (Bio-Rad Laboratories, Hercules CA, USA) was used to perform qPCR on sample cDNA. Oligonucleotide sequences are outlined in table 3. Oligonucleotides were designed using Beacon Designer 7.00 software (Premier Biosoft, Palo Alto, CA, USA). The oligonucleotide amplification efficiencies ranged between 96.3% and 103.3%. The reverse complement oligonucleotide used for amplification of NCAM1 hybridised within exon 18 of the gene NCAM1 sequence, such that mRNA for the NCAM-120 and NCAM140 isoforms would not be detected. The NCAM-180 oligonucleotides did not discriminate against the presence of the VASE exon within the transcript. qPCR reaction mixtures (50µL) contained 40ng of reverse transcription product, 0.4nM of complementary oligonucleotide pairs and 1× IQ SYBR green supermix (Bio-Rad Laboratories, Hercules CA, USA). The PCR cycle parameters were 95°C for 3 minutes and 40 cycles of 95°C for 10 seconds, 60°C for 15 seconds and 68°C for 15 seconds. qPCR data was acquired using IQ5 optical system 2.0 software (Bio-Rad Laboratories,Hercules CA, USA). All samples were run in triplicate and the expression of NCAM-180 calculated from the log 2 of the average triplicate value corrected for primer efficiency. Expression was normalised against the geometric mean of the levels of three reference genes; GAPDH, PPIA and SNCA, selected from a pool of potential reference genes using geNorm software (Ghent University Hospital Center for Medical Genetics, Ghent, Belgium) (Vandesompele et al., 2002). To assess inter-assay variation, cDNA samples derived from a cell line (SY5Y) were amplified in duplicate on each qPCR assay plate using GAPDH oligonucleotide primers and the variation calculated from the average CT values from each duplicate. Intra-assay variation was assessed by amplifying 6 replicates of the cell line cDNA on a single assay plate with the variation calculated from the CT values of each reaction. Inter- and intra-assay variation was 0.99% and 0.38% respectively.
Table 3.
A summary of the oligonucleotide primers used for real-time PCR analysis of NCAM1 expression.
| Gene | GenBank Accession # | Oligonucleotide sequence | Position 5’-3’ |
|---|---|---|---|
| SNCA | NM_07308 | F 5’-CTGCTGCTGAGAAAACCAAA-3’ | 63-82 |
| R 5’-CTGCTCCCTCCACTGTCTT-3’ | 269-251 | ||
| GAPDH | NM_002046 | F 5’-TGCACCACCAACTGCTTAGC-3’ | 556-575 |
| R 5’-GGCATGGACTGTGGTCATGAG -3’ | 642-622 | ||
| PPIA | NM_021130 | F 5’-ATGGTCAACCCCACCGTGTTCTTCG-3’ | 84-108 |
| R 5’-CGTGTGAAGTCACCACCCTGACACA-3 | 289-263 | ||
| NCAM-180 | NM_000615 | F 5’-GTCCTGCTCCTGGTGGTTGTG-3’ | 2507-2527 |
| R 5’-GGTCCTCTCCTCCTCCGTTCG-3 | 2704-2684 |
2.4 Statistics
Grubbs test was used to identify outliers within the data. The data was then analysed by the D'Agostino & Pearson omnibus normality test to assess the normality of distribution. Student’s T-test was used to analyse NCAM-180 expression in the cortical regions examined. Within BA46, variation between long and short DOI cohorts was compared using two way ANOVA. Further analysis using Student’s T tests were performed to determine the source of variation. Statistical significance was accepted at p<0.05. The relationship between demographic and tissue condition data were assessed using Pearson product moment correlation; a value of r2 > 0.25 was indicating the potential for a strong relationship between variables (Gliner et al., 2002). The statistical power of the data was assessed by power analysis with a power >80% indicating adequate power. Analyses were conducted using Prism 5.01 software (Graphpad Software, La Jolla, CA, USA).
3 Results
Analysis of the expression data using D'Agostino & Pearson omnibus normality test showed the data was normally distributed (K2=0.406 to 4.684; p=0.096–0.816) for all cohort groups used in this study. Consequently, parametric statistics were used to analyse the data.
