Abstract
In this work, we compare two methods for evaluating and quantifying pulmonary airspace enlargement in a mouse model of chronic cigarette smoke exposure. Standard stereological sample preparation, sectioning, and imaging of mouse lung tissues were performed for semi-automated acquisition of mean linear intercept (Lm) data. After completion of the Lm measurements, D2, a metric of airspace enlargement, was measured in a blinded manner on the same lung images using a fully automated technique developed in-house. An analysis of variance (ANOVA) shows that although Lm was able to separate the smoke-exposed and control groups with statistical significance (p = 0.034), D2 was better able to differentiate the groups (p<0.001) and did so without any overlap between the control and smoke-exposed individual animal data. In addition, the fully automated implementation of D2 represented a time savings of at least 24x over semi-automated Lm measurements. Although D2 does not provide 3D stereological metrics of airspace dimensions as Lm does, results show that it has higher sensitivity and specificity for detecting the subtle airspace enlargement one would expect to find in mild or early stage emphysema. Therefore, D2 may serve as a more accurate screening measure for detecting early lung disease than Lm.
Introduction
The development and use of animal chronic obstructive pulmonary disease (COPD) models requires sensitive methods of monitoring and quantifying the disease progression. Key components of COPD, as defined by the American Thoracic Society, are “abnormal, permanent enlargement of airspaces distal to terminal bronchioles, accompanied by destruction of their walls” [1]. In addition, destruction in emphysema, a major component of COPD, is defined as “nonuniformity in the pattern of respiratory airspace enlargement” [2]. In mild emphysema, it has been shown that increases in lung volume are not necessarily accompanied by decreases in total surface area [3]. The increase in volume may be due to the deterioration of elastic fibers in parenchymal tissue, which can lead to breakage of weakened alveolar walls that are under mechanical stress [4]. Although this breakage may result in a slight loss of total surface area, it will likely lead to a few enlarged airspaces that are surrounded by smaller, intact ones.
The mean linear intercept (Lm), a measure of the surface area to volume ratio, is by and large the most commonly reported metric of emphysema. However, its application and interpretation tend to vary among different laboratories, and results are often misused as an assessment of airspace diameter or airspace size [5]–[7]. In cases of mild emphysema, in which diseased areas of the lung may be small, dispersed, and heterogeneous with respect to distribution of airspace sizes (e.g. see Refs. [8]–[10]), it is generally difficult to quantify disease severity, as conventional methods, such as Lm, employ numerical averaging to extract a “central tendency” [6] and, hence, tend to underestimate the important influence of subtle localized changes or outliers. This was pointed out in Ref. [7]: “Lm is much more difficult to measure and fraught with danger of bias if the airspace size is very variable.” There are compelling arguments against abandoning Lm [11], although these views highlight that Lm may not be the most sensitive indicator for early emphysema diagnosis. Indeed, several studies have demonstrated that Lm often cannot distinguish mild emphysema from healthy controls [12]–[17]. Therefore, a histological method of measuring airspace enlargement that is specifically sensitive to the presence of the largest airspaces is desirable for detecting such a disease state.
Recently, Parameswaran et al. [18] introduced non-conventional metrics that could potentially be used as indicators of heterogeneously distributed airspace sizes characteristic of early lung disease. Briefly, these indexes, referred to as D1 and D2 (described in detail and derived in Ref. [18]), utilize the equivalent airspace diameters (i.e. diameter of a circle of equivalent area) and then incorporate higher moment factors from the airspace diameter distributions. Thus, the largest airspaces—potential indicators of early disease state—are weighted more heavily than smaller ones. We stress that D1 and D2 do not provide conventional 3D stereological information about average airspace dimensions – they simply emphasize the presence of a minority of enlarged airspaces. Nevertheless, as observed in Ref. [19], these new indexes may prove useful as indicators of physiology expected in early or mild emphysema but require rigorous validation.
Herein, as a validation effort, we have applied these indexes post factum to a study of airspace enlargement in smoke-exposed mice and compared the results to conventional Lm measurements on the same histological images.
Methods
Ethics Statement
Smoke exposure took place at Washington University in St. Louis. Experimental procedures were approved by the Institutional Animal Care and Use Committee of Washington University in St. Louis. Animals were allowed access to food and water ad libitum and were humanely sacrificed as necessary to ameliorate suffering.
