Abstract
Studies in mice lacking either classical or melanopsin photoreception have been useful in describing the photoreceptor contribution to irradiance detection in accessory visual responses. However, application of these findings to irradiance detection in intact animals is problematical because retinal degeneration or manipulation can induce secondary changes in the retina. Among responses dependent on irradiance detection, the suppression of activity by light (negative masking) has had limited study. To further understand the function of classical and melanopsin photoreceptors we studied irradiance and spectral sensitivity of masking by light, primarily in mice with intact retinae. The sensitivity of negative masking was equivalent for medium (~500nm) and short-wavelengths (~365nm) in three strains of wildtype mice, identifying a marked short-wavelength-sensitive-cone input. At medium wavelengths, spectral sensitivity above 500nm had closest fit to the nomogram for the medium-wavelength-sensitive-cone, but a combined input of cone and melanopsin photoreceptors in wildtype mice seems likely. Under white light a decompression of the irradiance range of masking in C3H rd/rd cl mice, lacking rods and cones, identified a functional deficiency presumably resulting from the absence of classical photoreceptor input. Together the evidence demonstrates a pronounced and sustained classical photoreceptor input to irradiance detection for negative masking, and suggests one role of classical photoreceptor input is to constrain dynamic range.
Keywords: light, locomotor activity, melanopsin, mice, photoreceptor
Introduction
Features of ambient light such as energy (irradiance) can be used to regulate responses to changes in the environment (Berson, Aug 2007). Detection of light in mammals is by three classes of ocular photoreceptor (Hattar et al., Jul 3 2003): rods, cones and a melanopsin photoreceptor subset of retinal ganglion cells (Berson et al., Feb 8 2002). The classical rods and cones have a rapid activation cycle and adapt to ambient light levels (Burns & Baylor, 2001). In contrast, melanopsin photoreceptors have a slower response with broad dynamic range and limited adaptation to light (Provencio et al., Jan 6 1998; Berson et al., Feb 8 2002; Sekaran et al., Jun 21 2005; Wong et al., Dec 22 2005).
The role and contribution of these photoreceptors to irradiance detection in accessory visual systems has been the subject of much attention (Berson, Aug 2007). The properties of the photoreceptors might suggest that melanopsin is more suited to sustained irradiance detection. Studies in mice with altered function of one or more of the photoreceptors identify the importance of melanopsin photoreceptors to irradiance detection (Panda et al., Dec 13 2002; Ruby et al., Dec 13 2002; Mrosovsky & Hattar, Nov 2003), but also support classical photoreceptor input.
However, the classical and melanopsin photoreceptor pathways directly interact, so retinal manipulation is unlikely to be limited or confined to the intended target (Belenky et al., Jun 2 2003; Ostergaard et al., Aug 2007; Wong et al., Jul 1 2007). For instance, classical photoreceptor degeneration or manipulation is associated with secondary changes in expression levels of melanopsin in the eye (Sakamoto et al., Oct 27 2004; Wan et al., May 29 2006; Dkhissi-Benyahya et al., Mar 1 2007). As a result, the form and purpose of the contribution from the different photoreceptors to irradiance detection in the non-degenerate retina remains unclear.
To understand how classical and melanopsin photoreceptors in the normal eye contribute to irradiance detection we made an investigation of the irradiance and spectral sensitivity of masking of activity by light. In nocturnal rodents an acute suppression of locomotion (negative masking) occurs in bright light (Aschoff, 1988) in an irradiance dependent manner (Mrosovsky, Dec 1994). In dim light as opposed to darkness, an increase in locomotion (positive masking) is seen because visual guidance of movement enhances locomotion. Previous studies of negative masking in mice with altered classical or melanopsin function respectively identified changes in the irradiance-response relationship (Mrosovsky et al., Apr 1999; Takao et al., 2000) and capacity to sustain responses (Mrosovsky & Hattar, Nov 2003). However, observations with photoreceptive deficient animals do not avoid the undefined complications associated with concomitant alteration of other aspects of retinal function. In the present paper, a primary investigation in wildtype mice was supported with experiments in mice that lack rods and cones (rd/rd cl) (Lucas & Foster, Apr 1999) but have unaltered melanopsin expression in the eye (Semo et al., May 2003).
Methods
Studies of irradiance and spectral sensitivity have been used extensively in identifying the contribution of photoreceptors to accessory visual responses (Brainard et al., 1983; Takahashi et al., 1984; Aggelopoulus & Meissl, 2000; Brainard et al., 2001). If the properties of the photoreceptors are sufficiently different, the dynamic range and spectral sensitivity of a response can identify the contribution of specific photoreceptors (MacNichol, 1986; Coohill, Nov 1991).
Four related experiments investigated interactions between photoreceptors in the regulation of negative masking. 1) To determine whether negative masking depends on more than one photoreceptor in the non-degenerate retina, and if the photoreceptor contribution changed with duration of light exposure, we tested the responses of C3H wildtype mice to light at 480 and 540nm. 2) To identify the photoreceptors contributing to negative masking, spectral sensitivity was defined in C3H wildtype, with a supporting study of responsiveness in C3H rd/rd cl mice. 3) To further examine the sensitivity to short and medium-wavelength light in the non-degenerate retina, we studied C57/BL6 and 129/SvEv mice. 4) To examine photoreceptor contribution under a broad-spectrum light we studied dynamic range and time course of negative masking sensitivity, with a comparison of C3H wildtype and C3H rd/rd cl mice.
All experiments were performed in accordance with the guidelines of the Canadian Council on Animal Care, or the University of Iowa Animal Care and Use Review.
