Abstract
The increasingly serious problem of acid rain is leading to increased potassium (K) loss from soils, and in our field investigation, we found that even congenerically relative Mosla species show different tolerance to K-deficiency. A hydroponic study was conducted on the growth of two Mosla species and their morphological, physiological and stoichiometric traits in response to limited (0.35 mmol K/L), normal (3.25 mmol K/L) and excessive (6.50 mmol K/L) K concentrations. Mosla hangchowensis is an endangered plant, whereas Mosla dianthera a widespread weed. In the case of M. hangchowensis, in comparison with normal K concentration, K-limitation induced a significant reduction in net photosynthetic rate (P n), soluble protein content, and superoxide dismutase (SOD) activity, but an increase in malondialdehyde (MDA) concentration. However, leaf mass ratio (LMR) and root mass ratio (RMR) were changed little by K-limitation. In contrast, for M. dianthera, K-limitation had little effect on P n, soluble protein content, SOD activity, and MDA concentration, but increased LMR and RMR. Critical values of N (nitrogen):K and K:P (phosphorus) ratios in the shoots indicated that limitation in acquiring K occurred under K-limited conditions for M. hangchowensis but not for M. dianthera. We found that low K content in natural habitats was a restrictive factor in the growth and distribution of M. hangchowensis, and soil K-deficiency caused by acid rain worsened the situation of M. hangchowensis, while M. dianthera could well acclimate to the increasing K-deficiency. We suggest that controlling the acid rain and applying K fertilizers may be an effective way to rescue the endangered M. hangchowensis.
Keywords: Ecophysiological response, Endangered species, Morphological plasticity, Mosla species, Weed
INTRODUCTION
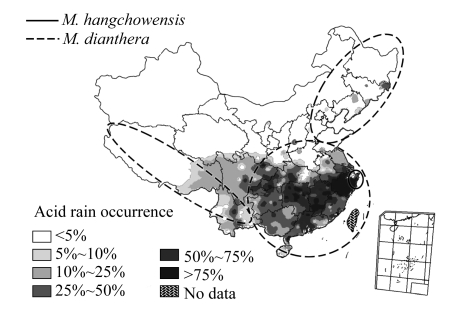
The increasingly serious problem of acid rain due to industrialization and anthropogenic activities has resulted in ecological concerns worldwide, especially in China during the past decades (Fig.1) (Zhang et al., 2007). The negatively charged nitrate and sulphate ions in acid rain may act as “counter-ions,” which allow cations such as potassium (K+), sodium (Na+), calcium (Ca2+) and magnesium (Mg2+) to be leached from the soil and become unavailable to plants (Ivring, 1983). This will probably lead to nutrient deficiency.
Fig.1.
Nationwide acid rain pattern in 2006 (MEP, 2007) and the distribution ranges of M. hangchowensis and M. dianthera in China
Potassium (K) is one of the most important metal elements influencing plant metabolism, growth and development (Marschner, 1995; Jia et al., 2008). Although the relationship between K supply and growth or physiological traits has been studied extensively in cultivated plants (Marschner, 1995; Basile et al., 2003), the relationship between K supply and growth, physiological or morphological traits in wild plants has received less attention (Chapin, 1980; Marschner, 1995). This is especially so for comparisons between endangered species and their weedy congeners.
Mosla hangchowensis Matsuda (Lamiaceae), an endangered annual in China, has only five small (each is less than 4 m2) local populations in the Yangtze River Delta region, where the acid rain problems are increasingly serious. Its habitats are limited to the top of rocky hills and/or roadsides beside gravel in higher mountainous regions (Chang et al., 1999; Ge and Chang, 2001). Its congeneric species, Mosla dianthera (Buch.-Ham.) Maxim, is widely distributed from northeast to southeast of Asia and in the center regions in China, and it is often a dominant species in the communities. Its habitats range from arid hills to the edge of wetlands and its distribution overlaps with that of M. hangchowensis (Fang et al., 1989).
In our previous analysis of 16 essential elements, we found that all but K concentrations in plant tissues of M. hangchowensis were within the normal ranges reported for most herbaceous species (Ge et al., 1997). We also found that soil-available K content (0~20 cm deep soil) under M. hangchowensis communities was higher than that in similar habitats without M. hangchowensis, (101.3±5.3) and (60.1±13.8) mg K/kg soil, respectively (P<0.05), with the soil-available K measured by Flame Atomic Absorption Spectrometry with the extraction solution of NH4OAc according to Bao (2005). However, the widespread M. dianthera that was distributed in similar habitat to that of M. hangchowensis had normal K concentrations in plant tissues.
M. hangchowensis is more restricted to comparatively higher K habitats than M. dianthera, one presumption of which is that this species has low capacity to assimilate soil K, regardless of soil K content in the habitats. According to Tilman (1980), the abilities of species in acquiring and using limiting nutrients determine their competitive success in the community. The reduced success of species with lower ability is especially obvious when the element content is low in their habitats. Therefore, it seems possible that M. hangchowensis is out-competed in habitats where the K content is low. The increasingly serious problem of acid rain in the Yangtze River Delta where the two Mosla species co-occur has a remarkable effect in reducing K content of habitats (Wang et al., 2003; Mou and Zhu, 2005), and thus has an ongoing influence on Mosla species.