Lung Sample Preparation
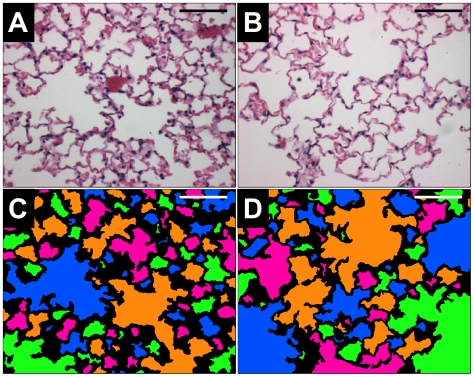
Lung tissue samples from 20 female AKR/J mice were used in this study, with 10 exposed to mainstream cigarette smoke (2–4 cigarettes/day, 6 days/week) for 24 weeks and 10 age-matched controls, as described previously [20], [21]. At the end of smoke exposure, the mice were sacrificed by CO2 asphyxiation and exsanguinated (the vasculature was not flushed with saline). Next, the chest cavity was opened and the diaphragm incised. Lungs were then inflated to 25 cmH2O with 10% neutral buffered formalin for ≈10 minutes, after which the trachea was tied off and the lungs excised and placed in a formalin bath for≥2 days. After fixation, lungs were trimmed and randomly oriented in preparation for sectioning. Lungs were embedded with paraffin and sectioned into 5 µm thick slices that were stained with hematoxylin and eosin (H&E). Slices were made in random directions, and eight random slices selected from all lobes of each mouse were placed on a slide. We note that this random method will result in some lobes being sampled multiple times, and the possibility exists that some lobes will avoid sampling altogether. Slices were then imaged at 200×magnification using a Nikon Optishot II microscope and Zeiss Axiocam digital camera; 12 images per mouse were acquired. Image locations were selected by using a random number generator (www.random.org) to determine image coordinates. Major airways and vasculature were generally avoided in selecting fields to focus on peripheral parenchyma, as reported by others (cf. Refs. [22]–[25]). When one of these was encountered, the microscope field was shifted in a randomly selected direction until the field included parenchymal tissue only. Digital images were 606×480 pixels and covered a field of approximately 1.0 mm×0.8 mm. Figure 1 shows representative H&E stained images from control (A) and smoke-exposed (B) mice, with color maps included to aid the eye in distinguishing airspaces (C and D, respectively). We note that gross examinations of morphometry of all healthy vs. smoke-exposed mice were insufficient for definitively determining the severity of disease.
Figure 1. Representative H&E stained images from a control mouse (A) and a smoke-exposed mouse (B).
Color maps of each image, (C) and (D) respectively, are shown to illustrate the different airspaces. The bars are 200 µm.
Mean Linear Intercept Measurements
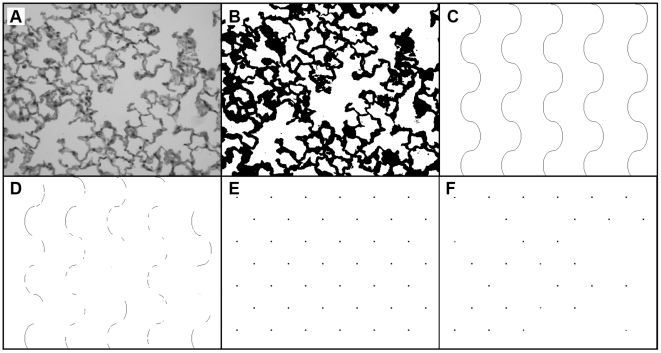
The mean linear intercept (Lm) was measured on the lung section images using Image-Pro® Plus (Media Cybernetics, Bethesda, MD) image analysis software as described previously [26]; see Figure 2. Briefly, a binary threshold mask of the alveolar septa was made, a grid of 5 cycloid lines was placed on the mask [10], [27], and the intercepts with the septa were counted. Next, a similar mask of the alveolar airspaces was made, a grid of 42 points was placed on the mask, and the points overlaying the airspaces were counted; truncation of airspaces by the optical boundary was ignored. Counts of non-alveolar airspace or tissues were manually removed and Lm was then calculated using the following equation:
| (1) |
where ΣPts is the sum of the points in the airspace mask, l is the cycloid length per point (including a geometrical correction for the curvature of the cycloid lines), and ΣInt is the sum of the intercepts of the cycloid lines with alveolar septa. The semi-automated Lm measurements required ≈2–3 minutes per image for complete analysis.
Figure 2. Image analysis steps for calculating Lm.
A) H&E stained image acquired at 200×magnification. B) Threshold of image distinguishes tissue from airspace. C) Cycloid grid lines. D) Intersection of cycloid lines with tissue in thresholded image (B). E) Grid points. F) Intersection of grid points with airspace in thresholded image.
D2 Measurements
Automated measurements of D2 were performed on the same images after the completion of the Lm measurements. To eliminate potential bias, neither the Lm data nor the exposure histories of the mice were available a priori to individuals calculating D2.