1) Identifying multi-variant photoreception
Animals and Housing
Mice were adult males on a C3H background from the laboratories of RGF. Animals were individually housed in running wheel cages with food and water available ad libitum. Temperature was maintained between 18 and 22°C. Activity was recorded as wheel revolutions in sequential 10-min bins using DataQuest 3 (Minimitter Respironics, Bend, OR). Cages had a circular base (25cm Ø) with a running wheel (17.5cm Ø) fitted centrally. Cage lids allowed illumination from the room lights for entrainment. A SpectraStar150 light source and fiber-optic apparatus (Schott AG, Mainz, Germany) allowed remote application of light pulses that were heat filtered, and could be controlled for wavelength and irradiance using neutral density and 10nm half bandwidth color filters (BFiOPTiLAS, Milton Keynes, UK). A hand-operated shutter was used to regulate the output and timing of light pulses. For each level of filter and cage, irradiance was measured in µWcm2 using a PM103 power meter (Macam Photometrics Ltd, Livingston, UK) and corrected for mouse lens transmission at the wavelengths applied (Hattar et al., Jul 3 2003).
Procedures
C3H/He wildtype mice (n = 8, 94 ± 1.3 days old, Mean and SEM at start) were treated at 9 irradiance levels at 480 and 540nm. Mice were stably entrained under ~120µWcm2 (~510 lux) fluorescent lights to a 16h light: 8h dark cycle. A 5-day experimental cycle of baseline (non-pulsed) days bracketing three consecutive pulse days was used. On pulse days, a 3-h light pulse was applied, starting 1-h after daily dark onset. The 18 color and irradiance combinations were randomly scheduled to avoid order effects.
Appropriate wavelengths for defining irradiance response curves were selected based on the relative sensitivity of the rod, medium-wavelength-sensitive (MWS) cone and melanopsin photoreceptors at given wavelengths: the absorption at the wavelength of maximal sensitivity was designated as 100% and the relative sensitivity at a given wavelength was derived from the defined absorption spectra for the photoreceptors (Govardovskii et al., Jul–Aug 2000). The two wavelengths selected were 480 and 540nm: melanopsin photoreceptor activation maximal around 480nm would be markedly reduced at 540nm (~26% of maximal), but the MWS-cone would have only a small difference in activation at 480nm (~82% of maximal) and 540nm (~74% of maximal).
Protocol controls
The entraining light cycle was used to maintain a consistent circadian phase despite application of the masking light pulses. To confirm entrainment under this protocol, onset of locomotor activity was checked on pulse days in all experiments. In addition, prior to these experiments we checked the suitability of this protocol. Mice (n = 5) were stably entrained and treated with bright white light pulses under the 5-day protocol then placed into constant darkness. Comparison of the phase alignment on entry into constant darkness after sham and bright white light pulses (~120µWcm2) confirmed that the phase delaying effect of light pulses was counter-balanced by the entraining light cycle.
Analysis
Data were normalized to activity in a given animal at corresponding times of the daily cycle on baseline days bracketing the light pulse. If technical issues meant that activity recording failed during a light treatment or a light pulse was not applied on time, repeat treatments were made at the end of the schedule. In total 144 scheduled, and 6 replacement treatments were completed. We also applied arbitrary exclusion criteria based on the bracketing baseline days to minimize the influence of aberrant activity. If activity was below 20 counts (10% of mean baseline for wildtype mice) in 4 consecutive bins or in 5 of the total 18 bins of the baseline period corresponding to the light treatment, the light treatment data were excluded. If an individual animal repeatedly failed to meet the exclusion criteria, the entire record for that animal was excluded. In experiment 1 no records were excluded.
Variable slope sigmoid functions at both wavelengths were fitted in Prism (Graphpad Software, San Diego, CA); constraints were applied for suppression maximum at 100% (Motulsky & Christapoulos, 2003). A modified F probability test was then used to compare differences in the slope independent of other variables and thereby identify multivariance, that is, input from more than one photoreceptor (Peirson et al., 2005). Differences in signal propagation efficiencies and relative activation with wavelength mean that changes in the slope of responses at two suitably separated wavelengths can identify multivariance (MacNichol, 1986; Peirson et al., 2005). Responses at the start and end of the long light pulse were compared to see if the contribution of photoreceptors changed with pulse duration.
In testing negative masking in this way, positive masking could influence tests for multivariance. However, data suggests that positive masking does not substantially compete downstream with negative masking for the control of activity (Redlin et al., Jan 2005), and positive masking would only be apparent when there was not an effect of negative masking on activity. In addition, any effect of positive masking on the sigmoid curve would be restricted to the dim light extremity of the curve. Therefore, any influence of positive masking on the measure of differences in slope in these tests would be minimal.
2) Spectral sensitivity
Animals and Housing
A detailed study in C3H wildtype mice was supported by a limited assessment of non-age-matched rd/rd cl mice under the same protocol. Housing was as in experiment 1. C3H rd/rd cl mice have complete rod and cone loss by 80 days of age (Lucas et al., Apr 1999). The rd/rd cl mice were older than the wildtype mice (see below), but age and loss of classical photoreceptors does not alter the expression of melanopsin or circadian photosensitivity in rd/rd cl mice (Semo et al., May 2003). To compensate for the limited numbers of rd/rd cl animals, treatments were repeated and the mean used in the analysis. The homozygous rd mutation was genotyped on an audit basis in animals selected for breeding pairs by PCR and restriction fragment size analysis as previously described (Lucas & Foster, Apr 1999). The heterozygous cl transgene was genotyped by PCR and product size analysis as previously described (Lucas & Foster, Apr 1999).