Here, we report a comparative study on the growth of M. hangchowensis and M. dianthera and their morphological, physiological and stoichiometrical responses to three levels of K supply representing limited, normal and excessive K availabilities. The particular objectives were (1) to compare the performance of the two species under the different K concentrations, (2) to establish whether M. hangchowensis has a lower ability to acquire K than M. dianthera, and (3) to predict the future population fates of the two congeneric species in face of soil K changes resulting from the increasingly serious problem of acid rain.
MATERIALS AND METHODS
Plant materials
Seedlings of the two species were collected in April 2003 from two co-occurring natural populations on Wuchao Mountain (120°00′ E, 30°12′ N), which is located in Hangzhou, southeastern China. The seedlings were grown at Hangzhou Botanical Garden (120°16′ E, 30°15′ N) under natural conditions until seeds could be harvested, and were then air-dried and stored in a refrigerator (4 °C) (SC-329GA, Haier Group, China) in November 2003. Seeds were germinated in early May 2004 in trays of peat (Sunshine Mix 6, Sun Gro Horticulture Canada Ltd.) and maintained in growth chambers with a 16-h photoperiod, day/night temperatures of 25/15 °C, relative humidity of 70%~80%, and irradiance (λ=400~700 nm) of approximately 250 μmol photon/(m2·s). When the height of plants reached about 4 cm, the seedlings were transplanted to plastic containers (39 cm×29 cm×12 cm), which were filled with different concentrations of Knop’s solution and continuously aerated by air pumps (Farr, 1925). Plants were grown for 10 weeks in a greenhouse in the College of Life Science, Zhejiang University, Hangzhou (120°05′ E, 30°18′ N) with 70% full ambient irradiance. The average daily temperature ranged from 20 to 36 °C during the experiment, which lasted from early May to mid July 2004.
Experimental design
The experiment used a 2×3×6 randomized complete block design (RCBD) with a total of 432 plants (432=2 species×3 K concentrations×6 blocks× 12 plants per box within each block). Twelve seedlings of each species were randomly selected and planted (supported by a foam plug) in each container, with one seedling in each grid cell. The foam plug provided room for stem expansion as the plant grew. Evaporation of water from the container was prevented by airproofing the foam plug with lanolin. The three K concentrations were: (1) control, referred to as the K3 treatment, continuously received normal Knop’s nutrient solution containing 3.25 mmol K/L, representing K concentrations in the soil solution under normal field conditions; (2) the K-limiting treatment, referred to as the K0 treatment, continuously received normal Knop’s nutrient solution containing 0.35 mmol K/L, representing K concentrations under field conditions for soils with low K availability (Nisbet and Shaw, 1996; Reisenauer, 1966); and (3) the excess K treatment, referred to as the K6 treatment, received the nutrient solution containing 6.50 mmol K/L, reflecting K concentrations in soils with high K availability due to dry K deposition (Luo et al., 2006). A combination of KCl, NaH2PO4, and NaNO3 was utilized to maintain equal K+, Na+ and NO3 − concentrations. The solutions were renewed once a week.
Measurements and calculation
Measurements were conducted in mid July when the species were growing vigorously. Crown dimension (defined as the product of crown width measured in two perpendicular directions) and above ground height of the plants were measured. Six out of the 12 plants in each container were selected randomly. The rate of net photosynthesis rate (P n) was measured between 09:00 and 11:00 a.m. using a portable gas exchange system (Model LCA-4, ADC Ltd., Hoddesdon, UK), and the CO2 concentration and temperature in the leaf chamber were maintained at 360 μmol CO2/mol and 25 °C, respectively. The extraction of soluble protein was carried out according to Jordan et al.(1992) and determined with Folin-Ciocalteu reagent according to Lowry et al.(1951). The activity of superoxide dismutase (SOD) was assayed by measuring its ability to inhibit the photochemical reduction of nitro blue tetrazolium (NBT) (Beauchamp and Fridovich, 1971). Malondialdehyde (MDA) concentration was measured by the thiobarbituric acid reaction (Stewart and Bewley, 1980). After these measurements the plants were harvested and separated into leaves, stems and roots. All samples were then dried in an oven at 80 °C for at least 48 h to constant weight. Height ratio (HR, height per unit biomass), leaf mass ratio (LMR, leaf mass/total mass) and root mass ratio (RMR, root mass/total mass) were calculated according to Hunt (1978) and Sakai (1995). Stem mass ratio (SMR, stem mass/total mass) was not examined, since only two of the three allocation traits (LMR, SMR and RMR) were independent variables.
The plant samples were then ground and analyzed for N concentration using Nessler’s reagent (Yuen and Pollard, 1952) after the samples were digested with H2SO4/H2O2 (Bao, 2005). The analyses of K and P concentrations in the digests were conducted using a Thermo Electron Corp. IRIS Intrepid II Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES) (Waltham, MA, USA). Nutrient concentrations are reported for the dry weights of leaf, shoot (leaves plus stems), and whole plant bases.