The indexes D1 and D2 are derived from the ratios of the distributions of the standardized moments of the mean equivalent airspace diameter (i.e. the diameter of a circle of equivalent area: 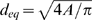 ). Hence, D1 is defined as the ratio of the second moment to the first moment, and D2 is the ratio of the third moment to the second moment [18]. The calculation of these indexes requires measurement of the areas of the individual airspaces and the calculation of the equivalent diameter deq of each airspace. Then the mean (D0), the variance (σ2), and the skewness (γ) of the deq distribution are used to calculate D1 and D2, according to [18]:
). Hence, D1 is defined as the ratio of the second moment to the first moment, and D2 is the ratio of the third moment to the second moment [18]. The calculation of these indexes requires measurement of the areas of the individual airspaces and the calculation of the equivalent diameter deq of each airspace. Then the mean (D0), the variance (σ2), and the skewness (γ) of the deq distribution are used to calculate D1 and D2, according to [18]:
| (2) |
| (3) |
Because D2 includes information about both the variance and skewness of the distribution, it is expected to be more sensitive than D1 or D0 to the presence of outliers. We note that higher order indexes Dn would include the (n+1)th standardized moment of the distribution, but their implementation may be unnecessary, as D2 may be sufficiently sensitive, or impractical, since Dn becomes increasingly complex with increasing n.
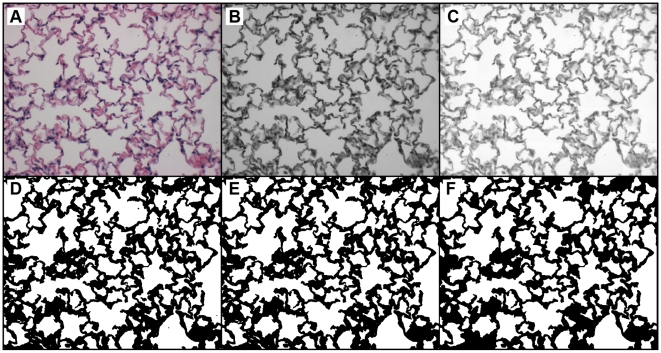
The following automated steps were used for calculating D2 from color RGB images of lung samples (see Figure 3). First, each 24-bit RGB image was converted to an 8-bit grayscale image by extracting the green channel, which provides the greatest contrast between the background and the red-blue H&E stained tissue. Then, a localized background normalization was performed to remove the differences in light intensity across each image [28]. This step linearly shifted the intensities of pixels in 30 local regions so that the maximum pixel value in each local region would be set to 255 (i.e., white). Next, each 8-bit grayscale image was converted to a binary image using a threshold of 225, with pixel values above 225 indicating airspace, and pixels at or below 225 indicating tissue (this level was set empirically). After thresholding, stray particles, or unconnected groups of edge-adjacent pixels, of area≤500 pixels were erased. Similarly, small white particles of area≤100 pixels within tissue walls were filled in. The remaining white regions represented the airspaces for the D2 calculation. Finally, the number of pixels in each region was measured as the area of each airspace. For each mouse, the airspace areas from all 12 images were assembled into a single data set. D2 was then calculated for each mouse using Eq. [3]. This automated procedure was implemented using the python programming language (www.python.org) and the python imaging library (www.pythonware.com). The fully automated D2 measurements required ≈20 minutes to process all 240 images, or ≈5 seconds per image, using a 3.2 MHz Pentium 4 desktop PC with 3 GB of RAM.
Figure 3. Automated steps for processing H&E stained images prior to calculating D2.
A) RGB color image acquired at 200×magnification. B) Green-channel of A. C) Localized background intensity normalization performed on B. D) Threshold of intensity converts C to a binary image. E) Small black particles removed from D. F) Small white particles removed from E.
D2 was measured manually (i.e. with little or no automation) on a subset of the images to validate that the automated thresholding method did not misinterpret features. Images were chosen using a random number generator to select one image per animal; thus, 20 images were analyzed manually. As with the automated D2 measurements, the manual measurements were performed blind with no knowledge of treatment history. Moreover, the computer-generated threshold images were not made available until after the completion of the manual analysis to prevent bias. All manual image processing was done using ImageJ [29] as previously described [30]. Images were first filtered with a 1.0 pixel radius Gaussian filter to eliminate speckle, then a 100 pixel radius rolling-ball background subtract filter was applied to minimize intensity variations. Next, images were thresholded, and unconnected particles were erased. Images were then manually repaired by filling in regions that did not threshold properly; this was done by directly comparing the thresholded image to the original image. Finally, the areas of the individual airways were measured and copied to a spreadsheet program for analysis. Regions with an area<50 pixels were not included in the analysis, as they generally resulted from incomplete thresholding or repair. The equivalent diameter of each airspace was calculated, and D0, σ2, and γ were then determined for each image from which D1 and D2 were then calculated. This manual technique required about 5–7 minutes per image, the bulk of which was used for the image repair (i.e. particle removal and correcting poorly thresholded regions).