Procedures
Wildtype (n = 12, 132 ± 9 days old, Mean and SEM at start) and rd/rd cl mice (n = 4, 281 ± 32 days old, Mean and SEM at start) were entrained and treated with light under the protocol described in experiment 1. Light treatments were a standard number of photons (Mean irradiance 7.05×1011 photons cm2 SEM 7.22×109, or <2%) at nine wavelengths (365, 400, 440, 480, 500, 520, 540, 560 and 580nm). The appropriate irradiance for defining spectral sensitivity was based on the irradiance producing sub-saturating negative masking (~80% of maximal responses) at 480nm in experiment 1. The number of neutral density filters required to produce this irradiance for each wavelength was determined by repeated trials with each monochromatic filters.
Analysis
No records were excluded. Relative sensitivity at each wavelength was calculated to give a spectral sensitivity profile in two steps: the mean responses for the first hour of the pulse were described as percent response relative to baselines, then mean sensitivity at each wavelength was calculated relative to the mean at the most sensitive wavelength. The spectral sensitivity of response was then compared to previously characterized photopigments or photoreceptors (MacNichol, 1986; Coohill, Nov 1991; Peirson et al., 2005). Above 480nm spectral sensitivities were compared to long-wavelength limb of the rod, MWS-cone and melanopsin photoreceptors. Short-wavelength data points were excluded from this analysis to avoid possible interference from the SWS-cone. Additionally, differences in sensitivity are most pronounced along the long-wavelength limb of the opsin-nomogram. The absorbance spectra for the photoreceptors were estimated by adjusting the standard absorbance template for an opsin-vitamin-A1 photopigment to the defined λmax of the photopigments or photoreceptors. Relative sensitivity was converted to log10 values to place the spectral sensitivity profile on a scale most-suited to comparison at the long-wavelength limb of visual pigment templates. Assessment of the fit between derived relative sensitivities and visual pigment templates was by least sum of squares.
3) Consistency of spectral sensitivity among strains
Animals and Housing
Adult male C57/BL6 and 129/SvEv wildtype mice from the laboratory of VCS were individually housed to record running wheel activity under defined light conditions using cages and environment control cabinets as previously described (Thompson et al., Sep 5 2007). Food and water was available ad libitum, and temperature was maintained between 19 and 21°C. The irradiance of applied light pulses was controlled using neutral density gel filters (Rosco, Stamford, CT). Controlling the color of applied light was dependent on the use of F40BL short-wavelength fluorescent bulbs (Peak 365nm, half bandwidth 55nm, GE Lighting), or standard fluorescent bulbs with Chroma green #389 light filters (Peak 505nm, half bandwidth 65nm, Rosco). Light measurement was as for experiment 1.
Procedure
C57/BL6 (n = 6, 72 ± 3 days old, Mean and SEM at start) and 129/SvEv mice (n = 6, 75 ± 4 days old, Mean and SEM at start) were stably entrained under 187µWcm2 fluorescent lights to a 12h light: 12h dark cycle and assessed using a previously tested 3-day experimental cycle of baseline (non-pulsed), pulsed and baseline days (Thompson et al., Sep 5 2007). On pulse days, a 3-h light pulse was applied, starting 1-h after daily dark onset. Light treatments were at 5 irradiance levels at short and medium-wavelengths (C57/BL 20.9, 3.03, 0.44, 0.064 and 0.009µWcm2; 129SvEv, 135, 20.9, 3.03, 0.44 and 0.064µWcm2).
Analysis
No records were excluded. Fitting and analysis of irradiance response curves was as in experiment 1, except the mean response for the first hour of the pulse was used.
4) Dynamic range and time course of responses under broad-spectrum light
Animals and Housing
C3H wildtype and rd/rd cl mice were individually housed in rectangular (44 × 23 × 20cm) running wheel cages and housed in a facility that allowed application of light pulses separate from entraining lights (Mrosovsky et al., Apr 1999). Temperature was maintained between 18 and 21°C. The irradiance of applied light pulses was controlled with gel neutral density filters (Rosco). Cages of wildtype and rd/rd cl mice were placed in alternate positions so that there would be no systematic variation in lighting. Light measurement was as for experiment 1, except lux was measured using an E2x luxmeter (B.Hagner AB, Solna, Sweden).
Procedures
C3H wildtype (n=8, 238 ± 43 days old, Mean and SEM at start) and rd/rd cl mice (n=6, 234 ± 66 days old, Mean and SEM at start) were tested with pulses of light at 12 irradiance levels. Mice were entrained to a ~120µWcm2 (~510 lux) 16h light: 8h dark cycle and assessed under a repeating 3-day experimental cycle of maintenance day, baseline day (non-pulsed) and pulse day. On pulse days, a 3-h defined irradiance light pulse was applied, starting 1-h after daily dark onset. Treatments were scheduled to avoid order effects and data normalized to the corresponding baseline values.
Analysis
For the wildtype with 12 treatments in 7 mice, 3 of 84 records did not meet the selection criteria and were excluded. For the rd/rd cl with 12 treatments in 6 mice, 7 of 12 baselines for one animal did not meet the selection criteria so the entire record for that animal was excluded, and 3 of the remaining 60 records did not meet the selection criteria. Fitting and analysis of irradiance response curves was as in experiment 1, based on the first 30-mins of the pulse and excluding the three lowest light intensities that were below the range of positive masking (Peirson et al., 2005). To assess the capacity to sustain responses, mean responses for 30-min blocks of the 3h light pulses were described as percent response relative to baselines. Effects of time course and genotype were assessed by repeated measures ANOVA.
