Abstract
Systemic lupus erythematosus (SLE) disproportionately affects females. Recent work demonstrates that men with Klinefelter's syndrome (47,XXY males) have a similar risk of developing SLE as do genotypic females. We present an unusual case of an African American family with two SLE affected individuals in which one of the SLE patients also has Turner's syndrome [46,X,del(X)(q13)]. While not definitive, this family raises interesting questions regarding the role of genes located on the X chromosome in the development of SLE. The paucity of case reports documenting the overlap of SLE with Turner's syndrome while there is and association of male SLE with Klinefelter's syndrome suggests a lower risk of SLE in Turner's females. These observations are consistent with a gene dose effect at X with two X chromosomes (46,XX or 47,XXY) conferring higher risk and one X chromosome (46,XY or 45,XO) conferring lower risk of SLE.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic and complex autoimmune disease, with both an environmental component and a heritable predisposition. The disease disproportionately affects females [9:1 female to male ratio (1)] and African American women in particular (European American females 1:1000; African American females 1:250) (2,3). Recent work indicates that the presence of two X chromosomes [genotypic females (3), Klinefelter's males (4)] or lack thereof (genotypic males and Turner's syndrome females) influences individual risk of developing the disease.
Turner's syndrome is a rare chromosomal disorder of females (1:2500 live births) first described in 1938 by H. H. Turner (5) and characterized by short stature and the lack of sexual development at puberty due to the partial or complete absence of a second X chromosome (6). The condition is confirmed through DNA karyotyping and/or fluorescence in situ hybridization (FISH).
Overlap of these two conditions appears to be rare, with only 2 cases documented (7,8). Although without cohort studies of Turner's syndrome among SLE patients or of SLE among Turner's syndrome patients, association can neither be confirmed nor refuted. Participation of SLE patients with sex chromosome disorders in genetic studies of lupus may help illuminate the location of lupus-related gene(s) on the X chromosome.
CASE REPORT
An African American family with two members diagnosed with systemic lupus erythematosus (SLE) was referred to the Lupus Genetics Studies for possible inclusion in the national Lupus Family Registry and Repository (LFRR) housed at the Oklahoma Medical Research Foundation. Per family and physician report, the younger sibling purported to have SLE also had Turner's syndrome.
At age 17 years, this African American woman was diagnosed with Turner's syndrome. Symptoms and signs revealed to the Lupus Genetics Studies staff included primary amenorrhea and short stature with a height of 145.8 cm (4ft 9½in, <5th percentile). No hormone replacement therapy or human growth hormone therapy was initiated.
Four months later, the same patient was admitted to the hospital with unintended weight loss (15−20 lbs), depression, and chest pain. She had a history of joint pain in her hands and right knee and was found to have rashes on the eyes and behind the ears which two rheumatologists observed and suspected of being discoid lupus. Laboratory tests revealed a positive antinuclear antibody and positive anti-double-stranded (ds)DNA. Family history of autoimmune illness included a sister with SLE and another relative with rheumatoid arthritis. The patient was diagnosed with SLE by the treating physician.
Medical record abstraction by the LFRR confirmed classification of SLE according to the American College of Rheumatology 1982 Classification Criteria for SLE as updated in 1997 (9,10) in both sisters. Classification criteria for the purported Turner's syndrome patient included a positive ANA (1:360, nuclear homogeneous pattern), positive anti-(ds)DNA (502, normal <10), positive antiphospholipid antibody, discoid lesions, and pleuritic chest with pleural effusion. Additional lupus-related findings included low complement (C3=52.5, normal 80 to 180) and low CH50 (23%, normal >50%). Classification criteria for the sibling included discoid rash, arthritis, anti-(ds)DNA (untitered) and a positive ANA (1:1080, nuclear speckled pattern). Additional lupus-related findings included positive anti-nuclear RNP and low complement (C4=12, normal 15 to 45).
