Abstract
Stroke can be categorized as ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage. Awakening with or experiencing the abrupt onset of focal neurologic deficits is the hallmark of the diagnosis of ischemic stroke. The most common presenting symptoms for ischemic stroke are difficulty with speech and weakness on one half of the body. Many stroke mimics exist; two of the most common are a postictal seizure and hypoglycemia. Taking a detailed history and performing ancillary testing will usually exclude stroke mimics. Neuroimaging is required to differentiate ischemic stroke from intracerebral hemorrhage, as well as to diagnose entities other than stroke. The choice of neuroimaging depends on its availability, eligibility for acute stroke interventions, and the presence of patient contraindications. Subarachnoid hemorrhage presents most commonly with severe headache and may require analysis of cerebrospinal fluid when neuroimaging is not definitive. Public education of common presenting stroke symptoms is needed for patients to activate emergency medical services as soon as possible after the onset of stroke.
The symptoms of stroke can sometimes be misleading and misinterpreted by physicians and patients. Family physicians are on the front line in their communities to recognize and manage acute cerebrovascular diseases. Accurate and prompt evaluation of cerebrovascular disease will increase eligibility of patients to receive acute therapy for stroke.
Classifying Stroke
Stroke can be subclassified by pathologic process and the vascular distribution affected. Defining the overall pathologic process is critical for decisions regarding thrombolysis, inpatient therapy, and prognosis. In the United States, 87 percent of all strokes are ischemic secondary to large-artery atherosclerosis, cardio embolism, small-vessel occlusion, and other or undetermined causes.1,2 The remaining 13 percent of strokes are hemorrhagic in intracerebral or subarachnoid locations.1,2 A common means of subclassifying ischemic stroke is by vascular distribution. Clinical determination of the affected vascular territory may aid rational evaluation and individualization of therapy.3 However, this type of subclassification has only fair to good interobserver agreement among stroke experts.4,5 Table 1 lists stroke subtypes by vascular distribution.6 The cause of subarachnoid hemorrhage is attributed to an aneurysm in approximately 85 percent of cases,7 with rarer causes accounting for the rest.
Table 1.
Oxfordshire Ischemic Stroke Subtypes and Clinical Features
| Stroke subtype | Clinical features |
|---|---|
| Total anterior circulation infarct (TACI) | Combination of new higher cerebral dysfunction (e.g., dysphasia, dyscalculia, visual-spatial disorder); homonymous visual field defect and ipsilateral motor and/or sensory defect involving two areas of the face, arm, or leg |
| Lacunar infarct (LACI) | Pure motor or pure sensory symptoms, sensorimotor stroke, or ataxic hemiparesis; face-arm and arm-leg syndromes included |
| Partial anterior circulation infarct (PACI) | Patients with only two of three TACI components, with higher cerebral dysfunction alone, or with a motor/sensory deficit more restricted than those classified as LACI (e.g., confined to one limb or to face and hand, but not the whole arm) |
| Posterior circulation infarct (POCI) |
Any one of the following: ipsilateral cranial nerve palsy with contralateral motor and/or sensory deficit; bilateral motor and/or sensory deficit; disorder of conjugate gaze; cerebellar dysfunction without ataxic hemiparesis; isolated homonymous visual field defect |
Information from reference 6.
Clinical Diagnosis
HISTORY AND PHYSICAL EXAMINATION
History and physical examination remain the pillars of diagnosing stroke. The most common historical feature of an ischemic stroke is its acute onset; the most common physical findings of ischemic stroke are focal weakness and speech disturbance.8 The most common and reliable symptoms and signs of ischemic stroke are listed in Table 2.4,8,9 Primary care physicians practicing in an emergency setting had a 92 percent sensitivity for diagnosis of stroke and transient ischemic attack (TIA) in a community-based study of diagnostic accuracy.10 The overall accuracy of a physician’s diagnosis of stroke is moderate to good, with lower reliability in less experienced or less confident examiners.4
Table 2.
Most Common Symptoms and Signs of Stroke and Their Reliability
| Symptom or sign | Prevalence (%)8 | Agreement between examiners (Kappa*)4 |
|---|---|---|
| Symptoms | ||
| Acute onset | 96 | Good (0.63)4 |
| Subjective arm weakness† | 63 | Moderate (0.59)4 |
| Subjective leg weakness† | 54 | Moderate (0.59)4 |
| Self-reported speech disturbance | 53 | Good (0.64)4 |
| Subjective facial weakness | 23 | — |
| Arm paresthesia‡ | 20 | Good (0.62)4 |
| Leg paresthesia‡ | 17 | Good (0.62)4 |
| Headache | 14 | Good (0.65)4 |
| Nonorthostatic dizziness | 13 | — |
| Signs | ||
| Arm paresis | 69 | Moderate to excellent (0.42 to 1.00)4,9 |
| Leg paresis | 61 | Fair to excellent (0.40 to 0.84)4,9 |
| Dysphasia or dysarthria |
57 | Moderate to excellent (0.54 to 0.84)4,9 Fair to excellent (0.29 to 1.00)4,9 |
| Hemiparetic or ataxic gait | 53 | Excellent (0.91)9 |
| Facial paresis | 45 | Poor to excellent (0.13 to 1.00)4,9 |
| Eye movement abnormality | 27 | Fair to excellent (0.33 to 1.00)9 |
| Visual field defect | 24 | Poor to excellent (0.16 to 0.81)4,9 |
NOTE: Symptoms and signs are arranged in order of prevalence.