Results
1) Identifying multi-variant photoreception
For the first 30-mins of the light pulses there was a significant difference between the slopes of the irradiance response curves at 480 and 540nm (Figure 1: 480nm slope = 1.09, 540nm slope = 0.65; F-test p<0.005), providing qualified support for multivariance at medium wavelengths. For the last 30-mins of light pulses, after sustained light treatment, there was marked variability in the response to a given stimulus (compare the poor R2 for the curves at this time). This meant that the same test of irradiance response curves would be uninformative: a non-significant result would normally suggest univariance, but in this case could equally result from the reduced power of the test with the high variability in the data set.
Figure 1.
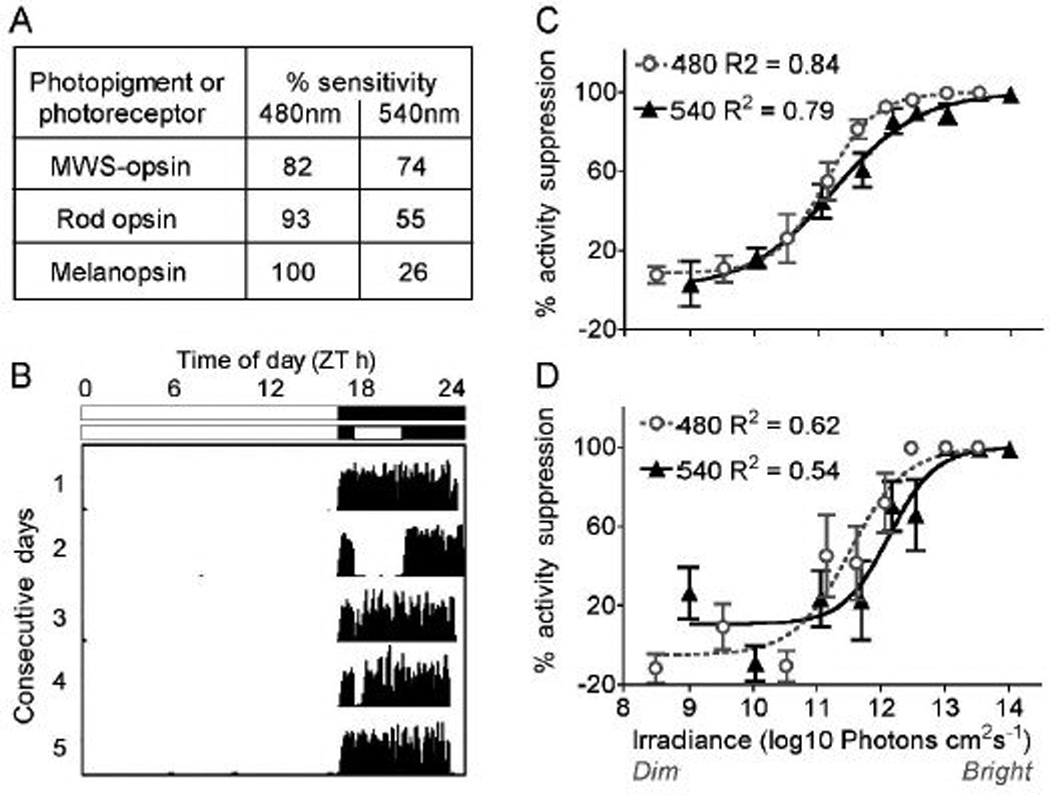
Multivariant photoreception in wildtype mice. (A) The percent maximal sensitivity for the medium-wavelength-sensitive cone, the rod and the melanopsin photoreceptors is shown for light at 480 and 540nm. (B) Example responses in a wildtype mouse are shown in actogram format. Open and filled bars above the main panel show the timing of the daily light cycle (upper bar, days 1 and 5), and the daily light cycle with a 3h pulse of light imposed 1-hour after lights off (lower bar, days 2, 3 and 4). The timing and amount of wheel running is shown on each day, primarily restricted to the dark period. Entrainment is maintained despite light pulses. On day 2, a light pulse at 480nm 1.35µWcm2 suppresses activity almost completely. On day 3, a light pulse at a less potent wavelength (540nm) and a lower irradiance (0.18µWcm2 suppresses activity to a lesser degree. On day 4, a light pulse at 540nm 1.29µWcm2 again only partially suppresses activity. Responses at 480 and 540nm are shown for the first (C) and last 30-minutes of light pulses (D) are shown as a percentage of baseline activity. Irradiance (dose) response curves are fitted with a variable slope sigmoid function (maximal suppression constrained at 100%).
2) Spectral sensitivity
Negative masking in wildtype mice was induced by short-wavelength light; this response was equivalent to that of medium wavelengths (Figure 2). At 365nm the SWS-cone has maximal sensitivity, but the other photoreceptors have only residual sensitivity at this wavelength. At longer wavelengths wildtype responses fell between the templates for the MWS-cone and the melanopsin photoreceptors but had best fit to the MWS-cone (best fit visual pigment template between 480 and 580nm: λmax 510nm, R2 = 0.92). The limited assessment of spectral sensitivity in the rd/rd cl was broadly consistent with the sensitivity of the melanopsin photoreceptors (R2 = 0.96): that is, limited sensitivity in the short-wavelength range and sensitivity at longer wavelengths peaking at ~480nm.
Figure 2.