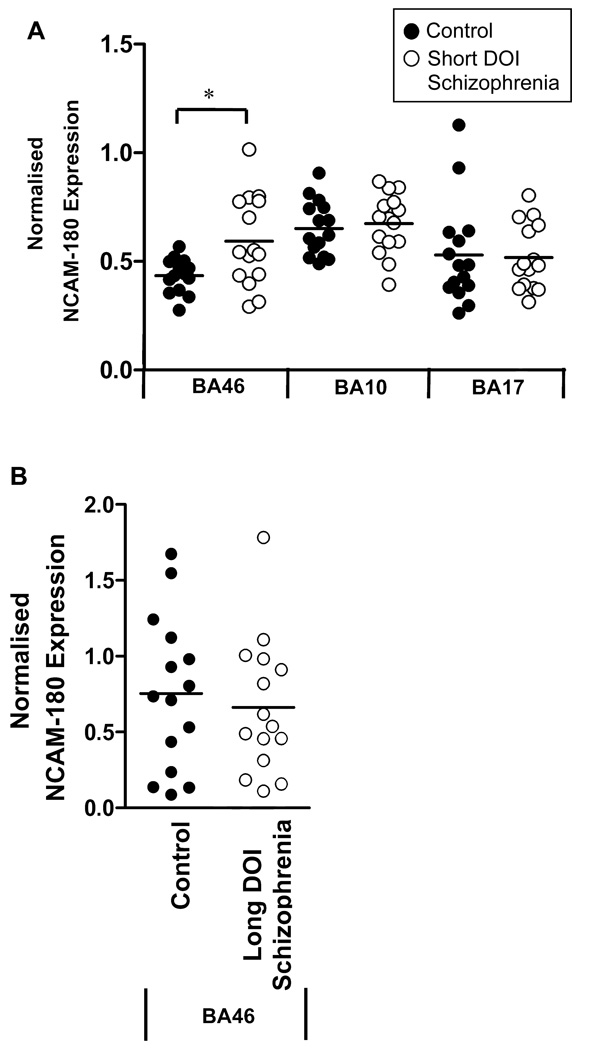
NCAM-180 mRNA levels were measured in three cortical regions (BA46, BA10 and BA17) from subjects with short-term DOI schizophrenia and age-and sex-matched controls. Grubbs test identified one control subject as an outlier for the BA46 data set (z=2.548, p<0.05). This data point was subsequently removed from analysis. The level of NCAM-180 mRNA was significantly increased in subjects with schizophrenia compared to controls in BA46 (mean±SEM: 0.593±0.052 vs 0.434±0.021 normalised expression levels; t=2.668, df=27, p=0.013). This increase remained significant following inclusion of the outlier (t=2.118, df=28, p=0.043). A power analysis showed our data had a statistical power of 82.6%. NCAM-180 mRNA levels were not significantly different between subjects with schizophrenia and controls in BA10 (t=−0.449, df=28, p=0.657) or BA17 (t=0.167, df=28, p=0.869) (figure 1A). Repeated exposure to the antipsychotic risperidone has been shown to increase the expression of NCAM-180 in rats (Mackowiak et al., 2009). Within our cohort, one subject with schizophrenia had been prescribed risperidone. Following removal of this subject from analysis, NCAM-180 expression in the schizophrenia group remained significantly increased compared to controls (t=2.801, df=26, p=0.009) suggesting prior medication with risperidone had no impact on our data.
Figure 1.
NCAM1 expression in the cortex of schizophrenia subjects. (A) Analysis of cortical NCAM1 mRNA expression in subjects who had been diagnosed with short duration of illness (DOI) schizophrenia showed a significant increase of NCAM1 mRNA levels in BA46 (P=0.013) associated with schizophrenia. No change in NCAM1 mRNA expression was observed in BA10 (P=0.575) or BA17 (P=0.772) of subjects with short duration of illness schizophrenia compared to controls. (B) Subsequent analysis of NCAM1 mRNA expression in BA46 from a separate cohort of long DOI schizophrenia subjects showed no change in NCAM1 expression compared to control subjects (P=0.602). NCAM1 expression data is expressed relative to the expression of PPIA, SNCA and GAPDH. *= P<0.05.
