Abstract
Objective: The aim of this study was to test the protective effect of mesenchymal stem cells (MSCs) on cardiomyocytes in vitro and to investigate the anti-apoptotic signaling pathway. Methods: MSCs from Sprague-Dawley (SD) rats were separated and cultured. MSC medium was collected from MSCs cultured in serum-free Dulbecco’s modified eagle medium (DMEM) under hypoxia. Cultured cardiomyocytes from neonatal SD rats were exposed to hypoxia/reoxygenation (H/R) and treated with MSC medium. The apoptotic cardiomyocytes were stained with Annexin-V-fluorescein isothiocyanate (FITC), Hoechst 33342 and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL). The mitochondrial transmembrane potential of cardiomyocytes was assessed using a fluorescence microscope. The expression of Bcl-2, Bax, cytochrome C, apoptosis-induced factor (AIF), and caspase-3 was tested by Western blot analysis. Results: Our data demonstrated that MSC medium reduced H/R-induced cardiomyocyte apoptosis, increased the Bcl-2/Bax ratio, and reduced the release of cytochrome C and AIF from mitochondria into the cytosol. Conclusion: MSCs protected the cardiomyocytes from H/R-induced apoptosis through a mitochondrial pathway in a paracrine manner.
Keywords: Mesenchymal stem cell (MSC), Apoptosis, Mitochondrial transmembrane potential, Hypoxia/reoxygenation (H/R)
INTRODUCTION
Bone marrow-derived mesenchymal stem cells (MSCs) are considered effective in myocardial infarction therapies both in basic research studies (Amado et al., 2005; Tang et al., 2006) and in clinical trials (Janssens et al., 2006; Meyer et al., 2006). MSCs repair the ischemic myocardium primarily by angioblast-mediated vasculogenesis (Kocher et al., 2001), prevention of apoptosis of native cardiomyocytes, or by direct regeneration of the lost cardiomyocytes (Shim et al., 2004; Takahashi et al., 2006). Gnecchi et al.(2005), Kinnaird et al.(2004) and Takahashi et al.(2006) recently reported that the cardio-protective effect of MSCs is related to a paracrine effect, which could be enhanced by hypoxia (Uemura et al., 2006).
However, the compound effect of the cytokines on cultured cardiomyocytes is still unknown. In this study, we use medium prepared from hypoxically cultured MSCs to investigate the effect of the secreted cytokines on cardiomyocytes suffering from hypoxia/reoxygenation (H/R), and further study the potential mechanisms involved.
MATERIALS AND METHODS
Animals
Neonatal and adult Sprague-Dawley (SD) rats were obtained from the Medical Institute Animal Center of Zhejiang University, China. The animal experiments were approved by the Animal Care and Use Committee of Zhejiang Provincial Medical Institute and were in compliance with the Guide for the Care and Use of Laboratory Animals as published by the US National Institutes of Health (Institute of Laboratory Animal Research, Commission on Life Science, National Research Council, 1996).
Cell culture
MSCs were obtained from the femora and tibiae of SD rats (each weighing about 80 g), using a modified method as previously described (Dobson et al., 1999; Xie et al., 2006). After 2~3 passages, MSCs, negative for CD45 and positive for CD44 and CD90, were used. A primary culture of neonatal SD rat cardiomyocytes was prepared using the method described originally by Simpson and Savion (1982) with minor modifications (Xie et al., 2006). After 10 d of incubation, cardiomyocytes grew to about 90% confluence.
MSC medium preparation
To prepare MSC medium, MSCs were incubated in a modular incubator chamber (Billups-Rothenberg, USA) for 6 h in serum-free Dulbecco’s modified eagle medium (DMEM), where normal air was replaced by 95% N2 and 5% CO2. The medium was collected and centrifuged at 4000 r/min to remove cell debris.
H/R-induced cardiomyocyte apoptosis
Cardiomyocytes were randomly divided into two groups: treated with DMEM or treated with MSC medium. Both groups of cells were treated with H/R. To mimic natural hypoxia, the cardiomyocytes were incubated at 37 °C in a modular incubator chamber for 24 h, where normal air was replaced by 95% N2 and 5% CO2. They were then moved into a normoxic incubator (95% air and 5% CO2) for 3 h to mimic the natural reoxygenation process. A control group was incubated in DMEM supplemented with 20% (w/v) fetal calf serum under standard cell culture conditions (95% air and 5% CO2).