We note that airspaces truncated by the borders of the image frame were included in the D2 analyses (both manual and automated). This was necessitated by the fact that Lm was calculated on the entire image frame (as is standard practice), and to make a fair comparison of Lm and D2 they must be calculated on the same exact images. We point out that the truncation may result in D2 measurements that are skewed to somewhat low values. However, the exclusion of these airspaces altogether only serves to filter out the largest airspaces – since they are most likely to border the edge – and thus further skew the results to even lower values. To verify this we eliminated the edge-bordering airspaces and reanalyzed D2 and Lm on all the images (data not shown). We found that although D2 dropped considerably for the smoke-exposed group, it was still significantly higher than for the control group (p-value<0.05). Lm for the smoke-exposed group, on the other hand, dropped so much that it became dramatically lower than for the control group (p-value<0.0005). Therefore, elimination of truncated airspaces clearly misrepresents the true nature of the lung tissue much worse than including the truncated airspaces. Hence, airspaces were defined by the edge of the optical image. Ideally, acquisition of larger image fields would be desirable so that truncated airspaces could be excluded without affecting results, as was done in Ref. [30]. This was not possible herein, as D2 was calculated after the completion of the Lm study.
Statistical Analysis
A statistical comparison was made between manual and automatic measurements of D0, D1, and D2 to determine how well the individual measurements for the two methods correlated and whether or not there was an overall difference in mean values for each variable. Pearson correlation coefficients were calculated, and analysis of variance (ANOVA) along with two sample t-tests were used to establish if differences existed between the measurement methods. ANOVA procedures were also performed to determine if D2 and Lm were equally effective in detecting significant differences between smoke-exposed mice and those in the control group. In these statistical analyses, a significance level (α) of 0.05 was used. Additionally, linear discriminate analysis was used to create classification rules to predict the specificity (i.e. ability to discern true negatives) and the sensitivity (i.e. ability to discern true positives) for D2 and Lm.
Results
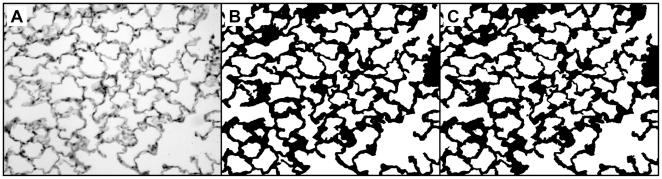
Figure 4 shows an example of a typical lung slide image (panel A) together with the results of manual thresholding (panel B) and automatic thresholding (panel C) used for D2 measurements. This image demonstrates that the automated method did not introduce artifacts or misinterpret features.
Figure 4. Comparison of thresholded images for manual and automated D2 analysis.
A) 606×480 pixel image of a lung slide acquired at 200×magnification (original images were acquired in RGB color). B) Manually thresholded and repaired image. C) Automatically thresholded image. Subtle differences between the thresholding methods can be seen upon inspection.
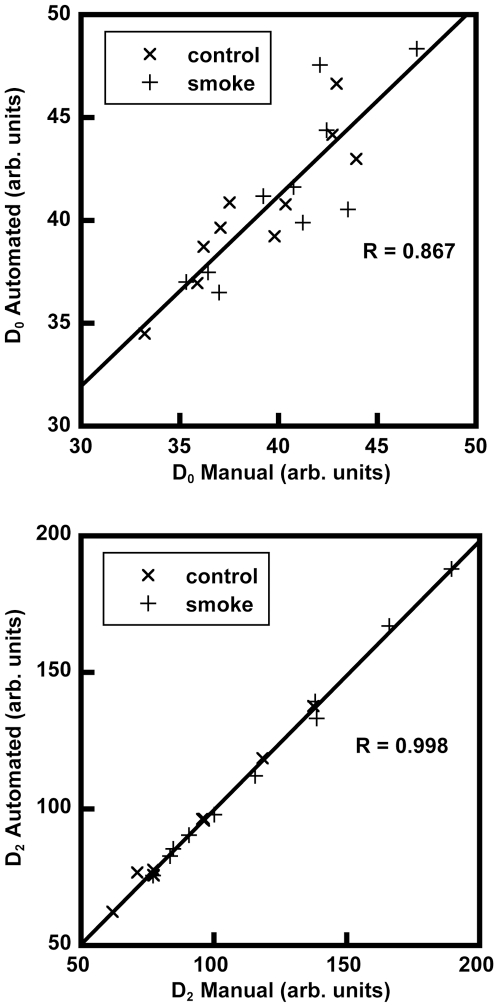
Graphs comparing the automatic vs. manual measurements of D0 and D2 for the 20 random images are shown in Figure 5. Results of the statistical analysis indicate that the two measurement types were highly correlated for each variable with Pearson correlation coefficients of R = 0.867 for D0, R = 0.994 for D1 (data not shown), and R = 0.998 for D2. The ANOVA showed that there was a significant interaction between measurement type (manual vs. automatic) and variable (D0, D1, or D2), indicating that the manual and automatic measurement values were inconsistently different across the variables (p-value<0.001). Two sample paired t-tests further explored this by showing that no significant differences existed between the measurement types for D1 (p-value = 0.652) and D2 (p-value = 0.374), but did show a significant difference between measurement types for D0 (p-value = 0.0108). This difference is shown in Figure 5, where D0 values are generally higher for the automatic measurements versus the manual measurements.