The LFRR undertook karyotyping (Figures 1a and 1b) and fluorescence in situ hybridization (FISH) (Figure 2) to confirm the family's and pediatrician's reports that the patient had Turner's syndrome. Testing demonstrated 46,X,del(X)(q13), a terminal long arm deletion for one of the two X chromosomes. In order to localize the deletion, DNA was typed on 1.8 million single nucleotide polymorphisms (SNPs) using the 6.0 GeneChip™ Array (Affymetrix Inc., Santa Clara, CA). We found that the most centromeric SNP deleted was at position 75,695,000 with a detected, i.e., undeleted, SNP only 20 base pairs centromeric. Therefore, the Xist gene, which determines X inactivation and is located near the start of the deletion on Xq13.1, was present. We determined by previously reported methods (11) that the full length X chromosome in this patient was active in some cells, but because the androgen receptor gene was deleted in the truncated X chromosome an estimation of percent inactivation of each X chromosome could not be made (11).
Figure 1A.
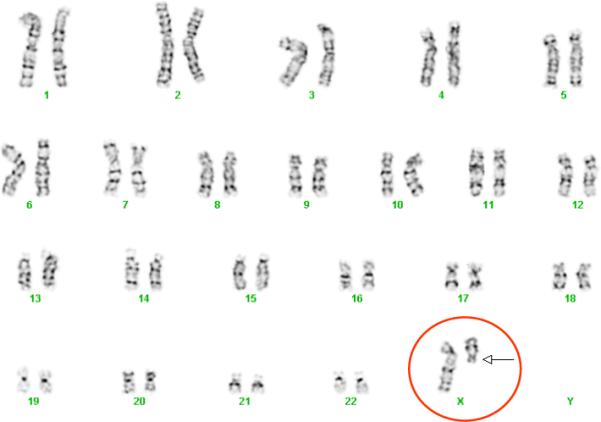
Karyotype of Turner's syndrome/SLE subject demonstrating 46,X,del(X)(q13).
Figure 1B.
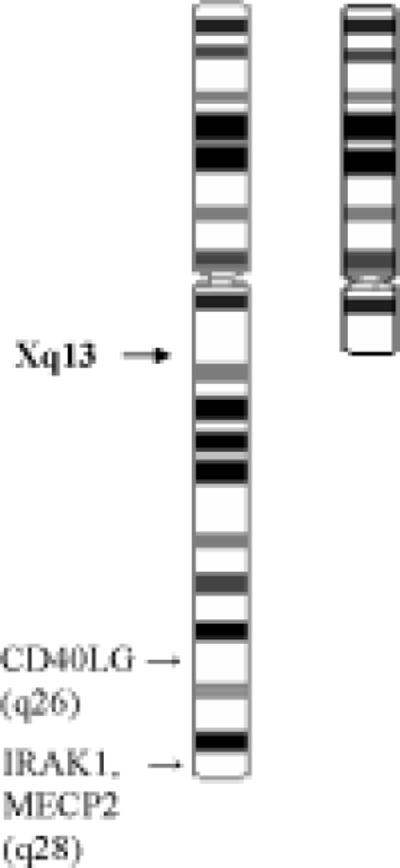
Ideogram of 46,X,del(X)(q13) demonstrating area of terminal chromosomal long-arm deletion and location of 3 lupus-linked genes: CD40LG, IRAK1, MECP2.
Figure 2.
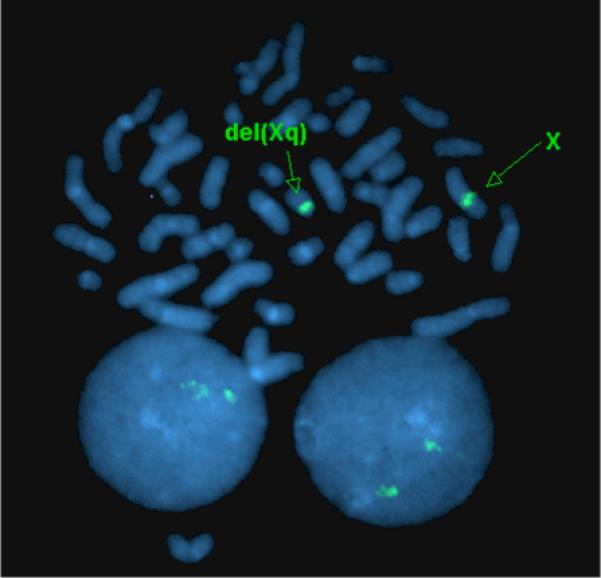
Fluorescence in situ hybridization (FISH) of Turner's syndrome/SLE overlap participant demonstrating 46,X,del(X)(q13).