We used three methods to determine apoptosis of cardiomyocytes. The Annexin-V-fluorescein isothiocyanate (FITC) apoptosis detection kit (BioVision, USA) was used according to the manufacturer’s instructions. Nuclei were stained with the chromatin dye Hoechst 33342 (Sigma, USA). Briefly, cells were fixed for 1 h in 4% (w/v) paraformaldehyde at room temperature, and then exposed to 5 μg/ml Hoechst 33342 for 30 min at 37 °C in the dark. Cells were observed using a fluorescence microscope. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL, Roche Diagnostic, Germany) assays were performed according to the manufacturer’s protocol. The percentage of TUNEL-positive cells was determined by counting at least 200 cells in 5 randomly selected fields.
Mitochondrial transmembrane potential assessment
Mitochondrial transmembrane potential was assessed using the lipophilic cationic probe JC-1 (BioVision, USA), a sensitive fluorescent dye, as we had described before (Chen et al., 2008). Red emission from the dye is attributed to a potential-dependent aggregation in the mitochondria. Green fluorescence reflects the monomeric form of JC-1, appearing in the cytosol after mitochondrial membrane depolarization. Cardiomyocytes were incubated with 10 μmol/L JC-1 for 15 min at 37 °C in the dark and monitored using a fluorescence microscope.
Isolation of mitochondria and cytosol
Mitochondrial and cytosolic fractions were prepared using a mitochondria/cytosol fractionation kit (BioVision, USA) according to the manufacturer’s protocol. Briefly, cells were collected and resuspended in 100 μl cytosol extraction buffer, incubated on ice for 10 min, and homogenized in an ice-cold tissue grinder. Cell homogenate was subjected to 700×g centrifugation for 10 min. The supernatant was further centrifuged at 10 000×g for 30 min. Supernatants were used as the cytosolic fraction, while the pellets, which were then resuspended in 20 μl of the mitochondrial extraction buffer, were used as the mitochondrial fraction.
Western blot analysis
Protein (20~100 μg) prepared from the disposed cells was separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred onto polyvinylidene difluoride (PVDF) immobilon-P membrane (Bio-Rad, CA, USA) using a transblot apparatus (Bio-Rad, CA, USA). The membranes were blocked in 10 mmol/L Tri-HCl (pH 8.0), 150 mmol/L NaCl and 0.05% (w/v) Tween 20 (Tris-buffered saline Tween 20, TBST) with 5% (w/v) non-fat milk at room temperature, followed by overnight incubation at 4 °C with primary antibodies diluted in TBST (1:1000 for Bcl-2, Bax, caspase-3 and β-actin, Cell Signal, USA; 1:1000 for cytochrome C, BD Pharmingen, USA; 1:1000 for AIF, Santa Cruz, USA). After washing with TBST, the membranes were incubated for 1 h with a horse radish peroxidase (HRP)-conjugated secondary antibody diluted 1:5000 in TBST, and the labeled proteins were detected using enhanced chemiluminescence reagents and exposed to film (Kodak, USA).
Data analysis
Data were expressed as the mean±SEM. Statistical significance between groups was assessed by one-way analysis of variance (ANOVA) followed by SNK using SPSS 11.5. P<0.05 was considered statistically significant.
RESULTS
Morphology of MSCs
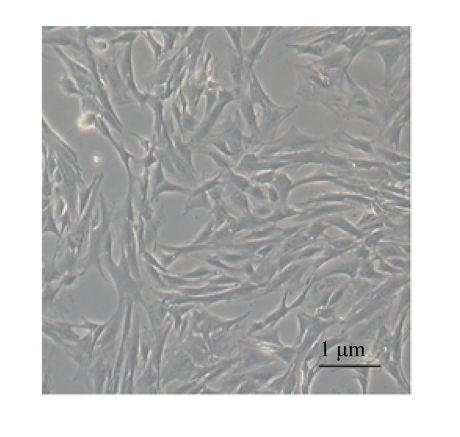
MSCs were attached to culture dishes and the majority displayed a spindle-like shape (Fig.1).
Fig.1.