Figure 5. Automated vs. manual calculation of the mean equivalent diameter D0 (top) and weighted index D2 (bottom) from 20 randomly selected images.
In spite of the subtle differences between thresholding methods (see Figure 4), the strong correlations indicate that there are no statistically significant differences between measurement techniques.
Table 1 shows the data used to calculate D2, in addition to the Lm results, for the 20 mice in the study. The mice are alphanumerically labeled according to control (C) or smoke-exposed (S). The average of the standard deviations of the treated mice is significantly greater than that of the control mice (p-value<0.001). This is an indicator of increased heterogeneity of airspace sizes in the treated mice.
Table 1. Data from the 20 mice in this study.
| Mouse | N | D0 (µm) | σ (µm) | γ | D1 (µm) | D2 (µm) | Lm (µm) |
| C1 | 989 | 31.844 | 24.72 | 2.69 | 51.027 | 80.756 | 45.7 |
| C2 | 1058 | 32.111 | 24.83 | 2.68 | 51.309 | 81.087 | 46.5 |
| C3 | 895 | 34.483 | 25.72 | 2.23 | 53.674 | 79.619 | 59.5 |
| C4 | 944 | 33.959 | 25.24 | 2.42 | 52.713 | 79.877 | 46.5 |
| C5 | 1048 | 32.966 | 24.94 | 2.79 | 51.834 | 82.258 | 47.5 |
| C6 | 1092 | 32.498 | 23.20 | 2.31 | 49.058 | 72.557 | 44.9 |
| C7 | 981 | 32.006 | 23.98 | 2.66 | 49.986 | 77.964 | 50.1 |
| C8 | 1077 | 30.690 | 22.01 | 2.55 | 46.475 | 70.556 | 45.7 |
| C9 | 1043 | 32.877 | 24.90 | 2.74 | 51.737 | 81.757 | 52.8 |
| C10 | 1025 | 31.489 | 23.86 | 2.80 | 49.558 | 78.828 | 47.0 |
| Mean (SD) | 1015 (62) | 32.5 (1.1) | 50.7 (2.1) | 78.5 (3.9) | 48.6 (4.5) | ||
| S1 | 770 | 37.453 | 32.39 | 3.02 | 65.456 | 111.35 | 57.7 |
| S2 | 722 | 37.445 | 33.31 | 2.73 | 67.078 | 110.69 | 59.6 |
| S3 | 896 | 32.934 | 27.25 | 2.92 | 55.481 | 91.998 | 53.5 |
| S4 | 827 | 35.000 | 31.76 | 2.84 | 63.809 | 107.36 | 53.6 |
| S5 | 1015 | 32.014 | 25.07 | 3.09 | 51.648 | 85.841 | 44.7 |
| S6 | 944 | 32.151 | 28.48 | 3.44 | 57.378 | 103.46 | 49.8 |
| S7 | 931 | 34.653 | 31.82 | 3.25 | 63.874 | 113.63 | 51.3 |
| S8 | 753 | 35.040 | 35.90 | 3.52 | 71.815 | 135.63 | 59.9 |
| S9 | 822 | 35.056 | 30.30 | 2.75 | 61.243 | 100.60 | 54.4 |
| S10 | 846 | 34.443 | 29.40 | 3.08 | 59.532 | 101.67 | 49.4 |
| Mean (SD) | 853 (93) | 34.6 (1.9) | 61.73 (5.9) | 106.2 (13.5) | 53.4 (4.8) |
C mice were control; S mice were smoke-exposed; N, total number of airspaces after thresholding; D0, mean equivalent airspace diameter; σ, standard deviation of the airspace distribution; γ, skewness of the airspace distribution; D1 and D2, weighted indexes; Lm, mean linear intercept.