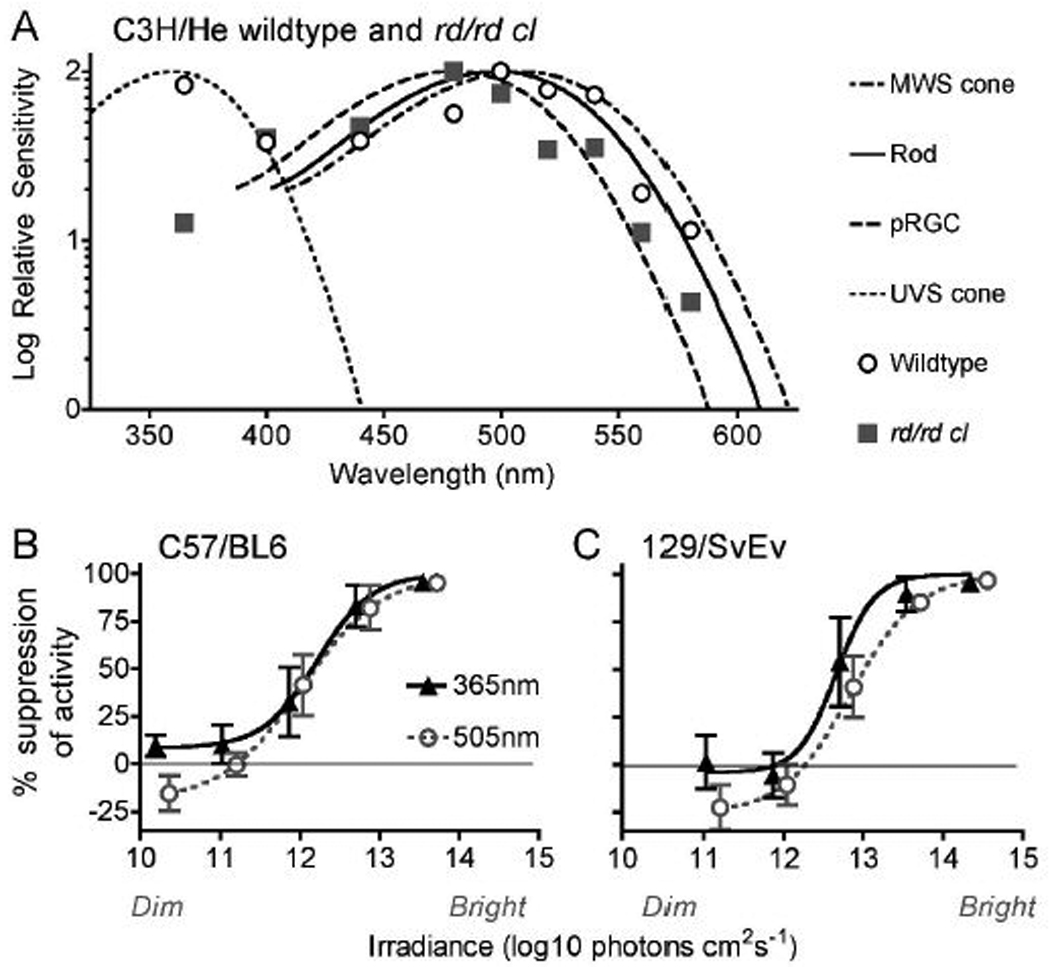
Spectral sensitivity of negative masking. (A) Spectral sensitivity profiles for the first hour of light pulses for C3H wildtype and rd/rd cl mice are plotted alongside A1 Govardovskii templates. Templates are fitted to the wavelengths of maximum sensitivity: for the rods (498nm), the short-wavelength-sensitive cone (359nm), the mediumwavelength-sensitive cone (508nm), and for the melanopsin photoreceptors (480nm). Responses during the first hour of short (~365nm) and medium (~505nm) wavelength light pulses are shown in (B) C57/BL6 and (C) 129/SvEv mice, fitted with irradiance response curves (maximal suppression constrained at 100%).
3) Consistency of spectral sensitivity among strains
The irradiance required to induce a half maximal suppression of activity (EC50) was also equivalent at short and medium-wavelength light in C57/BL6 and 129/SvEv mice (log10 photons EC50 ± SEM: C57/BL6 365nm, 12.21 ± 0.19, 505nm 12.02 ± 0.21, p = 0.52; 129SvEv, 365nm 12.66 ± 0.16, 505nm 12.84 ± 0.17, p = 0.52). In addition, C57/BL and 129SvEv mice showed positive masking with low irradiance medium-wavelength light but not with low irradiance short-wavelength light.
4) Dynamic range and capacity to sustain responses under broad-spectrum light
With white light wildtype mice had negative masking under high irradiance and positive masking under low irradiance (Figure 3). The irradiance range of negative masking in the wildtype, defined as the mainly linear part of the sigmoid function between 90 and 10% suppression of activity, was ~1-log unit of irradiance (16.5 to 3.4µWcm2). When the responses in the rd/rd cl were compared to the wildtype, EC50 was only marginally higher. Despite the similarity in this aspect of sensitivity, there were two genotype differences. First, positive masking was absent in rd/rd cl mice. Second, the irradiance range of negative masking was more than twice as broad in the rd/rd cl (121.9 to 0.56µWcm2, >2 log units of irradiance), evident as a significantly different slope in the fitted curves of the genotypes (F-test: F2,279 = 4.581, p=0.013).
Figure 3.