To determine whether the increase in NCAM-180 expression in BA46 detected during the early stages of schizophrenia are maintained throughout the progression of the illness, NCAM1 mRNA levels were measured in BA46 from subjects with long DOI schizophrenia. No significant difference in NCAM-180 expression in BA46 was observed between subjects with long DOI schizophrenia and matched control subjects (t=0.527, df=28, p=0.602) suggesting NCAM-180 dysfunction is associated with the earlier stages of schizophrenia (figure 1B). Analysis of NCAM-180 mRNA expression between the short and long DOI groups revealed significant variation with duration of illness (F=4.329, d.f.=1,55, p=0.042). This variation was due to lower NCAM-180 mRNA levels in the short DOI controls compared to long DOI controls (t=2.338, df=27, p<0.027) while there was no significant difference between the short and long DOI schizophrenia groups (t=0.442, df=27, p<0.662). Neither age nor duration of illness strongly correlated with NCAM-180 levels within the short and long DOI sample groups (0.224>r2>1.063 ×10−4).
There was no significant association between NCAM-180 mRNA levels and potential confounding factors such as tissue pH, PMI, gender, the incidence of suicide and antipsychotic medication for any sample group (0.232>r2>7.040×10−5).
4 Discussion
Our data shows a 27% increase in normalised expression levels of NCAM-180 mRNA in BA46 from subjects with schizophrenia during the early years following diagnosis of the illness. This finding is consistent reported increases in serum NCAM-180 protein levels associated with schizophrenia (Tanaka et al., 2007). Our data contrasts findings of increased NCAM-120 but not NCAM-180 protein expression in the CSF of subjects with schizophrenia (Poltorak et al., 1995). However, it is unlikely that a localised increase in NCAM-180 expression would be detected in the CSF.
Within the BA46, NCAM-180 protein expression has been reported to be unaltered in schizophrenia. However, an increase in the levels of the 105–115 kDa cNCAM isoform, a proteolytic cleavage product of the NCAM-180 protein, was observed. (Vawter et al., 2001; Vawter et al., 1998). This reported increase in cNCAM may reflect increased post-translational modification of NCAM-180 in schizophrenia in response to increased transcript levels. Significantly, previous reports of unaltered NCAM-180 protein in BA46 in schizophrenia used subjects with a mean age of over twenty years older than our short DOI cohort (Vawter et al., 2001) and may reflect the lack of change in NCAM-180 expression that we have shown is associated with late DOI schizophrenia.
The limited availability of post-mortem tissue is a constraint in this study, however, our sample size was sufficiently powerful to be confident in the differences in NCAM-180 expression seen between short DOI schizophrenia and controls. We were unable to ascertain data on the ethnicity of the subjects and thus we could not consider the impact this variable had on our data. Similarly, the limited sample size prevented a valid assessment of genotypic variation within the cohort. Therefore, there is the potential for the differences between our data and published studies to reflect genotypic differences between the cohorts. Substance abuse histories were also not available for the subjects included in our cohort. While toxicological analysis detected cannabinoids, opiates and ethanol within the blood of several subjects, the short half-lives of these drugs within the bloodstream makes accurate assessment of substance abuse history based on toxicology data difficult (Drummer, 2004). Thus, we cannot exclude any impact these factors may have had upon our data. The incidence of suicide did differ between the short DOI and long DOI groups used in this study. However, the lack of correlation between the incidence of suicide and levels of NCMA-180 levels suggest these differences are unlikely to affect data.
A recent microarray analysis has showed that only 2.2% of genes with altered expression in short DOI schizophrenia remain altered in subjects with long DOI schizophrenia (Narayan et al., 2008). From our data, abnormal NCAM-180 mRNA expression appears to be associated only with short DOI schizophrenia and may reflect changes in symptomatic aspects that develop early in the progression of the disorder, such as cognitive and negative symptoms, which have both been associated with NCAM1 dysfunction (Lyons et al., 1998, Sullivan et al., 2007). Despite apparent differences in NCAM-180 mRNA expression between duration of illness cohorts, no correlation between NCAM-180 mRNA levels and duration of illness was seen within the short or long DOI schizophrenia groups. Comparing the short and long DOI cohorts, NCAM-180 mRNA expression in the short DOI schizophrenia group appeared more comparable to that of the long DOI schizophrenia and control groups than to the short DOI control group. Thus, while NCAM-180 mRNA expression appears to increase between the mean aged 30 and 59 year old control groups, the progression of the illness appears to be associated with a constant level of NCAM-180 expression reflective of a more aged population. Interestingly, there was no correlation between age and NCAM-180 mRNA expression in either diagnostic group. Thus, while differences in NCAM-180 expression were evident between short and long DOI control groups, it may be naïve to assume that regulation of NCAM-180 expression in normal individuals is regulated solely as a function of age.