Characteristics of MSCs. Phase-contrast micrographs of the third passage of MSCs
MSC medium protected cardiomyocytes from H/R-induced apoptosis
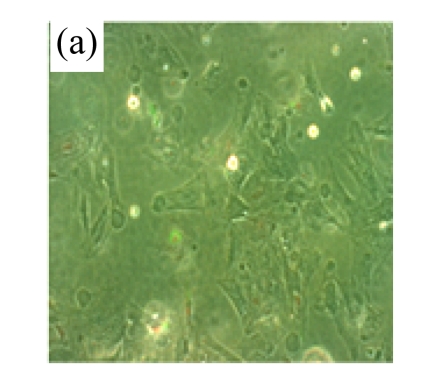
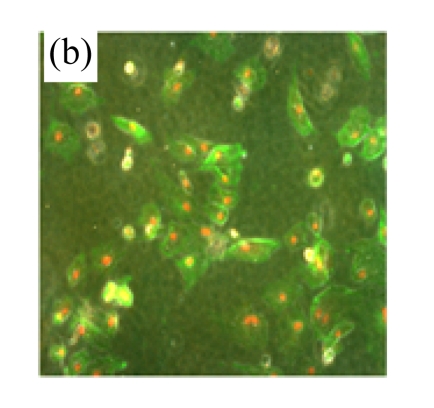
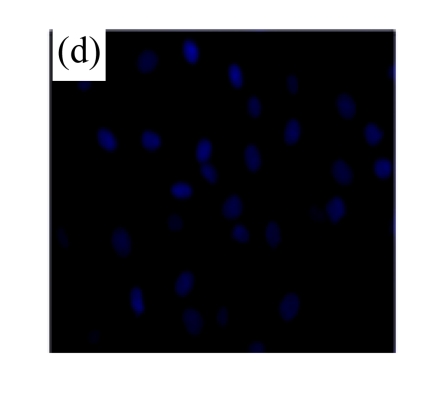
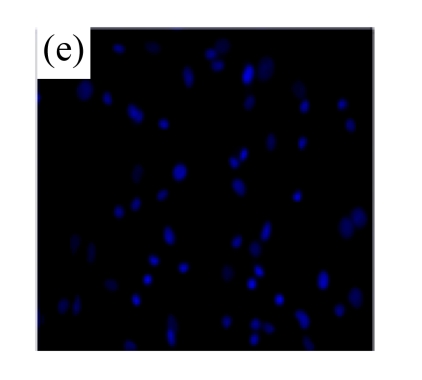
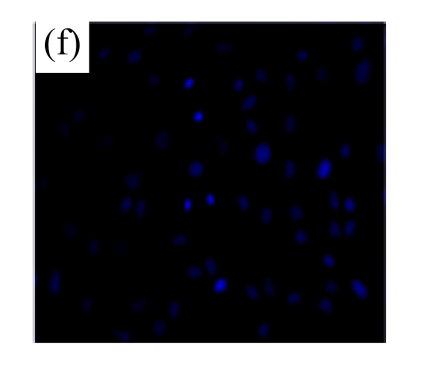
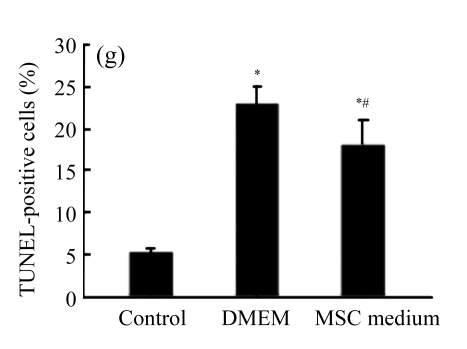
Exposure of cultured cardiomyocytes to H/R led to an increase of cell apoptosis, as assessed by three methods: Annexin V-FITC staining (Figs.2a~2c), Hoechst 33342 staining (Figs.2d~2f) and TUNEL assay (Fig.2g). MSC medium decreased the apoptosis (control: (5.2±0.5)%, DMEM: (23.0±2.1)%, P<0.05 vs control; MSC medium: (18.1±3.0)%, P<0.05 vs control and DMEM).
Fig.2.