Statistical analysis
Statistical analysis was conducted using SPSS 15.0 for Windows (SPSS Inc., Chicago, Illinois, USA). Normality of the dataset was tested by the Shapiro-Wilks test and the result showed that data transformations were not required. The Levene’s test conducted using the general linear model (GLM) procedure showed that the data had homogeneous variances. Since there was no significant species×K treatment interaction, the treatment effects on biomass production, crown dimension, height, and HR and the differences between species were tested using a two-way analysis of variance (ANOVA) followed by least significant difference (LSD) test to compare the main effect in GLM univariate procedure. The effects of species, K treatments and their interaction on LMR, RMR, P n, soluble protein concentration, MDA concentration, SOD activity, and tissue K, N and P concentrations were tested by general linear mixed model in SPSS, with species and K treatments as fixed factors. All data are presented as mean±standard error (SE), and statistical significance was determined at P<0.05.
RESULTS
Growth and morphological response to different K treatments
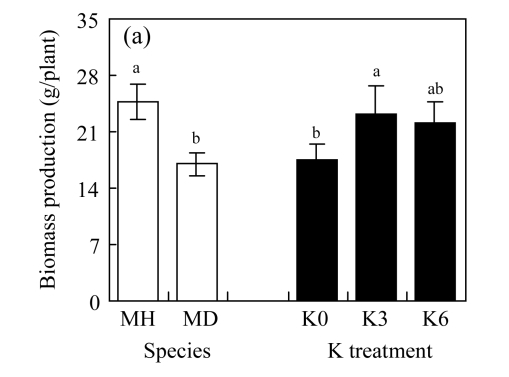
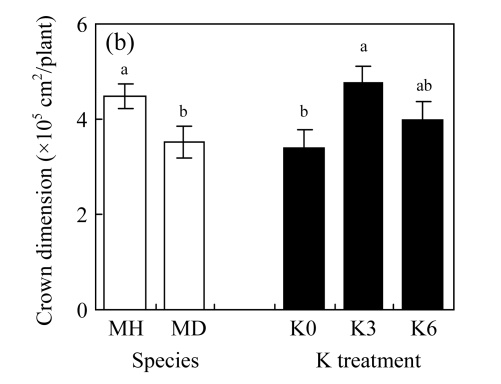
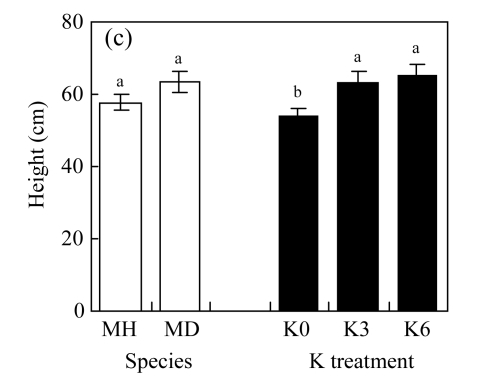
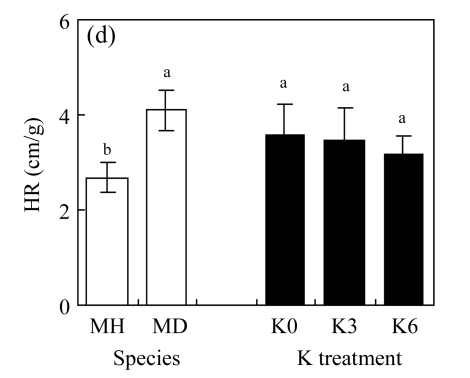
There were no significant differences between K3 and K6 in the four traits of either species (P>0.05, Fig.2; ANOVA data are shown in Table 1, same below). Biomass production and crown dimension were significantly higher, while HR was lower across the K treatments for M. hangchowensis than for M. dianthera (P<0.05). Across the two species, biomass production, crown dimension and height were significantly lower under K0 than under K3 (P<0.05).
Fig.2.
Main effects of species (MH: M. hangchowensis; MD: M. dianthera) and K treatments (K0: 0.35 mmol K/L; K3: 3.25 mmol K/L; K6: 6.50 mmol K/L) on (a) biomass production, (b) crown dimension, (c) height, and (d) HR (height ratio)
Error bars are standard errors. Different letters in each graph indicate significant differences (P<0.05) between species or among K treatments
Table 1.