Our findings of increased NCAM-180 expression in BA46 but not in BA10 or BA17 are important in light of a recent report of an association analysis suggesting a role for NCAM1 in cognitive deficit in schizophrenia (Sullivan et al., 2007) and previous reports implicating the dorsolateral prefrontal cortex in the cognitive dysfunction associated with schizophrenia (Levy and Goldman-Rakic, 1999; Perlstein et al., 2001; Yoon et al., 2008). NCAM-180 has been proposed to mediate synaptic plasticity via signalling through spectrin, potentially affecting long term potentiation and cognitive function (Doyle et al., 1992, Fux et al., 2003, Leshchyns'ka et al., 2003, Schuster et al., 1998). Notably, in light of our data, over expression of NCAM-180 has been shown to inhibit neurite outgrowth (Buttner et al., 2004). BA46 is known to be involved in cognitive functions, such as working memory (D'Esposito et al., 1998), while BA10 serves a major function of an attentional gateway for facilitating complex cognitive processing (Burgess et al., 2007; Gilbert et al., 2006). By contrast, while changes in cortical volume within BA17 have been associated with the incidence of schizophrenia, this region does not appear to be involved with cognitive processes (Dorph-Petersen et al., 2007). Thus, the aberrant NCAM-180 expression in BA46 of schizophrenia subjects observed in our study may play a role in deficits in select cognitive functions such as spatial working memory, which appears to be controlled by BA46 (McCarthy et al., 1994). Unfortunately, cognitive data was not available for the subjects used in our study and thus a direct comparison between NCAM-180 expression and cognitive deficits cannot be made.
NCAM1 related findings in schizophrenia, which include altered gene sequence (Aze et al., 2007; Sullivan et al., 2007), abnormal protein expression and post-translational cleaving (Tanaka et al., 2007; Vawter et al., 1998) and altered regulation of the genes controlling polysialylation of the NCAM1 protein (Tao et al., 2007; Arai et al., 2006), are complex. However, it is known that NCAM1 is also associated with a diverse range of functions in both the embryonic and adult brain (Ditlevsen et al., 2008). Furthermore, a recent restriction fragment differential display study showed increased mRNA levels of NCAM2 in BA46, suggestive of a broader involvement of neural cell adhesion molecules in the pathology of schizophrenia (Dean et al., 2007). Therefore, further studies are now required to better understand which of the diverse roles of NCAM molecules may be particularly affected in schizophrenia.
Acknowledgements
The authors gratefully acknowledge the assistance of Geoffrey Pavey for the preparation of post-mortem tissue, Won Je Jeon for his technical assistance and David Copolov, Christine Hill, Nicholas Keks, and Kenneth Opeskin for their roles in tissue collection and diagnostic confirmation.