MSC medium reduced H/R-induced apoptosis of cardiomyocytes
(a)~(c) Apoptotic cells were detected by Annexin V-FITC staining for labeling early-stage apoptotic cells (green) and necrotic cells (PI stained, red); (d)~(f) Hoechst 33342 staining of cardiomyocytes: apoptotic cells were characterized by nuclear shrinkage with condensed chromatin structure; (g) Quantification of apoptotic cardiomyocytes measured by TUNEL assay. The fraction of apoptotic cells was determined in five random microscopic fields totalling at least 1000 cells/group. Cardiomyocytes were hypoxic for 24 h and were reoxygenated for 3 h in serum-free DMEM (DMEM group) or the medium abstracted from MSCs (MSC medium group). Control cells were cultured in DMEM containing 20% (w/v) fetal calf serum. Results are representative of three independent experiments. Data are shown as mean±SEM. Control group: (a), (d); DMEM group: (b), (e); MSC medium group: (c), (f). * P<0.05 vs control group, # P<0.05 vs DMEM group
MSC medium protected cardiomyocytes from H/R-induced mitochondrial membrane potential loss
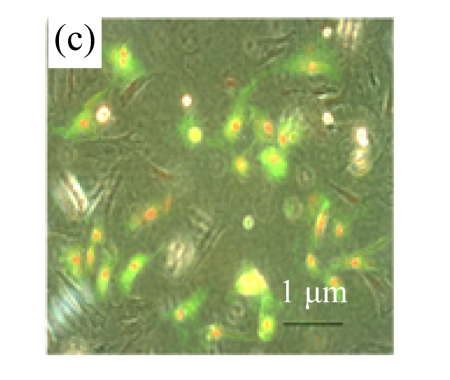
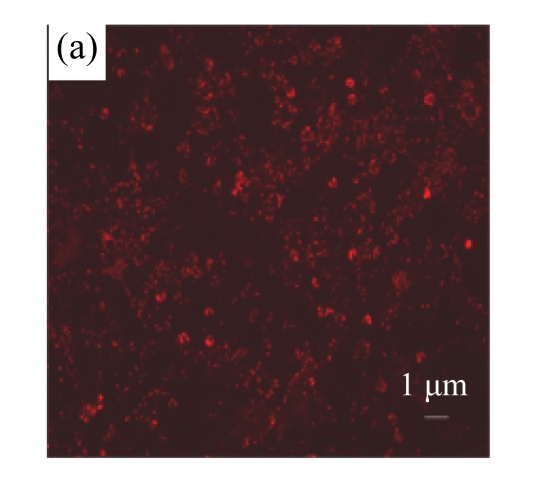
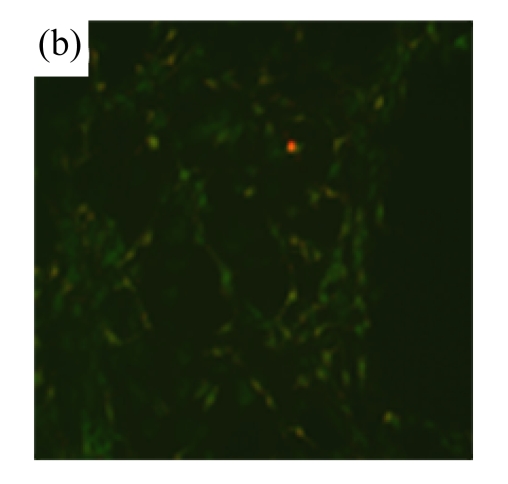
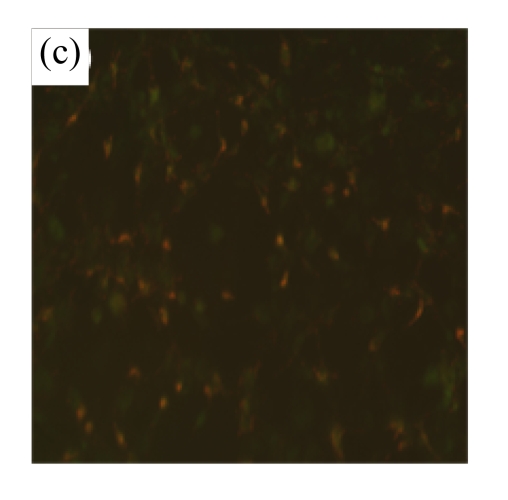
To determine whether MSC medium affected H/R-induced cardiomyocyte mitochondrial dysfunction, we assessed mitochondrial membrane potential using the potential-sensitive fluorescent probe JC-1. Normal cardiomyocytes exhibited red fluorescence (Fig.3a) whereas cardiomyocytes after H/R developed a diffuse green staining pattern (Fig.3b), indicative of reduced mitochondrial membrane potential. MSC medium had a marked effect on JC-1 staining, preserving mitochondrial membrane potential (Fig.3c).
Fig.3.