ANOVA results for the effects of species, K treatments and their interaction on biomass production, morphological and physiological traits, K, N, P concentrations in tissues, and shoot N:K and K:P ratios of M. hangchowensis and M. dianthera
| Parameters | Species |
K treatment |
Species×K |
||||
| F value | P value | F value | P value | F value | P value | ||
| Biomass production | 16.71 | 0.001 | 4.28 | 0.037 | 1.84 | 0.198 | |
| Crown dimension | 10.34 | 0.007 | 6.54 | 0.011 | 0.60 | 0.564 | |
| Height | 2.99 | 0.108 | 6.39 | 0.012 | 2.52 | 0.119 | |
| HR | 7.39 | 0.018 | 0.48 | 0.630 | 0.95 | 0.412 | |
| LMR | 26.35 | <0.001 | 31.82 | <0.001 | 8.56 | 0.004 | |
| RMR | 0.11 | 0.750 | 8.86 | 0.004 | 41.17 | <0.001 | |
| Pn | 1.49 | 0.232 | 25.25 | <0.001 | 5.74 | 0.008 | |
| Soluble protein concentration | 200.16 | <0.001 | 4.48 | 0.026 | 39.56 | <0.001 | |
| MDA concentration | 4.76 | 0.037 | 4.04 | 0.028 | 1.24 | 0.030 | |
| SOD activity | 37.19 | <0.001 | 3.44 | 0.046 | 11.48 | <0.001 | |
| K concentration | Leaf | 1.73 | 0.236 | 1747.79 | <0.001 | 106.70 | <0.001 |
| Shoot | 9.20 | 0.023 | 139.57 | <0.001 | 6.94 | 0.028 | |
| Plant | 28.02 | 0.002 | 375.26 | <0.001 | 14.99 | 0.005 | |
| N concentration | Leaf | 9.54 | 0.021 | 17.61 | 0.003 | 23.26 | 0.001 |
| Shoot | 0.12 | 0.743 | 1.45 | 0.307 | 27.09 | 0.001 | |
| Plant | 2.31 | 0.179 | 13.68 | 0.006 | 141.02 | <0.001 | |
| P concentration | Leaf | 0.41 | 0.544 | 1.29 | 0.341 | 2.92 | 0.130 |
| Shoot | 9.27 | 0.023 | 1.22 | 0.361 | 2.06 | 0.209 | |
| Plant | 2.10 | 0.198 | 9.44 | 0.014 | 7.19 | 0.026 | |
HR: height ratio; LMR: leaf biomass ratio; RMR: root biomass ratio; P n: net photosynthesis ratio; MDA: malondialdehyde; SOD: superoxide dismutase. The P values less than 0.05 are highlighted
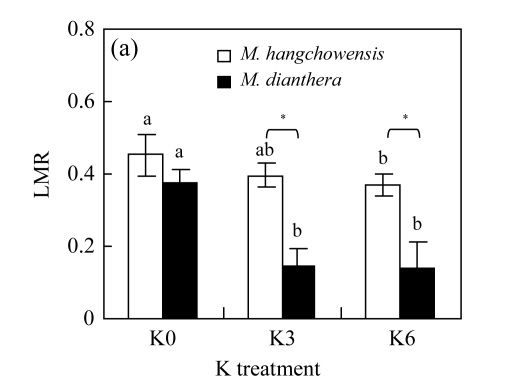
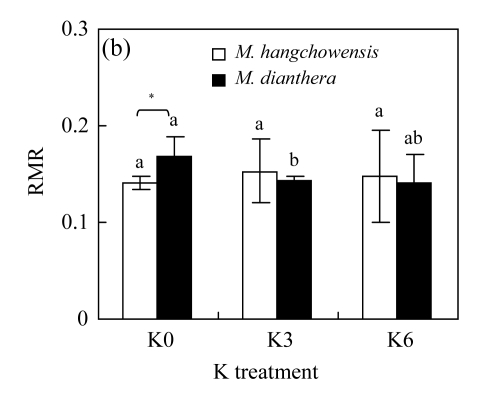
For M. hangchowensis, neither K0 nor K6 affected LMR in comparison with K3 (P>0.05, Fig.3). However, for M. dianthera, LMR was significantly higher under K0 than under K3 (P<0.05). Under K3 and K6, LMR of M. hangchowensis was significantly higher than that of M. dianthera (P<0.05). RMR did not change with increasing K supply for M. hangchowensis (P>0.05, Fig.3); while it was higher under K0 than under K3 for M. dianthera (P<0.05). Under K0, RMR of M. hangchowensis was significantly lower than that of M. dianthera (P<0.05).
Fig.3.
Comparison of (a) LMR (leaf mass ratio) and (b) RMR (root mass ratio) of M. hangchowensis and M. dianthera under different K treatments (K0: 0.35 mmol K/L; K3: 3.25 mmol K/L; K6: 6.50 mmol K/L)
Error bars are standard errors. Different letters in each graph indicate significant differences (P<0.05) among K treatments for the same species; * indicates significant difference (P<0.05) between species under the same K treatment
Response of physiological parameters to different K treatments
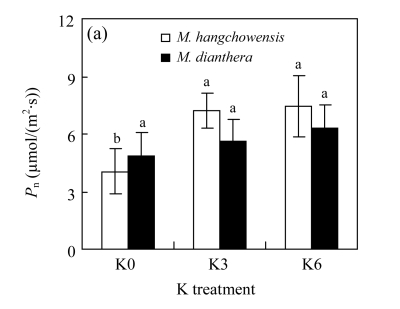
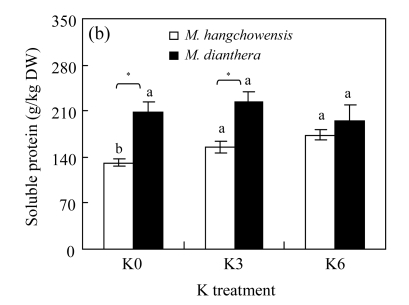
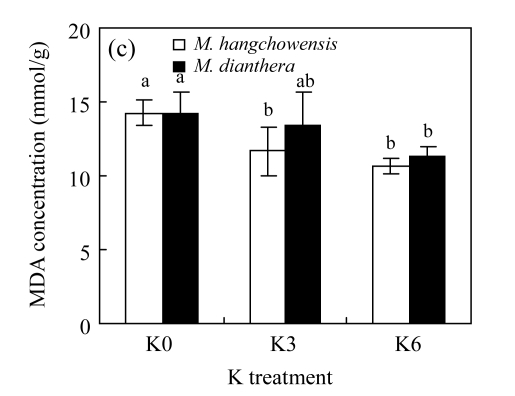
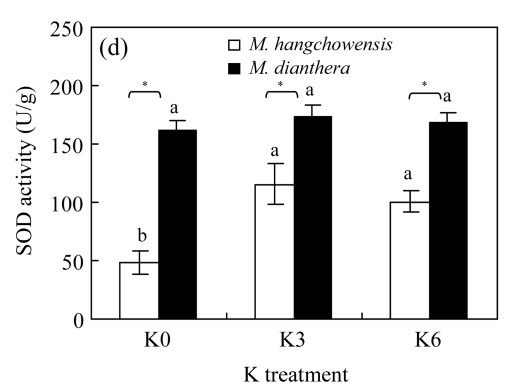