The study was supported by Operational Infrastructure Support (OIS) from the Victorian State Government and by the funding grants; NIH RO1 MH069696-01 and NHMRC project grant 3503441. Brian Dean is a NHMRC Senior Research Follow (400016). The funding bodies had no further role in study design, in the collection, analysis or interpretation of the data, in the writing of the report or in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There is no conflict of interest associated with the submission of this manuscript.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Arai M, Yamada K, Toyota T, Obata N, Haga S, Yoshida Y, Nakamura K, Minabe Y, Ujike H, Sora I, Ikeda K, Mori N, Yoshikawa T, Itokawa M. Association between polymorphisms in the promoter region of the sialyltransferase 8B (SIAT8B) gene and schizophrenia. Biol. Psychiatry. 2006;59:652–659. doi: 10.1016/j.biopsych.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Atz ME, Rollins B, Vawter MP. NCAM1 association study of bipolar disorder and schizophrenia: polymorphisms and alternatively spliced isoforms lead to similarities and differences. Psychiatr. Genet. 2007;17:55–67. doi: 10.1097/YPG.0b013e328012d850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas JA, Chaix JC, Steinmetz M, Goridis C. Differential splicing and alternative polyadenylation generates distinct NCAM transcripts and proteins in the mouse. EMBO J. 1988;7:625–632. doi: 10.1002/j.1460-2075.1988.tb02856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau D, Liang JJ, Robitaille Y, Quirion R, Srivastava LK. Decreased expression of the embryonic form of the neural cell-adhesion molecule in schizophrenic brains. PNAS USA. 1995;92:2785–2789. doi: 10.1073/pnas.92.7.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn. Sci. 2007;11:290–298. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Buttner B, Reutter W, Horstkorte R. Cytoplasmic domain of NCAM 180 reduces NCAM-mediated neurite outgrowth. J. Neurosci. Res. 2004;75:854–860. doi: 10.1002/jnr.20049. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cremer H, Chazal G, Goridis C, Represa A. NCAM is essential for axonal growth and fasciculation in the hippocampus. Mol. Cell. Neurosci. 1997;8:323–335. doi: 10.1006/mcne.1996.0588. [DOI] [PubMed] [Google Scholar]
- Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S, Barthels D, Rajewsky K, Wille W. Inactivation of the N-CAM gene in mice results in size-reduction of the olfactory-bulb and deficits in spatial-learning. Nature. 1994;367:455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res. Cogn. Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Dean B, Keriakous D, Scarr E, Thomas EA. Gene expression profiling in Brodmann's area 46 from subjects with schizophrenia. Aust. N. Z. J. Psychiatry. 2007;41:308–320. doi: 10.1080/00048670701213245. [DOI] [PubMed] [Google Scholar]
- Ditlevsen DK, Povlsen GK, Berezin V, Bock E. NCAM-induced intracellular signaling revisited. J. Neurosci. Res. 2008;86:727–743. doi: 10.1002/jnr.21551. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Pierri JN, Wu Q, Sampson AR, Lewis DA. Primary visual cortex volume and total neuron number are reduced in schizophrenia. J. Comp. Neurol. 2007;501:290–301. doi: 10.1002/cne.21243. [DOI] [PubMed] [Google Scholar]
- Doyle E, Nolan PM, Bell R, Regan CM. Hippocampal NCAM180 transiently increases sialylation during the acquisition and consolidation of a passive avoidance response in the adult rat. J. Neurosci. Res. 1992;31:513–523. doi: 10.1002/jnr.490310315. [DOI] [PubMed] [Google Scholar]
- Drummer OH. Postmortem toxicology of drugs of abuse. Forensic Sci. Int. 2004;142:101–113. doi: 10.1016/j.forsciint.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Frost G, Patel K, Bourne S, Coakham HB, Kemshead JT. Expression of alternative isoforms of the neural cell-adhesion molecule (NCAM) on normal brain and a variety of brain-tumors. Neuropathol. Appl. Neurobiol. 1991;17:207–217. doi: 10.1111/j.1365-2990.1991.tb00716.x. [DOI] [PubMed] [Google Scholar]