DISCUSSION
When casually considered, gender may appear dichotomous and discrete. However, because of a wide range of disorders of sex differentiation (reviewed in 12), biological sex is a continuum. A class of disorders of sex differentiation is those with sex chromosomal abnormalities. The common diseases classified as such include Turner's syndrome (45,XO) and Klinefelter's syndrome (47,XXY). We hypothesize that study of disorders of sex differentiation may shed light upon the gender predilection of SLE.
Study of other X chromosome abnormalities has been illuminating in autoimmune disease. For example, acquired X monosomy in peripheral blood cells has been found in autoimmune thyroid disease, systemic sclerosis and primary biliary cirrhosis, but not in SLE (13). Skewing of X inactivation has been reported in several female-predominate autoimmune diseases (reviewed in 14), but one study found no skewing in SLE (15). However, this study also found no skewing in systemic sclerosis, while others have found marked X inactivation skewing in this disease (16). Skewed X chromosome inactivation has not been found in identical twins discordant for SLE (17). Skewing of X inactivation could not be determined in our SLE and Turner's syndrome patient because techniques for this determination rely on the androgen receptor gene, which is deleted from the truncated X chromosome in our patient.
Turner's syndrome has been associated with several autoimmune diseases. These include autoimmune thyroid disease and Crohn's disease. Of interest, both these diseases are found among Turner's syndrome patients with particular X chromosome abnormalities, namely, one normal X chromosome and one X isochromosome with two copies of the long arm of X (18,19). However, only the study of autoimmune thyroid disease represents a true cohort study of Turner's syndrome patients (19), while the work describing Crohn's disease in Turner's syndrome is merely a collection of case reports (18). The other autoimmune disease that reported data most strongly suggest is found in excess among girls with Turner's syndrome is celiac disease, which can be present in up to 10% (20,21). While a lack of association of Tuner's syndrome and SLE is not certain, the possibility that Turner's syndrome, skewed X inactivation and acquired X monosomy are associated with some but not all autoimmune diseases is intriguing, and potentially highly informative if mechanisms for these differences can be determined. In addition, the relationship of Turner's karyotype to autoimmune disease may be important.
We have found that Klinefelter's syndrome occurs in excess among men with SLE such that 47,XXY Klinefelter's men are at the same risk of SLE as ethnically similar women (4). In this same study, we found that no woman with SLE had Turner's syndrome (4). Further, while there are numerous case reports of Klinefelter's men with SLE (4, reviewed in 22), we find only two previous reports of SLE in women with Turner's syndrome: one confirmed and characterized case (7) and one alleged case (8). We identified the present family by specifically canvassing our collaborators for patients with both Turner's syndrome and SLE. Thus, these data suggest that Turner's syndrome is certainly not over-represented among SLE patients and may in fact be under-represented, consistent with a gene dose effect for the X chromosome (4).
The previous characterized patient with Turner's syndrome/SLE overlap has 45,X/46,XXq+ mosaicism, or a mosaic short-arm deletion of one X chromosome (7) (Table 1). The present report of Turner's syndrome/SLE overlap documents a partial long arm deletion of the second X chromosome [46,X,del(X)(q13)] without mosaicism. Genes found on X that escape X inactivation [∼15% of X genes (reviewed in 23)] are potential gene dose candidates because normal (46,XX) women have two transcribed and translated copies while men have only one active copy. Three potential candidates are CD40LG (Xq26) which is demethylated (i.e., not inactivated) and overexpressed in T cells from women with SLE (24), IRAK1 (Xq28) (25), and MECP2 (Xq28) (26). However, the present reported case of Turner's syndrome/SLE overlap has only one copy of each of these genes due to the Xq13 terminal deletion on 46,X (Figure 1B). While many genes are involved in the susceptibility to SLE, further study of individuals with Turner's syndrome/SLE overlap may help illuminate the location of lupus-related gene(s) on the X chromosome, while further delineating the roles of gene dose effect. Our data suggest that a gene effect could reside on the p arm of X or on the q arm centromeric to q13.1; however, other genes on X for which an allelic difference is important, instead of dose, could still reside on the X chromosome.