Kappa statistic: 0 to 0.20 = poor agreement; 0.21 to 0.40 = fair agreement; 0.41 to 0.60 = moderate agreement; 0.61 to 0.80 = good agreement; 0.81 to 1.00 = excellent agreement.
Noted as “loss of power.”4
Noted as “loss of sensation.”4
Physicians need to quickly assess persons with suspected acute ischemic stroke because acute therapies for stroke have a narrower time window of effectiveness than therapies for myocardial infarction. The National Institute of Health Stroke Scale (NIHSS;11,12 available at http://www.ninds.nih.gov/doctors/NIH_Stroke_Scale.pdf) was designed to be completed in five to eight minutes.
The exact time of onset of symptoms is critical for determining eligibility for thrombolysis. However, a community-based study found that examiners agreed to the minute less than 50 percent of the time,4 suggesting the need to corroborate time of symptom onset with a witness or known event.
Reliably distinguishing between intracerebral hemorrhage and ischemic stroke can only be done through neuroimaging. Both entities are characterized by acute onset of focal symptoms. Persons with intracerebral hemorrhage may have gradual worsening of symptoms after the abrupt onset, reflecting an increasing size of the hematoma. Persons with hemorrhage also may have a decreased level of consciousness.
Subarachnoid hemorrhage presents differently from intracerebral hemorrhage and ischemic stroke. The most common symptom described by the patient is the “worst headache of my life.” Symptoms may also include vomiting, seizures, meningismus, and a decreased level of consciousness.13 Persons with subarachnoid hemorrhage may not exhibit focal signs because the bleeding occurs outside the brain, except when an aneurysm bleeds into a focal location, such as a posterior communication artery aneurysm compressing the third cranial nerve.
VALIDATED DECISION SUPPORT TOOLS
The NIHSS is one of the most common classifications of early stroke severity;12 it provides a structured neurologic examination that has diagnostic11,14 and prognostic value.11 Current guidelines recommend the use of the NIHSS,11 but no trial data exist to show its use improves outcomes. In general, combinations of signs and symptoms are more useful than single findings. Table 38,9,15–19 describes operating characteristics for several validated stroke diagnostic tools. Common signs across stroke diagnostic tools include acute onset of unilateral weakness or numbness and speech disturbance.8,15–19 One of the validated instruments, the Recognition of Stroke in the Emergency Room (ROSIER) scale, adds a visual field defect on examination.8 Most of these tools were designed for prehospital care, but emergency department physicians using the ROSIER scale correctly classified 90 percent of all patients in a community-based validation study of consecutive patients seen in the United Kingdom.8 Physicians using ROSIER missed patients with ischemic posterior or lacunar lesions, which emphasizes the need for an examination more thorough than a scale alone. No head-to-head trials have been performed to demonstrate improved patient outcomes using a validated stroke scale versus global clinical impression. In practice, since most of these tools demonstrate good clinical accuracy, a physician should become familiar with one of them to help confirm their overall clinical impression of stroke.
Table 3.
Operating Characteristics of Selected Stroke Screening Tools
| Name | Components | Sensitivity (% [95% CI]) |
Specificity (% [95% CI]) |
LR (95% CI) |
|---|---|---|---|---|
| Cincinnati Prehospital Stroke Scale15 |
Facial paralysis; arm drift; abnormal speech |
1 item: 66 (49 to 80) 2 items: 26 (14 to 43) 3 items: 11 (3 to 26) ≥ 1 item: 85 (80 to 90)8 |
1 item: 87 (80 to 92) 2 items: 95 (90 to 98) 3 items: 99 (95 to 100) ≥ 1 item: 79 (73 to 85)8 |
LR+ 0 items: 0.39 (0.25 to 0.61)9 ≥ 1 item: 5.5 (3.3 to 9.1)9 1 item: 5.2 (2.6 to 11)9 2 items: 4.2 (1.4 to 13)9 3 items: 14 (1.6 to 121)9 |
| Face, Arm, Speech Test16 |
In patients with Glasgow Coma Scale > 6 and presence of at least one of the following: facial paralysis; arm weakness; speech impairment |
82 (76 to 88)8 | 83 (77 to 89)8 | — |
| Los Angeles Prehospital Stroke Screen |
Presence of all six items is positive for stroke: Age > 45 years; no seizure history; symptoms present < 24 hours; ambulatory at baseline; serum glucose > 60 mg per dL (3.35 mmol per L) and < 400 mg per dL (22.20 mmol per L); unilateral deficit of one of three items (facial paresis, arm drift, weak handgrip) |
91 (76 to 98)17 7818 59 (52 to 66)8 |
97 (93 to 99)17 85 (80 to 90)8,18 |
LR+ = 31 (13 to 75)17 LR− = 0.09 (0.03 to 0.27)17 |
| Melbourne Ambulance Stroke Screen18 |