- Fux CM, Krug M, Dityatev A, Schuster T, Schachner M. NCAM180 and glutamate receptor subtypes in potentiated spine synapses: an immunogold electron microscopic study. Mol. Cell. Neurosci. 2003;24:939–950. doi: 10.1016/j.mcn.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J. Cogn. Neurosci. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Gliner JA, Morgan GA, Harmon RJ. Basic associated designs: analysis and interpretation. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41:1256–1258. doi: 10.1097/00004583-200210000-00017. [DOI] [PubMed] [Google Scholar]
- He HT, Barbet J, Chaix JC, Goridis C. Phosphatidylinositol is involved in the membrane attachment of NCAM-120, the smallest component of the neural cell-adhesion molecule. EMBO J. 1986;5:2489–2494. doi: 10.1002/j.1460-2075.1986.tb04526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honer WG, Falkai P, Young C, Wang T, Xie J, Bonner J, Hu L, Boulianne GL, Luo Z, Trimble WS. Cingulate cortex synaptic terminal proteins and neural cell adhesion molecule in schizophrenia. Neurosci. 1997;78:99–110. doi: 10.1016/s0306-4522(96)00489-7. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, Meltzer HY, Green MF, Capuano G, Stroup TS, McEvoy JP, Swartz MS, Roscnheck RA, Perkins DO, Davis CE, Hsiao JK, Lieberman JA. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Arch. Gen. Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Keks N, Hill C, Opeskin K, Copolov DL, Dean B. Psychiatric diagnosis after death: the problems of accurate diagnosis. In: Dean B, Hyde TM, Klienman JE, editors. Using CNS Tissue in Psychiatry Research: a Practical Guide. Sydney: J. Gordon & Breach Science Publishers; 1999. pp. 19–37. [Google Scholar]
- Kingsbury AE, Foster OJ, Nisbet AP, Cairns N, Bray L, Eve DJ, Lees AJ, Marsden CD. Tissue pH as an indicator of mRNA preservation in human post-mortem brain. Brain Res. Mol. Brain Res. 1995;28:311–318. doi: 10.1016/0169-328x(94)00219-5. [DOI] [PubMed] [Google Scholar]
- Leshchyns'ka I, Sytnyk V, Morrow JS, Schachner M. Neural cell adhesion molecule (NCAM) association with PKC beta(2) via beta I spectrin is implicated in NCAM-mediated neurite outgrowth. J. Cell Biol. 2003;161:625–639. doi: 10.1083/jcb.200303020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Association of storage and processing functions in the dorsolateral prefrontal cortex of the nonhuman primate. J. Neurosci. 1999;19:5149–5158. doi: 10.1523/JNEUROSCI.19-12-05149.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons F, Martin ML, Maguire C, Jackson A, Regan CM, Shelley RK. The Expression of an N-Cam Serum Fragment Is Positively Correlated with Severity of Negative Features in Type-Ii Schizophrenia. Biol. Psychiatry. 1988;23:769–775. doi: 10.1016/0006-3223(88)90065-0. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, Stenger VA, Cohen JD. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am. J. Psychiatry. 2005;162:475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- Mackowiak M, Dudys D, Chocyk A, Wedzony K. Repeated risperidone treatment increases the expression of NCAM and PSANCAM protein in the rat medial prefrontal cortex. Eur. Neuropsychopharmacol. 2009;19:125–137. doi: 10.1016/j.euroneuro.2008.10.001. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Blamire AM, Puce A, Nobre AC, Bloch G, Hyder F, Goldman-Rakic P, Shulman RG. Functional magnetic resonance imaging of human prefrontal cortex activation during a spatial working memory task. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8690–8694. doi: 10.1073/pnas.91.18.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan S, Tang B, Head SR, Gilmartin TJ, Sutcliffe JG, Dean B, Thomas EA. Molecular profiles of schizophrenia in the CNS at different stages of illness. Brain Res. 2008;1239:235–248. doi: 10.1016/j.brainres.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybroe O, Linnemann D, Bock E. Heterogeneity of soluble neural cell-adhesion molecule. J. Neurochem. 1989;53:1372–1378. doi: 10.1111/j.1471-4159.1989.tb08527.x. [DOI] [PubMed] [Google Scholar]
- Parnas J. From predisposition to psychosis: progression of symptoms in schizophrenia. ACTA Psychiatr. Scand. 1999;99:20–29. doi: 10.1111/j.1600-0447.1999.tb05979.x. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am. J. Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Pollerberg GE, Burridge K, Krebs KE, Goodman SR, Schachner M. The 180-kd component of the neural cell-adhesion molecule NCAM is involved in cell-cell contacts and cytoskeleton-membrane interactions. Cell Tissue Res. 1987;250:227–236. doi: 10.1007/BF00214676. [DOI] [PubMed] [Google Scholar]