There was no significant difference between K3 and K6 in any of the four physiological traits for either species (P>0.05, Fig.4). For M. hangchowensis, P n, soluble protein content and SOD activity were lower while MDA content was higher under K0 than under K3 (P<0.05). However, for M. dianthera, no differences were evident between K0 and K3 for the four traits measured (P>0.05). Under K0 and K3, soluble protein content of M. hangchowensis was higher than that of M. dianthera (P<0.05). Under the same K treatment, the SOD activity of M. hangchowensis was lower than that of M. dianthera (P<0.05).
Fig.4.
Comparison of (a) net photosynthesis rate (P n), (b) soluble protein concentration, (c) malondialdehyde (MDA) content, and (d) superoxide dismutase (SOD) activity of M. hangchowensis and M. dianthera under different K treatments (K0: 0.35 mmol K/L; K3: 3.25 mmol K/L; K6: 6.50 mmol K/L)
Error bars are standard errors. Different letters in each graph indicate significant differences (P<0.05) among K treatments for the same species; * indicates significant difference (P<0.05) between species under the same K treatment
Response of stoichiometrical traits to different K treatments
For M. hangchowensis, K concentrations in the leaf, shoot (leaves plus stem) and whole plant increased with K supply (P<0.05, Table 2), while for M. dianthera, only leaf K concentration was higher under K6 than under K3 (P<0.05). K concentrations in the leaf, shoot and whole plant were all lower in M. hangchowensis than in M. dianthera under K0 (P<0.05), while leaf K concentration was higher in M. hangchowensis than in M. dianthera under K3 (P<0.05).
Table 2.
Comparison of N, P and K concentrations in M. hangchowensis and M. dianthera under different K treatments
| K concentration (g/kg DW) |
N concentration (g/kg DW) |
P concentration (g/kg DW) |
|||||||
| Leaf | Shoot | Plant | Leaf | Shoot | Plant | Leaf | Shoot | Plant | |
| M. hangchowensis | |||||||||
| K0 | 7.35±0.68c | 6.07±0.56c | 6.60±1.14b | 17.05±2.55b | 9.70±1.37b | 12.36±1.78a | 2.17±0.74 | 1.92±0.66 | 3.21±0.30a |
| K3 | 30.66±0.44b | 22.94±1.30b | 24.02±2.41a | 20.96±1.52b | 8.53±0.64b | 9.25±0.94b | 2.23±0.81 | 1.98±0.44 | 2.69±0.19ab |
| K6 | 38.79±3.90a | 30.43±2.51a | 24.79±3.65a | 31.18±2.16a | 14.10±0.73a | 12.19±1.02a | 2.31±0.64 | 1.95±0.55 | 2.37±0.27b |
| M. dianthera | |||||||||
| K0 | 29.85±2.32a* | 28.70±2.19* | 26.21±2.96* | 21.79±2.70b | 10.59±1.09b | 10.87±1.30b | 2.69±0.35 | 2.50±0.33 | 3.24±0.41a |
| K3 | 26.08±2.28b* | 26.13±4.75 | 22.63±4.83 | 36.71±0.42a* | 14.60±0.37a* | 13.03±0.43a* | 2.30±0.07 | 2.02±0.09 | 1.85±0.09b* |
| K6 | 33.12±1.68a | 31.39±2.84 | 27.20±2.85 | 23.62±2.17b* | 7.85±0.64c* | 6.94±0.67c* | 1.99±1.14 | 2.36±0.95 | 2.09±0.96a |
K0: 0.35 mmol K/L; K3: 3.25 mmol K/L; K6: 6.50 mmol K/L. All data are expressed as mean±SE. Different lowercase letters following the values in each column indicate significant differences (P<0.05) among K treatments for the same species and no letter means no significant inter-treatment difference. DW=dry weight
indicates significant difference (P<0.05) between species under the same K treatment
For M. hangchowensis, N concentrations in the leaf, shoot and whole plant were all higher under K6 than under K3 (P<0.05, Table 2). N concentration in the whole plant was higher under K0 than under K3 (P<0.05). For M. dianthera, N concentrations in the leaf, shoot and whole plant were all lower under K0 and K6 than under K3 (P<0.05). N concentrations of the leaf, shoot and whole plant were all lower in M. hangchowensis than in M. dianthera under K3, while higher under K6 (P<0.05).