Table 1.
Description of X-chromosome anomalies associated with Turner's syndrome/SLE overlap in 3 cases.
| Turner's syndrome/SLE Case Study | X Chromosome Anomaly |
|---|---|
| Takegami et al. | 45,X/46,XXq+ (mosaic) |
| Rakfal and Deutsch | alleged case, not characterized |
| Cooney et al. | 46,X,del(X)(q13) |
ACKNOWLEDGEMENTS
We give special thanks to the Lupus Family Registry and Repository staff for their hard work and data collection, and to all our referring physicians whose lupus patient referrals make this work possible. Ms. Ramirez was an OMRF Fleming Scholar while portions of this work were carried out.
Funding: This study was funded by the following NIH grants: AR62277, AR053734, AI24717 and AR42460.
REFERENCES
- 1.Wallace DJ, Hahn BH. Dubois’ Lupus Erythematosus. 7th Edition Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 2.Fessel WJ. Systemic lupus erythematosus in the community. Incidence, prevalence, outcome, and first symptoms; the high prevalence in black women. Arch Intern Med. 1974;134:1027–1035. [PubMed] [Google Scholar]
- 3.McCarty DJ, Manzi S, Medsger TA, Jr., Ramsey-Goldman R, LaPorte RE, Kwoh CK. Incidence of systemic lupus erythematosus. Race and gender differences. Arthritis Rheum. 1995;38:1260–1270. doi: 10.1002/art.1780380914. [DOI] [PubMed] [Google Scholar]
- 4.Scofield RH, Bruner GR, Namjou B, Kimberly RP, Ramsey-Goldman R, Petri M, Reveille JD Alarcon GS, Vila LM, Reid J, Harris B, Li S, Kelly JA, Harley JB. Klinefelter's Syndrome, 47,XXY, in Male Systemic Lupus Erythematosus Supports a Gene Dose Effect from the X Chromosome. Arthritis Rheum. 2008;58:2511–2517. doi: 10.1002/art.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner HH. A syndrome of infantilism, congenital webbed neck, and cubitus valgus. Endocrinol. 1938;23:566. [PubMed] [Google Scholar]
- 6.Ford CE, Jones KW, Polani PE, De Almeida JC, Briggs JH. A sex-chromosome anomaly in a case of gonadal dysgenesis (Turner's syndrome). Lancet. 1959;1(7075):711–713. doi: 10.1016/s0140-6736(59)91893-8. [DOI] [PubMed] [Google Scholar]
- 7.Takegami T, Nakao K, Nagayama Y, Fujita T, Hoshino T, Tsunematsu T, et al. [A case of SLE associated with Turner's syndrome of 45, XO/46, XXq+ mosaicism (author's transl)]. Nippon Naika Gakkai Zasshi. 1980;69:861–866. doi: 10.2169/naika.69.861. [DOI] [PubMed] [Google Scholar]
- 8.Rakfal SM, Deutsch M. Radiotherapy for malignancies associated with lupus: case reports of acute and late reactions. Am J Clin Oncol. 1998;21:54–57. doi: 10.1097/00000421-199802000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 10.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 11.Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992;51:1229–1239. [PMC free article] [PubMed] [Google Scholar]
- 12.MacLaughlin DT, Donahoe PK. Sex determination and differentiation. N Engl J Med. 2004;350:367–378. doi: 10.1056/NEJMra022784. [DOI] [PubMed] [Google Scholar]
- 13.Invernizzi P, Miozzo M, Oertelt-Prigione S, Meroni PL, Persani L, Selmi C, Battezzati PM, Zuin M, Lucchi S, Marasini B, Zeni S, Watnik M, Tabano S, Maitz S, Pasini S, Gershwin ME, Podda M. X monosomy in female systemic lupus erythematosus. Ann N York Acad Sci. 2007;1110:84–91. doi: 10.1196/annals.1423.010. [DOI] [PubMed] [Google Scholar]