MSC medium attenuated the reduction of mitochondrial membrane potential of H/R-induced cardiomyocytes
Mitochondrial membrane potential was determined using the potential-sensitive fluorescent probe JC-1. (a) Normally cultured cardiomyocytes contained red fluorescent mitochondria in the cytoplasm; (b) Cardiomyocytes after treatment with H/R and serum-free DMEM culture showed green fluorescence, indicating the loss of mitochondrial membrane potential; (c) Cardiomyocytes with H/R and MSC medium culture showed red fluorescent mitochondria in the cytoplasm, indicating the preservation of the mitochondrial membrane potential. Results are representative of three independent experiments
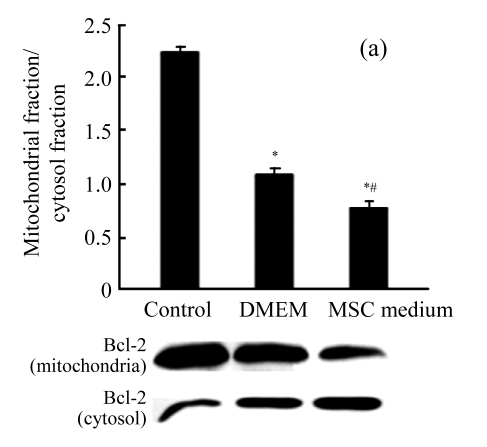
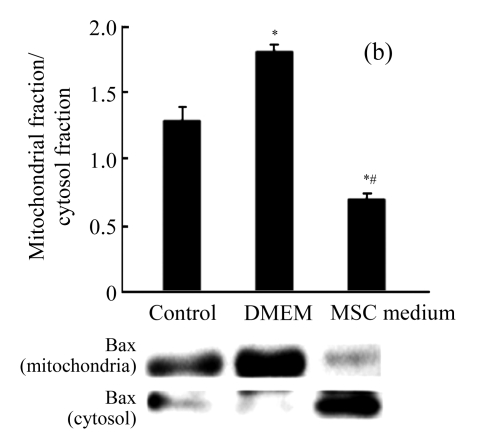
MSC medium increased the ratio Bcl-2/Bax in mitochondria
To explore the signaling pathway upstream of the mitochondria, we investigated whether MSC medium would have any impact on the proapoptotic Bcl-2-family members Bcl-2 and Bax. Mitochondria were prepared and analyzed for the expression of Bcl-2 and Bax. Treatment with MSC medium during H/R decreased the Bax level in the mitochondria, resulting in a significant increase of about 3.3-fold in the Bcl-2/Bax ratio (Figs.4a and 4b).
Fig.4.
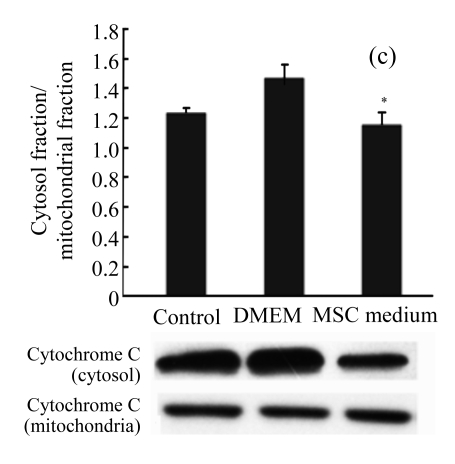
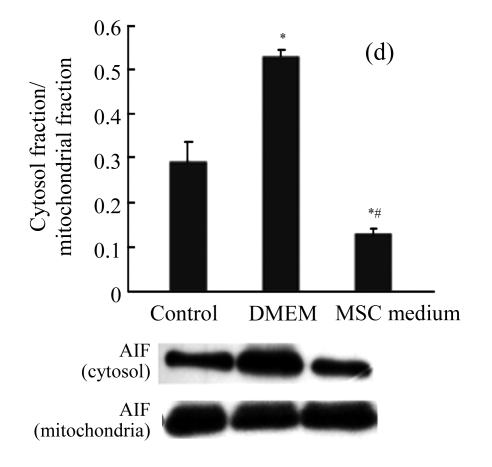
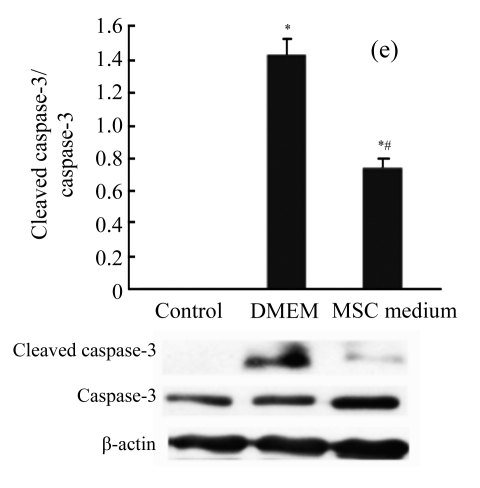
The effect of MSC medium on the mitochondrial signaling pathway
MSC medium increased the ratio of Bcl-2 (a) to Bax (b) in mitochondria, reduced the release of cytochrome C (c) and AIF (d) into the cytoplasm, and reduced the activation of caspase-3 and cleaved caspase-3 (e). 50 μg of the cytosolic proteins and 20 μg of mitochondrial proteins were analyzed by Western blotting analysis with anti-Bcl-2, anti-Bax, anti-cytochrome C, and anti-AIF. 100 μg of total cell lysates were analyzed with anti-caspase-3. Results are representative of three independent experiments. * P<0.05 vs control group, # P<0.05 vs DMEM group
MSC medium reduced the mitochondrial release of apoptosis inducing factor (AIF)
As H/R could impact the mitochondrial transmembrane potential, we testified whether AIF was involved in the caspase-independent proapoptotic effect. The level of AIF in the cytosol increased after H/R, and treatment with MSC medium during H/R prevented AIF release from the mitochondria (Fig.4d).
MSC medium reduced cytochrome C release and caspase-3 activation