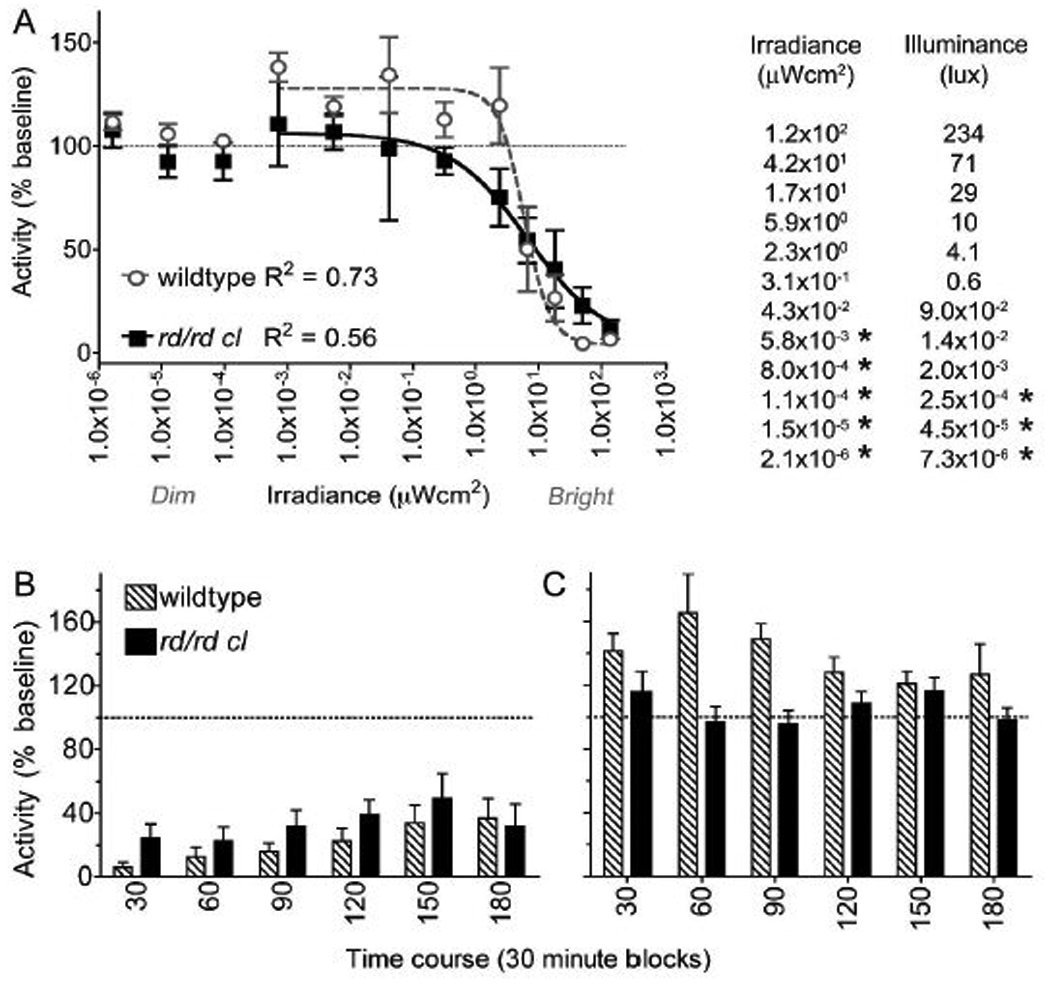
Time course and dynamic range of masking. (A) The dynamic range of responses to broad-spectrum light (first 30-minutes of light pulse) is shown in wildtype and rd/rd cl, fitted with irradiance response curves (maximal suppression constrained at 100%). Alongside the panel, treatment irradiance (µWcm2) is shown against illuminance (lux): measurements highlighted with a star (*) are derived from regression based on the increase in neutral density filters. The effect of duration of the pulse on change in activity during the light pulse is shown as 30-minute bins at example irradiances for (B) negative masking 16.5µWcm2 and (C) positive masking 8.0×10-4µWcm2.
Analysis of negative masking over the course of the 3-h pulse showed a partial reduction in the degree of activity suppression, but no genotype dependent difference in the capacity to sustain responses (Two-way ANOVA of genotypes over time at 16.5µWcm2: variation with time p < 0.005; variation with genotype p = 0.68). Positive masking was sustained in wildtype, but absent altogether in the rd/rd cl.
Discussion
Earlier work in mice with altered retinal function has partially informed our understanding of classical and melanopsin photoreceptor input to irradiance detection in accessory visual responses. To minimize the problem of secondary changes in retained photoreceptors with altered retinal function we made a study of irradiance and spectral sensitivity of negative masking primarily in wildtype animals. We also made supporting experiments in rd/rd cl mice that have melanopsin expression levels similar to the wildtype (Semo et al., May 2003), a contrast to some previously used rodent models (Sakamoto et al., Oct 27 2004; Wan et al., May 29 2006; Dkhissi-Benyahya et al., Mar 1 2007). However, interpretation of rd/rd cl responses must still be qualified because of the possibility of undefined changes in the sensitivity of the retained melanopsin photoreceptors.
Negative masking - photoreceptor input
The evidence that melanopsin photoreceptors have only residual sensitivity at short wavelengths is convincing (Lucas et al., 2001; Berson et al., Feb 8 2002; Hattar et al., Jul 3 2003; Newman et al., Nov 11 2003; Melyan et al., Feb 17 2005; Panda et al., Jan 28 2005; Qiu et al., Feb 17 2005; Mure et al., Oct 2007). Therefore, the equivalent sensitivity in three strains of wildtype mice at 365 and 480nm can be reasonably identified with a pronounced SWS-cone contribution to negative masking. As supporting evidence for this interpretation, the rd/rd cl had sensitivity similar to the wildtype at 480nm but only ~10% as sensitive at 365nm.