P concentration in whole plant was unaffected by K0 and K6 in comparison with K3 for M. hangchowensis (P>0.05), while it was higher under K0 and K6 than under K3 for M. dianthera (P<0.05, Table 2). Under K3, P concentration in the whole plant of M. hangchowensis was higher than that of M. dianthera (P<0.05).
DISCUSSION
Induced variation in stoichiometrical traits by different tissue K concentrations
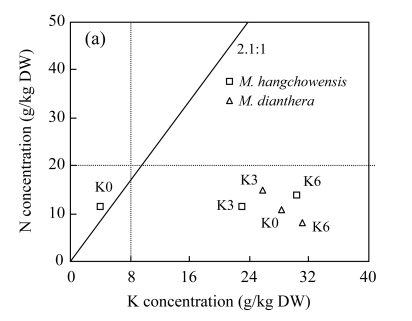
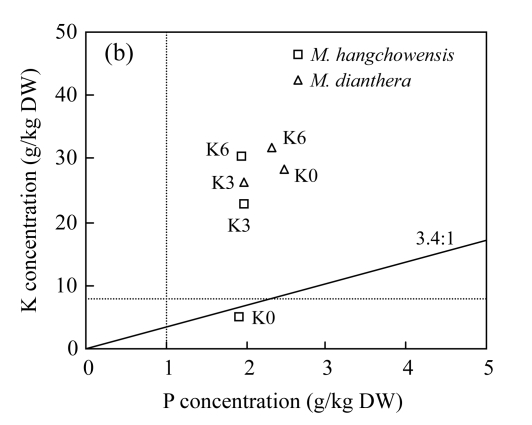
Critical values for assessing nutrient limitation have been developed for different plant species (Marschner, 1995; Koerselman and Meuleman, 1996; Aerts and Chapin, 2000). According to the often-used critical values for general plants, tissue K concentration of 8 g/kg is considered the lowest amount for plant growth (Marschner, 1995). We found that tissue K concentration of M. hangchowensis under limited K supply was below the critical value, 8 g K/kg dry weight (DW) (Table 2 and Fig.5). However, for M. dianthera, tissue K concentrations were all higher than 20 g/kg DW under K-limited condition (Table 2 and Fig.5). It suggests that two species have different K uptake ability when K supply is limited. While under normal and excessive K conditions, tissue K concentrations in both species were all within the normal range found by Marschner (1995) for many plant species (Table 2).
Fig.5.
Relationships between nutrient concentrations with regard to critical tissue concentrations of nitrogen (N), phosphorus (P), potassium (K) and the N:K, K:P ratios in shoots (leaves plus stems) of M. hangchowensis and M. dianthera under different K treatments. (a) N vs K; (b) K vs P
Dotted lines represent critical tissue nutrient concentrations, 8 g/kg DW for K, 20 g/kg DW for N, and 1 g/kg DW for P (Aerts and Chapin, 2000). DW: dry weight. The solid line indicates the critical nutrient ratio (N:K=2.1:1, K:P=3.4:1)
To further assess the limitation that occurred in M. hangchowensis under K-limited condition, we evaluated tissue N:K and K:P ratios. Olde Venterink et al.(2003) proposed that if shoot (leaves plus stem) N:K ratio >2.1 and K:P ratio <3.4, then K-limitation is implied. In current study, for M. hangchowensis, N:K was higher than 2.1 and K:P was lower than 3.4, showing that K-limitation was induced in M. hangchowensis under K-limited condition (Fig.5). However, for M. dianthera, the higher K concentration in the shoot and the normal critical N:K and K:P ratios both indicate its tolerance to low K, and no K-limitation was implied.
Physiological response to variation in K concentrations
The basic roles that K plays in plants are to promote osmotic adjustment, photosynthesis and protein synthesis (Ashraf and Naz, 1994; Quintero et al., 1998). We found that P n and K concentrations in the leaf of M. hangchowensis, but not M. dianthera, were significantly reduced by low K (Fig.4 and Table 2). In contrast, P n was not affected by the limited K supply for M. dianthera, suggesting that the two species had different physiological response patterns to K supply. The lack of K-limitation effect on P n in M. dianthera might be due to its efficient K uptake mechanism because its tissue K concentrations were little affected by the K-limitation and remained normal under K-limited condition (Table 2).
Soluble protein plays an important role in reducing damage to plants under environmental stress (Öncel et al., 2004). SOD reduces damage to the electron transfer system (Halliwell, 1987) and restrains the formation of free radicals to activate the antioxidant defense mechanism, which protects the unsaturated membrane lipids, nucleic acids, enzymes and other cellular structures from the harmful effects of free radicals (Larson, 1988; Foyer et al., 1994). MDA is a secondary end product of the oxidation of polyunsaturated fatty acids and its amount may reflect general lipid peroxidation (Stewart and Bewley, 1980). In the current study, K-limitation reduced the soluble protein content and SOD activity, and increased the MDA concentration of M. hangchowensis, which indicates that lipid peroxidation was enhanced, while the activity of defense enzymes was reduced. Hence, M. hangchowensis was probably injured by K-limitation. In contrast, M. dianthera showed well adaptation to both K-limitation and K-excess and maintained a well-regulated metabolism, which indicates good tolerance to a wide ecological range of K supplies.