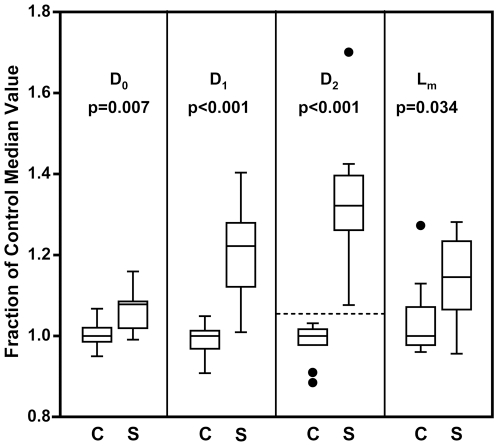
Boxplots of the results are shown in Figure 6, comparing the control (C) and smoking (S) groups with accompanying ANOVA p-values. The difference between the control and smoking groups is statistically significant in Lm (p-value = 0.034) but with a clear overlap in individual values between the two groups. By comparison, the D2 results showed a higher degree of significance from an ANOVA model (p-value<0.001) with a clear separation between the control and smoking groups (as emphasized by the horizontal dashed line). This was verified by a significance of interaction test from an ANOVA model, which indicated that the separation of treatment groups was significantly more pronounced for D2 than it was for Lm (p-value<0.001). ANOVA tests determined that D0 and D1 were also better at distinguishing treatment groups than Lm but were not better than D2. In addition, the sensitivity and specificity results from the linear discriminate analysis showed that the sensitivity of D2 exceeded that of Lm (80% vs. 60%) as did the specificity (100% vs. 80%), indicating that D2 was better at predicting which mice belonged to the control vs. smoke-exposed group.
Figure 6. Boxplots of histological data from the 10 control (C) and 10 smoke-exposed (S) mice.
D0 is the mean equivalent diameter, D1 and D2 are weighted indexes of airspace size distribution, and Lm is the mean linear intercept. Data were normalized to the median values of the control group. The p-values indicate the significance of the ability of each method to discern between the treatment groups. The dotted line was added to illustrate the lack of overlap between groups in the D2 results. In these boxplots, the box vertical dimensions represent the first and third quartiles, the line inside the box represents the median (second quartile), the bars represent the largest and smallest non-outliers (within 1.5 times the interquartile range), and the points represent outliers (beyond 1.5 times the interquartile range).
Discussion
In this study we compared two methods for quantifying airspace enlargement in smoke-exposed mice. We followed standard procedures for lung tissue sample preparation, image acquisition, and Lm analysis. Following this, we calculated the new index, D2, on the same images to compare how well the two methods separate the smoke-exposed and control groups. Our results show that D2 was better able to distinguish between the groups (see Figure 6), and this is attributed to the fact that D2 is weighted by enlarged airspaces and is therefore a reflection of the airspace size distribution. Lm, on the other hand, is a measure of the intra-alveolar septal wall mean free path and tends to mask the presence of sparse, enlarged airspaces. We emphasize that D2 does not provide information about the actual airspace geometries; rather, it simply offers a more sensitive metric of airspace enlargement.
A manual validation of the automated D2 measurements was performed to assure that the automation did not misinterpret features and would not adversely affect the results. Figures 4 and 5, with accompanying statistical analysis, confirm that full automation did not introduce appreciable errors. We note that the difference in scatter in the top panel of Figure 5 (the D0 comparison) versus that of the bottom panel (the D2 comparison) illustrates that small discrepancies in thresholding, particularly of the smallest airspaces (cf. Figures 4B and 4C), are outweighed by the effects of the largest airspaces and are, therefore, generally not significant. This point underscores the robustness of the automated method. Still, there may be cases when a semi-automated implementation may be necessary, such as situations of poor image quality or images that include large blood vessels or conducting airways. We note that the image processing method employed herein differs somewhat from that originally used in Ref. [18]. There, the authors applied a watershed segmentation to the lung histology images to define the airspace boundaries. Although easy to automate, this type of segmentation may not realistically represent the airway architecture. For example, airway walls were represented as thin lines while the tissue itself was either incorporated into the airways or was segmented into additional “airspaces.” Another problem is that this segmentation does not allow for “free ends” which are generally alveolar openings from alveolar ducts [6]; rather, it connects the free ends, resulting in artificial subdivision of airspaces. Herein, we implemented and automated the method of simple thresholding to more faithfully define the tissue boundaries as depicted in the histology images [31].
The full automation of D2 calculations has eliminated intermediate, time-intensive steps, such as point counting, without sacrificing accuracy. This has two primary advantages over manual or semi-automated methods. 1) Full automation eliminates the potential for operator bias by removing the opportunity to make decisions that might skew the results. The only prospects for bias would be in the tissue sampling or acquisition of the images themselves, which can be avoided through strict implementation of random and blinded means. 2) Full automation is much faster and is relatively simple to employ using existing technology and computational methods. In this study, calculation of D2 starting from the raw images was at least 24x faster than the semi-automated Lm measurements, as D2 required approximately 5 seconds per image and Lm required 2–3 minutes per image.