At longer wavelengths the rod, MWS-cone and melanopsin photoreceptors are all sensitive, and interpretation of results is less straightforward, but taken altogether, the evidence for a contribution to negative masking by more than one photoreceptor type is convincing. First, input by SWS-cone and an additional photoreceptor is identified by the similar sensitivity at short and long wavelengths in wildtype mice. Second, the slopes of irradiance response curves are different at 480 and 540nm. Third, wildtype spectral sensitivity at long-wavelengths fitted best with the MWS-cone but was actually intermediate to the sensitivity predicted by the melanopsin or the MWS-cone photoreceptors alone. Furthermore, evidence for input from both classical and melanopsin photoreceptors to negative masking comes from previous studies (Panda et al., Dec 13 2002; Mrosovsky & Hattar, Nov 2003). It therefore seems likely that there is combined melanopsin and MWS-cone input to negative masking at longer wavelengths.
The time course of negative masking sensitivity under broad-spectrum light was similar in wildtype and rd/rd cl mice, adding to evidence that melanopsin function underlies the capacity to sustain irradiance detection in negative masking (Mrosovsky & Hattar, Nov 2003). However, the extent of the SWS-cone contribution shows that classical photoreceptor input to irradiance detection can, at least in this instance, be sustained: if classical photoreception only provided input in the initial stages of the response, then we would not expect such a pronounced contribution to the mean response over an hour. In support of this, in vitro studies have recently suggested that extrinsic stimulation of the melanopsin cells at higher irradiances is relatively independent of light adaptation (Wong et al., Jul 1 2007). As melanopsin function is necessary for sustaining negative masking responses, it seems plausible that the presence of melanopsin photoreception may facilitate or enable sustained classical photoreceptor input.
Negative masking - basis for combined input
Additional input from classical photoreception would most obviously allow for a response under a wider set of circumstances. Several aspects can be identified. First, input from the SWS-cone broadens spectral sensitivity and this might be important in the context of the relative increase in short-wavelength light during twilight (Roenneberg & Merrow, Mar 5 2002). Second, the classical and melanopsin photoreceptors have very different temporal properties (Burns & Baylor, 2001; Berson et al., Feb 8 2002; Sekaran et al., Jun 21 2005), so combining input might provide a necessary balance between rapid and sustained responses. Third, the classical and melanopsin photoreceptors have different irradiance sensitivities (Burns & Baylor, 2001; Berson et al., Feb 8 2002), and combining input might be necessary for obtaining an appropriate dynamic range of the response. Each of these remains a non-exclusive possibility. Our study did identify changes in dynamic range in the rd/rd cl, where the response reflects the broad dynamic range of the melanopsin photoreceptors. In the wildtype, the combination of classical and melanopsin photoreceptors restrict dynamic range to a narrower band of irradiance in a way not possible with melanopsin alone.
Positive masking
Positive masking most obviously represents the use of vision to detect and negotiate obstacles, allowing increased movement through the environment. Therefore photoreceptor input should be confined to the image-forming rods and cones. Indeed, enhanced wheel running activity is abolished by lesions of the classical photoreceptors (Mrosovsky et al., Apr 1999) or the lateral geniculate nucleus (Edelstein & Mrosovsky, Nov 9 2001). Moreover, under broad-spectrum light we observed positive masking in wildtype but not rd/rd cl mice. In wildtype mice, there was also positive masking under dim medium-wavelength light (activating rods and possibly MWS-cones) but not under dim short-wavelength light (activating SWS-cones only). Therefore, our findings suggest that SWS-cones and possibly MWS-cones do not contribute to positive masking in mice. A rod only basis for visual guidance of movement in positive masking seems more likely in mice.
Irradiance detection in masking and other tasks
The main contribution of this paper is to provide evidence that in intact mice, the classical photoreceptors provide an important contribution to negative masking. It is not a matter of irradiance detection in accessory visual systems being mainly mediated by melanopsin photoreceptors. In other accessory visual responses, although the contribution of the classical photoreceptors varies, the same principle appears to apply. For instance, in the pupil light reflex melanopsin function is necessary for persistence and high irradiance responsiveness (Lucas et al., Jan 2003; Zhu et al., Mar 2007), but classical photoreceptor input extends the dim light dynamic range of the pupil light reflex (Lucas et al., 2001), and therefore supports lens accommodation in scotopic vision.
In this context, it should be kept in mind that there are interactions among the photoreceptors in the eye, and our findings show that despite the enthusiasm for melanopsin, classical photoreceptors do play a role in irradiance detection.
Acknowledgements
We thank Peggy Salmon and Geraldine Ryan for assistance with experiments. Funds for this work came from a CIHR grant to NM, a Wellcome Trust grant to RGF, and HHMI awards to EMS and VCS.