Immunoblots of cytochrome C were also studied. There was a significant increase in cytosolic cytochrome C after H/R. MSC medium reduced H/R-induced cytochrome C release (Fig.4c) and the downstream activation of caspase-3 (Fig.4e).
DISCUSSION
The paracrine effect of MSCs has been the focus of many recent studies. It has been reported that MSCs can secrete many growth factors and proteases (Kemp et al., 2005; Wang et al., 2004), including vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), insulin-like growth factors (IGF)-1, stromal cell-derived factor (SDF), basic fibroblast growth factor (bFGF), matrix metalloproteinases (MMP), transforming growth factor (TGF)-β, and platelet derived growth factor (PDGF). In this study, we did not target the specific factors, but we found that MSC medium protected cardiomyocytes from H/R-induced apoptosis, inhibited the release of cytochrome C from the mitochondria and reduced caspase-3 activation, suggesting that MSC medium protects cardiomyocytes by interfering with a mitochondria-mediated apoptotic pathway.
The mechanism by which MSC medium interfered with cytochrome C release was probably by regulating the Bcl-2/Bax ratio. The roles of the Bcl-2 family proteins are oppositional (Fehlberg et al., 2003), either antiapoptotic or proapoptotic. Bcl-2 is localized dominantly in the mitochondrial membranes and blocks the mitochondial membrane permeabilization, exerting an antiapoptotic effect. Contrarily, Bax is translocated from the cytosol to mitochondria, and increases the mitochondial membrane permeabilization, which could be blocked by antiapoptotic protein Bcl-2. The ratio of Bcl-2/Bax could be a key factor in determining the regulation of mitochondrial cytochrome C release, further activation of caspase-3 and in the initiation of apoptosis (Murphy et al., 1999).
There are two kinds of apoptotic pathways: caspase-dependent or -independent apoptotic pathways. Mitochondria play an important role between them, like a crossover point (Fehlberg et al., 2003). AIF is a key trigger of caspase-independent apoptosis (Daugas et al., 2000; Susin et al., 1999), which is located in the mitochondrial intermembrane space and is released from mitochondria into the cytosol and nucleus in response to death stimuli. The release of AIF results in the generation of apoptotic phenotypes such as chromatin condensation and phosphatidylserin exposure. As Bax was reported to induce mitochondrial AIF release (Antonsson, 2001) and MSC medium could reduce the Bax level in mitochondria, we concluded that MSC medium protected cardiomyocytes from H/R-induced apoptosis by reducing the translocation of AIF to the cytosol.