- 14.Invernizzi P, Pasini S, Selmi C, Miozzo M, Podda M. Skewing of X chromosome inactivation in autoimmunity. Autoimmunity. 2008;41:272–277. doi: 10.1080/08916930802024574. [DOI] [PubMed] [Google Scholar]
- 15.Chitnis S, Monteiro J, Glass D, Apatoff B, Salmon J, Concannon P, Gregersen PK. The role of X-chromosome inactivation in female predisposition to autoimmunity. Arthritis Res. 2000;2:399–406. doi: 10.1186/ar118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selmi C, Invernizzi P, Gershwin ME. The X chromosome and systemic sclerosis. Curr Op Rheumatol. 2006;18:601–605. doi: 10.1097/01.bor.0000245718.56770.a4. [DOI] [PubMed] [Google Scholar]
- 17.Huang Q, Parfitt A, Grennan DM, Manolios N. X-chromosome inactivation in monozygotic twins with systemic lupus erythematosus. Autoimmunity. 1997;26:85–93, 1997. doi: 10.3109/08916939709003851. [DOI] [PubMed] [Google Scholar]
- 18.Hayward PA, Satsangi J, Jewell DP. Inflammatory bowel disease and the X chromosome. QJM. 1996;89:713–718. doi: 10.1093/qjmed/89.9.713. [DOI] [PubMed] [Google Scholar]
- 19.Elsheikh M, Wass JA, Conway GS. Autoimmune thyroid syndrome in women with Turner's syndrome--the association with karyotype. Clin Endocrinol. 2001;55:223–226. doi: 10.1046/j.1365-2265.2001.01296.x. [DOI] [PubMed] [Google Scholar]
- 20.Bonamico M, Bottaro G, Pasquino AM, Caruso-Nicoletti M, Mariani P, Gemme G, Paradiso E, Ragusa MC, Spina M. Celiac disease and Turner syndrome. J Ped Gastroenterol Nutrit. 1998;26:496–499. doi: 10.1097/00005176-199805000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Bettendorf M, Doerr HG, Hauffa BP, Lindberg A, Mehls O, Partsch CJ, Schwarz HP, Stahnke N, Ranke MB. Prevalence of autoantibodies associated with thyroid and celiac disease in Ullrich-Turner syndrome in relation to adult height after growth hormone treatment. J Ped Endocrinol. 2006;19:149–154. doi: 10.1515/jpem.2006.19.2.149. [DOI] [PubMed] [Google Scholar]
- 22.Rovensky J. Rheumatic diseases and Klinefelter's syndrome. Autoimmun Rev. 2006;6:33–36. doi: 10.1016/j.autrev.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Sidhu SK, Minks J, Chang SC, Cotton AM, Brown CJ. X chromosome inactivation: heterogeneity of heterochromatin. Biochem Cell Biol. 2008;86:370–379. doi: 10.1139/o08-100. [DOI] [PubMed] [Google Scholar]
- 24.Lu Q, Wu A, Tesmer L, Ray D, Yousif N, Richardson B. Demethylation of CD40LG on the inactive X in T cells from women with lupus. J Immunol. 2007;179:6352–6358. doi: 10.4049/jimmunol.179.9.6352. [DOI] [PubMed] [Google Scholar]
- 25.Jacob CO, Reiff A, Armstrong DL, Myones BL, Silverman E, Klein-Gitelman M, et al. Identification of novel susceptibility genes in childhood-onset systemic lupus erythematosus using a uniquely designed candidate gene pathway platform. Arthritis Rheum. 2007;56:4164–4173. doi: 10.1002/art.23060. [DOI] [PubMed] [Google Scholar]
- 26.Sawalha AH, Webb R, Han S, Kelly JA, Kaufman KM, Kimberly RP, Alarcón-Riquelme ME, James JA, Vyse TJ, Gilkeson G, Choi CB, Scofield RH, Bae SC, Nath SK, Harley JB. Common variants within MECP2 confer risk of systemic lupus erythematosus. PLoS One. 2008;3:e1727. doi: 10.1371/journal.pone.0001727. [DOI] [PMC free article] [PubMed] [Google Scholar]