- Pollerberg GE, Schachner M, Davoust J. Differentiation state-dependent surface mobilities of 2 forms of the neural cell-adhesion molecule. Nature. 1986;324:462–465. doi: 10.1038/324462a0. [DOI] [PubMed] [Google Scholar]
- Poltorak M, Khoja I, Hemperly JJ, Williams JR, El-Mallakh R, Freed WJ. Disturbances in cell recognition molecules (N-CAM and Ll Antigen) in the CSF of patients with schizophrenia. Exp. Neurol. 1995;131:266–272. doi: 10.1016/0014-4886(95)90048-9. [DOI] [PubMed] [Google Scholar]
- Santoni MJ, Barthels D, Vooper G, Boned A, Gordis C, Wille W. Differential exon usage involving an unusual splicing mechanism generates at least eight types of NCAM cDNA in mouse brain. EMBO J. 1989;8:385–392. doi: 10.1002/j.1460-2075.1989.tb03389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster T, Krug M, Hassan H, Schachner M. Increase in proportion of hippocampal spine synapses expressing neural cell adhesion molecule NCAM180 following long-term potentiation. J. Neurobiol. 1998;37:359–372. doi: 10.1002/(sici)1097-4695(19981115)37:3<359::aid-neu2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Keefe RSE, Lange LA, Lange EM, Stroup TS, Lieberman J, Maness PF. NCAM1 and neurocognition in schizophrenia. Biol. Psychiatry. 2007;61:902–910. doi: 10.1016/j.biopsych.2006.07.036. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Yoshida S, Shimada Y, Ueda H, Asai K. Alteration in serum neural cell adhesion molecule in patients of schizophrenia. Hum. Psychopharmacol. Clin. Exp. 2007;22:97–102. doi: 10.1002/hup.828. [DOI] [PubMed] [Google Scholar]
- Tao R, Li C, Zheng YL, Qin W, Zhang J, Li XW, Xu YF, Shi YY, Feng GY, He L. Positive association between SIAT8B and schizophrenia in the Chinese Han population. Schizophr. Res. 2007;90:108–114. doi: 10.1016/j.schres.2006.09.029. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Bonhomme R, Vitiello S, Rougon G, Poulain DA. Cell surface expression of polysialic acid on NCAM is a prerequisite for activity-dependent morphological neuronal and glial plasticity. J. Neurosci. 1999;19:10228–10236. doi: 10.1523/JNEUROSCI.19-23-10228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryu K, Butler AK, Chesselet MF. Synaptogenesis and ultrastructural localization of the polysialylated neural cell adhesion molecule in the developing striatum. J. Comp. Neurol. 1999;405:216–232. doi: 10.1002/(sici)1096-9861(19990308)405:2<216::aid-cne6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- van Kammen DP, Poltorak M, Kelley ME, Yao JK, Gurklis JA, Peters JL, Hemperly JJ, Wright RD, Freed WJ. Further studies of elevated cerebrospinal fluid neuronal cell adhesion molecule in schizophrenia. Biol. Psychiatry. 1998;43:680–686. doi: 10.1016/s0006-3223(97)00324-7. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vawter MP, Cannon-Spoor HE, Hemperly JJ, Hyde TM, VanderPutten DM, Kleinman JE, Freed WJ. Abnormal expression of cell recognition molecules in schizophrenia. Exp. Neurol. 1998;149:424–432. doi: 10.1006/exnr.1997.6721. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Usen N, Thatcher L, Ladenheim B, Zhang P, VanderPutten DM, Conant K, Herman MM, van Kammen DP, Sedvall G, Garver DL, Freed WJ. Characterization of human cleaved N-CAM and association with schizophrenia. Exp. Neurol. 2001;172:29–46. doi: 10.1006/exnr.2001.7790. [DOI] [PubMed] [Google Scholar]
- Wood GK, Tomasiewicz H, Rutishauser U, Magnuson T, Quirion R, Rochford J, Srivastava LK. NCAM-180 knockout mice display increased lateral ventricle size and reduced prepulse inhibition of startle. Neuroreport. 1998;9:461–466. doi: 10.1097/00001756-199802160-00019. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Minzenberg MJ, Ursu S, Walters R, Wendelken C, Ragland JD, Carter CS. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: Relationship with impaired cognition, behavioral disorganization, and global function. Am. J. Psychiatry. 2008;165:1006–1014. doi: 10.1176/appi.ajp.2008.07060945. [DOI] [PMC free article] [PubMed] [Google Scholar]