In conclusion, in the present study, we demonstrated that H/R induced apoptosis of cardiomyocytes through a mitochondria-mediated pathway, and that the treatment with MSC medium reduced the injury by increasing the ratio Bcl-2/Bax.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30670868) and the Natural Science Foundation of Zhejiang Province, China (No. R206007)
References
- 1.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA. 2005;102(32):11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonsson B. Bax and other pro-apoptotic Bcl-2 family “killer-proteins” and their victim, the mitochondrion. Cell Tissue Res. 2001;306(3):347–361. doi: 10.1007/s00441-001-0472-0. [DOI] [PubMed] [Google Scholar]
- 3.Chen TL, Wang JA, Shi H, Gui C, Luo RH, Xie XJ, Xiang MX, Zhang X, Cao J. Cyclosporin a pre-incubation attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Scand J Clin Lab Invest. 2008;68(7):585–593. doi: 10.1080/00365510801918761. [DOI] [PubMed] [Google Scholar]
- 4.Daugas E, Susin SA, Zamzami N, Ferri KF, Irinopoulou T, Larochette N, Prevost MC, Leber B, Andrews D, Penninger J, et al. Mitochondrio-nuclear translocation of aif in apoptosis and necrosis. Faseb J. 2000;14(5):729–739. [PubMed] [Google Scholar]
- 5.Dobson KR, Reading L, Haberey M, Marine X, Scutt A. Centrifugal isolation of bone marrow from bone: an improved method for the recovery and quantitation of bone marrow osteoprogenitor cells from rat tibiae and femurae. Calcif Tissue Int. 1999;65(5):411–413. doi: 10.1007/s002239900723. [DOI] [PubMed] [Google Scholar]
- 6.Fehlberg S, Gregel CM, Göke A, Göke R. Bisphenol A diglycidyl ether-induced apoptosis involves Bax/Bid-dependent mitochondrial release of apoptosis-inducing factor (AIF), cytochrome C and Smac/DIABLO. Br J Pharmacol. 2003;139(3):495–500. doi: 10.1038/sj.bjp.0705275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11(4):367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Laboratory Animal Research, Commission on Life Science, National Research Council. Guide for the Care and Use of the Laboratory Animal. Washington, DC, USA: National Academic Press; 1996. pp. 1–140. [Google Scholar]
- 9.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, et al. Autologous bone marrow-derived stem-cell transfer in patients with St-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367(9505):113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 10.Kemp KC, Hows J, Donaldson C. Bone marrow-derived mesenchymal stem cells. Leuk/Lymphoma. 2005;46(11):1531–1544. doi: 10.1080/10428190500215076. [DOI] [PubMed] [Google Scholar]
- 11.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 12.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves. Cardiac Function Nat Med. 2001;7(1):430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 13.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months follow-up data from the randomized, controlled boost (bone marrow transfer to enhance St-elevation infarct regeneration) trial. Circulation. 2006;113(10):1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 14.Murphy KM, Streips UN, Lock RB. Bax membrane insertion during Fas(Cd95)-induced apoptosis precedes cytochrome C release and is inhibited by Bcl-2. Oncogene. 1999;18(44):5991–5999. doi: 10.1038/sj.onc.1203001. [DOI] [PubMed] [Google Scholar]
- 15.Shim WS, Jiang S, Wong P, Tan J, Chua YL, Tan S, Sin YK, Lim T, Chua CH, Teh M, et al. Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells. Biochem Biophys Res Commun. 2004;324(2):481–488. doi: 10.1016/j.bbrc.2004.09.087. [DOI] [PubMed] [Google Scholar]
- 16.Simpson P, Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ Res. 1982;50(1):101–116. doi: 10.1161/01.res.50.1.101. [DOI] [PubMed] [Google Scholar]
- 17.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397(6718):441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, Matsuzaki M, Hamano K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol. 2006;291(2):H886–H893. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- 19.Tang J, Xie Q, Pan G, Wang J, Wang M. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur J Cardiothorac Surg. 2006;30(2):353–361. doi: 10.1016/j.ejcts.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 20.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98(11):1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 21.Wang PP, Wang JH, Yan ZP, Hu MY, Lau GK, Fan ST, Luk JM. Expression of hepatocyte-like phenotypes in bone marrow stromal cells after HGF induction. Biochem Biophys Res Commun. 2004;320(3):712–716. doi: 10.1016/j.bbrc.2004.05.213. [DOI] [PubMed] [Google Scholar]
- 22.Xie XJ, Wang JA, Cao J, Zhang X. Differentiation of bone marrow mesenchymal stem cells induced by myocardial medium under hypoxic conditions. Acta Pharmacol Sin. 2006;27(9):1153–1158. doi: 10.1111/j.1745-7254.2006.00436.x. [DOI] [PubMed] [Google Scholar]