Growth and morphological response to different K treatments
High biomass allocation to the root may be beneficial to nutrient and water uptake while high HR is often regarded as a light-seeking strategy for species under dense canopies (Sakai, 1995; Pothier and Prévost, 2002). In addition, high LMR is an advantage to maintain high P n for supplying energy for K uptake. We found that M. hangchowensis did not adjust its morphological traits to alleviate the adverse effects caused by K-limitation (Fig.3). In contrast, M. dianthera increased LMR and RMR to keep K-uptake capacity as well as photosynthetic capacity relatively unaffected. This seems to be a competitive strategy for M. dianthera under K-limitation condition.
Actuality and prospect of two Mosla species in response to environmental changes
Species with lower element utilization ability will be defeated in competition in environments with low element availability (Tilman, 1980). The lack of ability of M. hangchowensis to acclimate well to K-limited condition might lead to a decrease in population in the community and probably aggravates its endangerment. In contrast, M. dianthera can acclimate well to a broad range of K concentrations and the wide ecological adaptation for K resources may have helped M. dianthera distribute widely in the subtropical and tropical zones of China and in other countries of East and Southeast Asia (Fang et al., 1989). It therefore seems probable that the low K content in natural habitats is a restrictive factor in the growth and distribution of M. hangchowensis and the decline of K supply in relation to acid rain will worsen the situation of M. hangchowensis, while M. dianthera can well acclimate to the increasing K-deficiency. This idea needs to be further tested in the field, and if established, controlling the acid rain and applying K fertilizers may be an effective way to relieve the increasingly serious problem of endangerment of M. hangchowensis.
Acknowledgments
We thank Dr. Barry Madison and Dr. Ashton Duvall of Queen’s University, Canada for their help on the language on an earlier version of this manuscript.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30570113) and the Research Fund for the Doctoral Program of Higher Education (No. 20060335008), China
References
- 1.Aerts R, Chapin FS. The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res. 2000;30:1–67. doi: 10.1016/S0065-2504(08)60016-1. [DOI] [Google Scholar]
- 2.Ashraf M, Naz F. Responses of some arid zone grasses to K-deficiency. Acta Physiol Plant. 1994;16:69–80. [Google Scholar]
- 3.Bao SD. Soil and Agricultural Chemistry Analysis. Beijing, China: China Agriculture Press; 2005. pp. 106–268. (in Chinese) [Google Scholar]
- 4.Basile B, Reidel EJ, Weinbaum SA, DeJong TM. Leaf potassium concentration, CO2 exchange and light interception in almond trees (Prunus dulcis (Mill) D.A. Webb) Sci Hortic-AMSTERDAM. 2003;98(2):185–194. doi: 10.1016/S0304-4238(02)00214-5. [DOI] [Google Scholar]
- 5.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 6.Chang J, Liu K, Ge Y, Qin GQ. Features of the photosynthesis of Mosla hangchowensis and the response of photosynthesis to soil water status. Acta Phytoecol Sin. 1999;23:62–70. (in Chinese) [Google Scholar]
- 7.Chapin FSIII. The mineral nutrition of wild plants. Annu Rev Ecol Syst. 1980;11:233–260. doi: 10.1146/annurev.es.11.110180.001313. [DOI] [Google Scholar]
- 8.Fang YY, Wang JX, Wei Z, et al. Flora of Zhejiang (V) Hangzhou, China: Science and Technology Publishing House; 1989. pp. 289–290. (in Chinese) [Google Scholar]
- 9.Farr CH. Root-hair elongation in Knop’s solution and in tap water. Am J Bot. 1925;12(7):372–383. doi: 10.2307/2435496. [DOI] [Google Scholar]
- 10.Foyer CH, Lelandais M, Kunert KJ. Photooxidative stress in plants. Physiol Plant. 1994;92(4):696–717. doi: 10.1111/j.1399-3054.1994.tb03042.x. [DOI] [Google Scholar]
- 11.Ge Y, Chang J. Existence analysis of populations of Molsa hangchowensis, an endangered plant. Bot Bul Acad Sin. 2001;42:141–147. [Google Scholar]
- 12.Ge Y, Chang J, Qin GQ, et al. The Correlation of Element Uptake in Mosla Hangchowensis . In: Yan LJ, Bao YX, Qian JD, editors. Research on and Probe into Ecology. Beijing, China: China Environmental Science; 1997. pp. 137–141. (in Chinese) [Google Scholar]
- 13.Halliwell B. Oxidative damage, lipid peroxidation and antioxidant protection in chloroplasts. Chem Phys Lipids. 1987;44(2-4):327–340. doi: 10.1016/0009-3084(87)90056-9. [DOI] [Google Scholar]