Both Lm and D2 have strengths that can be exploited in studies of lung structure. Lm has the advantage of providing a quantitative measure of the volume to surface-area ratio. D2, on the other hand, has advantages of sensitivity, reliability, and speed when measuring airspace enlargement. With rigorous D2 validation studies such as presented herein, we anticipate that D2 and Lm can be used in tandem as quantitative measures in emphysema assessment to provide high sensitivity to disease state, as well as quantitative information about average airspace dimensions, respectively. By further probing the sensitivity limitations of D2, a useful lower bound of its practical implementation can be determined. Therefore, future work should investigate the limits of D2 sensitivity in, for example, disease states of minimal severity. The ability to detect very early stages of airway enlargement may provide additional biomarker candidates associated with disease onset and progression.
Acknowledgments
The authors would like to thank D. Kobayashi of Washington University in St. Louis for smoke exposures of mice and H. Bresler of Battelle for helpful conversations in preparation of this manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by Battelle Memorial Institute (www.battelle.org) as part of a Pulmonary Systems Biology Initiative, a competitive internally funded program; the individuals making funding decisions had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This work was also supported by National Heart, Lung, and Blood Institute (www.nhlbi.nih.gov) Grant R01-HL-073598.
References
- 1.Celli BR, Snider GL, Heffner J, Tiep B, Ziment I, et al. Standards for the Diagnosis and Care of Patients with Chronic Obstructive Pulmonary-Disease. Am J Respir Crit Care Med. 1995;152:S77–S121. [PubMed] [Google Scholar]
- 2.Snider GL, Kleinerman JL, Thurlbeck WM, Bengali ZH. The Definition of Emphysema - Report of a National-Heart-Lung-and-Blood-Institute, Division of Lung-Diseases Workshop. American Review of Respiratory Disease. 1985;132:182–185. doi: 10.1164/arrd.1985.132.1.182. [DOI] [PubMed] [Google Scholar]
- 3.Coxson HO, Rogers RM, Whittall KP, D'Yachkova Y, Pare PD, et al. A quantification of the lung surface area in emphysema using computed tomography. Am J Respir Crit Care Med. 1999;159:851–856. doi: 10.1164/ajrccm.159.3.9805067. [DOI] [PubMed] [Google Scholar]
- 4.Suki B, Lutchen KR, Ingenito EP. On the progressive nature of emphysema: roles of proteases, inflammation, and mechanical forces. Am J Respir Crit Care Med. 2003;168:516–521. doi: 10.1164/rccm.200208-908PP. [DOI] [PubMed] [Google Scholar]
- 5.Fehrenbach H. Animal models of pulmonary emphysema: a stereologist's perspective. Eur Respir Rev. 2006;15:136–147. [Google Scholar]
- 6.Weibel ER. Morphological quantitation of emphysema: A debate. J Appl Physiol. 2006;100:1419–1420. doi: 10.1152/japplphysiol.01301.2005. [DOI] [PubMed] [Google Scholar]
- 7.Weibel ER, Hsia CCW, Ochs M. How much is there really? Why stereology is essential in lung morphometry. J Appl Physiol. 2007;102:459–467. doi: 10.1152/japplphysiol.00808.2006. [DOI] [PubMed] [Google Scholar]
- 8.Ito S, Ingenito EP, Arold SP, Parameswaran H, Tgavalekos NT, et al. Tissue heterogeneity in the mouse lung: effects of elastase treatment. J Appl Physiol. 2004;97:204–212. doi: 10.1152/japplphysiol.01246.2003. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin RF, Tueller EE. Anatomic and Histologic Changes of Early Emphysema. Chest. 1971;59:592–&. doi: 10.1378/chest.59.6.592. [DOI] [PubMed] [Google Scholar]
- 10.March TH, Barr EB, Finch GL, Hahn FF, Hobbs CH, et al. Cigarette smoke exposure produces more evidence of emphysema in B6C3F1 mice than in F344 rats. Toxicol Sci. 1999;51:289–299. doi: 10.1093/toxsci/51.2.289. [DOI] [PubMed] [Google Scholar]
- 11.Mitzner W. Use of mean airspace chord length (Lm) to assess emphysema. J Appl Physiol. 2008 doi: 10.1152/japplphysiol.90968.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emami K, Cadman RV, Macduffie Woodburn JP, Fischer MC, Kadlecek SJ, et al. Early Changes of Lung Function and Structure in an Elastase Model of Emphysema - A Hyperpolarized 3He MRI Study. J Appl Physiol. 2007 doi: 10.1152/japplphysiol.00482.2007. [DOI] [PubMed] [Google Scholar]