Abbreviations
- MWS
medium wavelength sensitive
- rd/rd cl
rodless/coneless mouse
- SWS
short wavelength sensitive
References
- Aggelopoulus NC, Meissl H. Responses of neurones of the rat suprachiasmatic nucleus to retinal illumination under photopic and scotopic conditions. J Physiol. 2000;523:211–222. doi: 10.1111/j.1469-7793.2000.t01-1-00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschoff JavGC. Masking of circadian activity rhythms in hamsters by darkness. Journal Comparative Physiology. 1988;162:559–562. doi: 10.1007/BF00612521. [DOI] [PubMed] [Google Scholar]
- Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- Berson DM. Phototransduction in ganglion-cell photoreceptors. Pflugers Arch. 2007;454:849–855. doi: 10.1007/s00424-007-0242-2. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard GC, Richardson BA, King TS, Matthews SA, Reiter RJ. The suppression of pineal melatonin content and N-acetyltransferase activity by different light irradiances in the Syrian hamster: a dose-response relationship. Endocrinology. 1983;113:293–296. doi: 10.1210/endo-113-1-293. [DOI] [PubMed] [Google Scholar]
- Burns ME, Baylor DA. Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu. Rev. Neurosci. 2001;24:779–805. doi: 10.1146/annurev.neuro.24.1.779. [DOI] [PubMed] [Google Scholar]
- Coohill TP. Action spectra again? Photochem Photobiol. 1991;54:859–870. doi: 10.1111/j.1751-1097.1991.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Dkhissi-Benyahya O, Gronfier C, De Vanssay W, Flamant F, Cooper HM. Modeling the role of mid-wavelength cones in circadian responses to light. Neuron. 2007;53:677–687. doi: 10.1016/j.neuron.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein K, Mrosovsky N. Behavioral responses to light in mice with dorsal lateral geniculate lesions. Brain Res. 2001;918:107–112. doi: 10.1016/s0006-8993(01)02966-3. [DOI] [PubMed] [Google Scholar]
- Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K. In search of the visual pigment template. Vis Neurosci. 2000;17:509–528. doi: 10.1017/s0952523800174036. [DOI] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:75–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas R, Douglas R, Foster R. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nature Neuroscience. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Foster RG. Neither functional rod photoreceptors nor rod or cone outer segments are required for the photic inhibition of pineal melatonin. Endocrinology. 1999;140:1520–1524. doi: 10.1210/endo.140.4.6672. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- MacNichol EFJ. A unifying presentation of photopigment spectra. Vision Res. 1986;26:1543–1556. doi: 10.1016/0042-6989(86)90174-4. [DOI] [PubMed] [Google Scholar]
- Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- Motulsky H, Christapoulos A. Fitting models to biological data using linear and nonlinear regression. GraphPad Software, Inc.; 2003. [Google Scholar]
- Mrosovsky N. In praise of masking: behavioural responses of retinally degenerate mice to dim light. Chronobiol Int. 1994;11:343–348. doi: 10.3109/07420529409057251. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Foster RG, Salmon PA. Thresholds for masking responses to light in three strains of retinally degenerate mice. J Comp Physiol [A] 1999;184:423–428. doi: 10.1007/s003590050341. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int. 2003;20:989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- Mure LS, Rieux C, Hattar S, Cooper HM. Melanopsin-dependent nonvisual responses: evidence for photopigment bistability in vivo. J Biol Rhythms. 2007;22:411–424. doi: 10.1177/0748730407306043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, Walker MT, Brown RL, Cronin TW, Robinson PR. Melanopsin forms a functional short-wavelength photopigment. Biochemistry. 2003;42:12734–12738. doi: 10.1021/bi035418z. [DOI] [PubMed] [Google Scholar]
- Ostergaard J, Hannibal J, Fahrenkrug J. Synaptic contact between melanopsin-containing retinal ganglion cells and rod bipolar cells. Invest Ophthalmol Vis Sci. 2007;48:3812–3820. doi: 10.1167/iovs.06-1322. [DOI] [PubMed] [Google Scholar]
- Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- Peirson SN, Thompson S, Hankins MW, Foster RG. Mammalian photoentrainment: results, methods, and approaches. Methods Enzymol. 2005;393:697–726. doi: 10.1016/S0076-6879(05)93037-1. [DOI] [PubMed] [Google Scholar]
- Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci U S A. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- Redlin U, Hattar S, Mrosovsky N. The circadian Clock mutant mouse: impaired masking response to light. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:51–59. doi: 10.1007/s00359-004-0570-z. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Merrow M. Light reception: discovering the clock-eye in mammals. Curr Biol. 2002;12:R163–R165. doi: 10.1016/s0960-9822(02)00731-5. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O'Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Liu C, Tosini G. Classical photoreceptors regulate melanopsin mRNA levels in the rat retina. J Neurosci. 2004;24:9693–9697. doi: 10.1523/JNEUROSCI.2556-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekaran S, Lupi D, Jones SL, Sheely CJ, Hattar S, Yau KW, Lucas RJ, Foster RG, Hankins MW. Melanopsin-dependent photoreception provides earliest light detection in the mammalian retina. Curr Biol. 2005;15:1099–1107. doi: 10.1016/j.cub.2005.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semo M, Peirson S, Lupi D, Lucas RJ, Jeffery G, Foster RG. Melanopsin retinal ganglion cells and the maintenance of circadian and pupillary responses to light in aged rodless/coneless (rd/rd cl) mice. Eur J Neurosci. 2003;17:1793–1801. doi: 10.1046/j.1460-9568.2003.02616.x. [DOI] [PubMed] [Google Scholar]
- Takahashi J, DeCoursey P, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- Takao M, Morigiwa K, Sasaki H, Miyoshi T, Shima T, Nakanishi S, Nagai K, Fukuda Y. Impaired behavioral suppression by light in metabotropic glutamate receptor subtype 6-deficient mice. Neuroscience. 2000;97:779–787. doi: 10.1016/s0306-4522(00)00053-1. [DOI] [PubMed] [Google Scholar]
- Thompson S, Philp AR, Stone EM. Visual function testing: A quantifiable visually guided behavior in mice. Vision Res. 2007 doi: 10.1016/j.visres.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Wan J, Zheng H, Hu BY, Xiao HL, She ZJ, Chen ZL, Zhou GM. Acute photoreceptor degeneration down-regulates melanopsin expression in adult rat retina. Neurosci Lett. 2006;400:48–52. doi: 10.1016/j.neulet.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Wong KY, Dunn FA, Berson DM. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:1001–1010. doi: 10.1016/j.neuron.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol. 2007;582:279–296. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tu DC, Denner D, Shane T, Fitzgerald CM, Van Gelder RN. Melanopsin-dependent persistence and photopotentiation of murine pupillary light responses. Invest Ophthalmol Vis Sci. 2007;48:1268–1275. doi: 10.1167/iovs.06-0925. [DOI] [PubMed] [Google Scholar]