- 14.Hunt R. Plant Growth Analysis. London, UK: Edward Arnold; 1978. [Google Scholar]
- 15.Ivring PM. Acidic precipitation effects on crops: a review and analysis of research. J Environ Qual. 1983;12:442–453. [Google Scholar]
- 16.Jia YB, Yang XE, Feng Y, Jilani G. Differential response of root morphology to potassium deficient stress among rice genotypes varying in potassium efficiency. J Zhejiang Univ Sci B. 2008;9(5):427–434. doi: 10.1631/jzus.B0710636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan BR, He J, Chow WS, Anderson JM. Changes in mRNA levels and polypeptide subunits of ribulose-1,5-bisphosphate carboxylase in response to supplementary ultraviolet-B radiation. Plant Cell Environ. 1992;15(1):91–98. doi: 10.1111/j.1365-3040.1992.tb01461.x. [DOI] [Google Scholar]
- 18.Koerselman W, Meuleman AM. The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol. 1996;33(6):1441–1450. doi: 10.2307/2404783. [DOI] [Google Scholar]
- 19.Larson RA. The antioxidants of higher plants. Phytochemistry. 1988;27(4):969–978. doi: 10.1016/0031-9422(88)80254-1. [DOI] [Google Scholar]
- 20.Lowry OH, Rosebrough NJ, Farr AL, Randal RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 21.Luo YH, Dai TG, Liang K. Study on distribution of the atmospheric dust fall and its metal element contents in shaoguan city, Guangdong province. Geol Surv Res. 2006;29(1):64–68. (in Chinese) [Google Scholar]
- 22.Marschner H. Mineral Nutrition of Higher Plants. San Diego, USA: Academic Press; 1995. [Google Scholar]
- 23.MEP (Ministry of Environmental Protection of the People’s Republic of China) Report on the State of the Environment in China 2006. 2007 Available from: http://english.mep.gov.cn/standards_reports/soe/SOE2006/200711/t20071105_112565.htm [Accessed 08/02/2009]
- 24.Mou YM, Zhu GL. The study of acid rain distribution of Zhejiang Province based on GIS. Bul Sci Tech. 2005;21(3):356–359. (in Chinese) [Google Scholar]
- 25.Nisbet AF, Shaw S. Technical Report, NRPB-M674. Chilton, UK: National Radiological Protection Board; 1996. Dynamics of Radionuclide Behaviour in Soil Solution: Compilation of Data from Lysimeter and Field Studies. [Google Scholar]
- 26.Olde Venterink H, Wassen MJ, Verkroost A, Ruiter PC. Species richness-productivity patterns differ between N-, P- and K-limited wetlands. Ecology. 2003;84(8):2191–2199. doi: 10.1890/01-0639. [DOI] [Google Scholar]
- 27.Öncel I, Yurdakulol E, Keleş Y, Kurt L, Yıldız A. Role of antioxidant defense system and biochemical adaptation on stress tolerance of high mountain and steppe plants. Acta Oecol-Int J Ecol. 2004;26(3):211–218. doi: 10.1016/j.actao.2004.04.004. [DOI] [Google Scholar]
- 28.Pothier D, Prévost M. Photosynthetic light response and growth analysis of competitive regeneration after partial cutting in a boreal mixed stand. Trees-Structure and Function. 2002;16(4-5):365–373. doi: 10.1007/s00468-001-0158-y. [DOI] [Google Scholar]
- 29.Quintero JM, Fournier JM, Ramos J, Benlloch M. K+ status and ABA affect both exudation rate and hydraulic conductivity in sunflower roots. Physiol Plant. 1998;102(2):279–284. doi: 10.1034/j.1399-3054.1998.1020216.x. [DOI] [Google Scholar]
- 30.Reisenauer HM. Mineral Nutrients in Soil Solution. In: Altman PL, Dittmer DS, editors. Environmental Biology. Bethesda: Federation of American Societies for Experimental Biology; 1966. pp. 507–508. [Google Scholar]
- 31.Sakai S. Evolutionarily stable growth of a sapling which waits for future gap formation under closed canopy. Evol Ecol. 1995;9(4):444–452. doi: 10.1007/BF01237766. [DOI] [Google Scholar]
- 32.Stewart RC, Bewley JD. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980;65(2):245–248. doi: 10.1104/pp.65.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tilman D. Resources: A Graphical-Mechanistic Approach to Competition and predation. Am Nat. 1980;116(3):362–393. doi: 10.1086/283633. [DOI] [Google Scholar]
- 34.Wang DZ, Jiang X, Bian YR, Lang YH, Wang F, He JZ. K+ adsorption characteristics and reaction kinetics in red soft under acid deposition. China Environ Sci. 2003;23(2):171–175. (in Chinese) [Google Scholar]
- 35.Yuen SH, Pollard AG. The determination of nitrogen in agricultural materials by the Nessler reagent: preparation of the reagent. J Sci Food Agric. 1952;3(10):441–447. doi: 10.1002/jsfa.2740031002. [DOI] [Google Scholar]
- 36.Zhang JE, Ouyang Y, Ling DJ. Impacts of simulated acid rain on cation leaching from the Latosol in south China. Chemosphere. 2007;67(11):2131–2137. doi: 10.1016/j.chemosphere.2006.12.095. [DOI] [PubMed] [Google Scholar]