- 13.Foronjy RF, Mirochnitchenko O, Propokenko O, Lemaitre V, Jia YX, et al. Superoxide dismutase expression attenuates cigarette smoke- or elastase-generated emphysema in mice. Am J Respir Crit Care Med. 2006;173:623–631. doi: 10.1164/rccm.200506-850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madani A, Keyzer C, Gevenois PA. Quantitative computed tomography assessment of lung structure and function in pulmonary emphysema. Eur Respir J. 2001;18:720–730. doi: 10.1183/09031936.01.00255701. [DOI] [PubMed] [Google Scholar]
- 15.Robbesom AA, Versteeg EMM, Veerkamp JH, van Krieken JHJM, Bulten HJ, et al. Morphological quantification of emphysema in small human lung specimens: Comparison of methods and relation with clinical data. Modern Pathology. 2003;16:1–7. doi: 10.1097/01.MP.0000043519.29370.C2. [DOI] [PubMed] [Google Scholar]
- 16.Thurlbeck WM. Internal Surface Area and Other Measurements in Emphysema. Thorax. 1967;22:483–&. doi: 10.1136/thx.22.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thurlbeck WM, Muller NL. Emphysema - Definition, Imaging, and Quantification. Am J Roent. 1994;163:1017–1025. doi: 10.2214/ajr.163.5.7976869. [DOI] [PubMed] [Google Scholar]
- 18.Parameswaran H, Majumdar A, Ito S, Alencar AM, Suki B. Quantitative characterization of airspace enlargement in emphysema. J Appl Physiol. 2006;100:186–193. doi: 10.1152/japplphysiol.00424.2005. [DOI] [PubMed] [Google Scholar]
- 19.Mitzner W. Morphologic quantification of heterogeneous parenchyma - Comment. J Appl Physiol. 2006;100:1421–1422. doi: 10.1152/japplphysiol.01301.2005. [DOI] [PubMed] [Google Scholar]
- 20.Pemberton PA, Cantwell JS, Kim KM, Sundin DJ, Kobayashi D, et al. An inhaled matrix metalloprotease inhibitor prevents cigarette smoke-induced emphysema in the mouse. COPD. 2005;2:303–310. doi: 10.1080/15412550500218171. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro SD, Goldstein NM, Houghton AM, Kobayashi DK, Kelley D, et al. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol. 2003;163:2329–2335. doi: 10.1016/S0002-9440(10)63589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkinson JJ, Holmbeck K, Yamada S, Birkedal-Hansen H, Parks WC, et al. Membrane-type 1 matrix metalloproteinase is required for normal alveolar development. Developmental Dynamics. 2005;232:1079–1090. doi: 10.1002/dvdy.20267. [DOI] [PubMed] [Google Scholar]
- 23.Robertson CH, Hall DL, Hogg JC. A description of lung distortion due to localized pleural stress. J Appl Physiol. 1973;34:344–350. doi: 10.1152/jappl.1973.34.3.344. [DOI] [PubMed] [Google Scholar]
- 24.Soutiere SE, Tankersley CG, Mitzner W. Differences in alveolar size in inbred mouse strains. Respir Physiol Neurobiol. 2004;140:283–291. doi: 10.1016/j.resp.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 25.le Cras TD, Markham NE, Morris KG, Ahrens CR, McMurtry IF, et al. Neonatal dexamethasone treatment increases the risk for pulmonary hypertension in adult rats. Am J Physiol Lung Cell Mol Physiol. 2000;278:L822–829. doi: 10.1152/ajplung.2000.278.4.L822. [DOI] [PubMed] [Google Scholar]
- 26.Hodge-Bell KC, Lee KM, Renne RA, Gideon KM, Harbo SJ, et al. Pulmonary inflammation in mice exposed to mainstream cigarette smoke. Inhal Toxicol. 2007;19:361–376. doi: 10.1080/08958370601144076. [DOI] [PubMed] [Google Scholar]
- 27.Weibel ER. Stereological methods volume 1: Practical methods for biological morphometry. London; New York: Academic Press; 1979. [Google Scholar]
- 28.Gonzalez RC, Wintz PA. Digital image processing. xviii. Reading, Mass: Addison-Wesley; 1987. p. 503. [Google Scholar]
- 29.Rasband WS. 1997. ImageJ. U. S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/
- 30.Jacob RE, Minard KR, Laicher G, Timchalk C. 3D 3He diffusion MRI as a local in vivo morphometric tool to evaluate emphysematous rat lungs. J Appl Physiol. 2008 doi: 10.1152/japplphysiol.90375.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato A, Hirai T, Imura A, Kita N, Iwano A, et al. Morphological mechanism of the development of pulmonary emphysema in klotho mice. Proc Natl Acad Sci U S A. 2007;104:2361–2365. doi: 10.1073/pnas.0607882104. [DOI] [PMC free article] [PubMed] [Google Scholar]