Age > 45 years; no seizure history; symptoms present < 24 hours; ambulatory at baseline; serum glucose > 60 and < 400 mg per dL; presence of ≥ one of four items (facial droop, arm drift, weak handgrip, speech impairment) |
90 (81 to 96) | 74 (53 to 88) | LR+ = 3.49 (1.83 to 6.63) LR− = 0.13 (0.06 to 0.27) |
| Recognition Of Stroke In the Emergency Room scale8 |
A score of 1 point or higher is positive for stroke:* History of syncope or loss of consciousness (−1 pt) History of seizure activity (−1 pt) New acute onset of: Asymmetric facial weakness (+1 pt) Asymmetric arm weakness (+1 pt) Asymmetric leg weakness (+1 pt) Speech disturbance (+1 pt) Visual field defect (+1 pt) |
93 (89 to 97) | 83 (77 to 89) | LR+ = 5.49 (3.11 to 9.68) LR− = 0.083 (0.04 to 0.17) |
| von Arbin19 | Acute onset of focal neurologic deficit; onset < seven days go; no recent head trauma |
86 (81 to 91) | 99 (98.5 to 99.4) | LR+ = 94 (59 to 152) LR− = 0.14 (0.095 to 0.20) |
STROKE MIMICS AND DIFFERENTIAL DIAGNOSIS
Physicians need to consider a broad differential diagnosis when evaluating a patient presenting with a suspected stroke (Table 4).8,11,14,20–24 The two most common stroke mimics are hypoglycemia and seizure.8,14,20,21
Table 4.
Stroke Mimics and Distinguishing Features
| Condition | Distinguishing features |
|---|---|
| Seizure | History of loss of consciousness, seizure activity, or post-ictal state14,20 |
| Systemic infection | Chest most common source;14 acute illness exacerbating an old deficit21 |
| Syncope/presyncope or hypotension | Hypotension unusual in acute stroke; prevalence of blood pressure < 120/80 at initial stroke presentation = 7.1 percent;22 symptoms may be transient or respond to hydration |
| Toxic-metabolic disturbances | Hypoglycemia most common14,21 |
| Tumor | Mass noted on neuroimaging |
| Acute confusional state | May be related to alcohol intoxication, medication adverse effect, or other encephalopathy |
| Vertigo or dizziness | Imbalance, but not vertigo, increases the likelihood of stroke;23 prevalence of stroke or transient ischemic attack in adults older than 44 years with isolated dizziness symptoms in emergency setting = 0.7 percent23 |
| Migraine | History of similar events, preceding aura and headache11 |
| Functional or medically unexplained symptoms | Reported incidence is 0.2 to 4.3 percent of patients admitted for stroke;8,24 only history of headache or pre/post presentation functional syndrome was associated with unexplained symptoms in a United Kingdom case-control study; dysarthria, vertigo, and/or ataxia were less likely to be unexplained; motor, sensory, and visual field symptoms, stroke risk factors, and history of pre- post-presentation depression or anxiety equally likely in patients with stroke and unexplained symptoms24 |
| Dementia | Presence of known cognitive impairment was one of two factors that independently predicted a stroke mimic in a Australian prospective study of patients admitted with suspected stroke14 |
One potential area of confusion is among patients presenting with a symptom of dizziness. In a population-based study of adults older than 44 years presenting to the emergency department or directly admitted to the hospital with a principal symptom of dizziness, only 0.7 percent of patients with isolated dizziness symptoms had an ultimate diagnosis of stroke or TIA.23 Vertigo from a central cause such as stroke is normally associated with nystagmus or other cerebellar signs.
The rates of overdiagnosis of stroke in studies of consecutive patients vary from 19 to 31 percent.14,20,21 Known history of cognitive impairment,14 non-neurologic abnormal physical findings,14 and decreased level of consciousness20 are independent predictors of a stroke mimic in patients with suspected stroke. Patient factors such as confusion, aphasia, and presentation more than 48 hours after the event also make diagnostic information less reliable.14
Duration of symptoms distinguishes stroke from TIA, which has been traditionally defined as a focal ischemic neurologic event resolving within 24 hours. Subsequent observations have shown that a majority of TIAs resolve within one hour.25 The National Institute for Neurologic Disorders and Stroke trial found that placebo-treated patients without resolution in one hour or improvement in three hours had only a 2 percent chance of resolving in 24 hours.26
Diagnostic Tests and Imaging
Figure 1 presents an algorithm for the diagnosis of acute stroke.2,4,11 Table 5 lists initial diagnostic studies recommended by current guidelines for patients with suspected stroke.11 These studies help exclude stroke mimics, uncover critical comorbidities (e.g., myocardial ischemia), and establish the safety of thrombolytic therapy.
Figure 1.
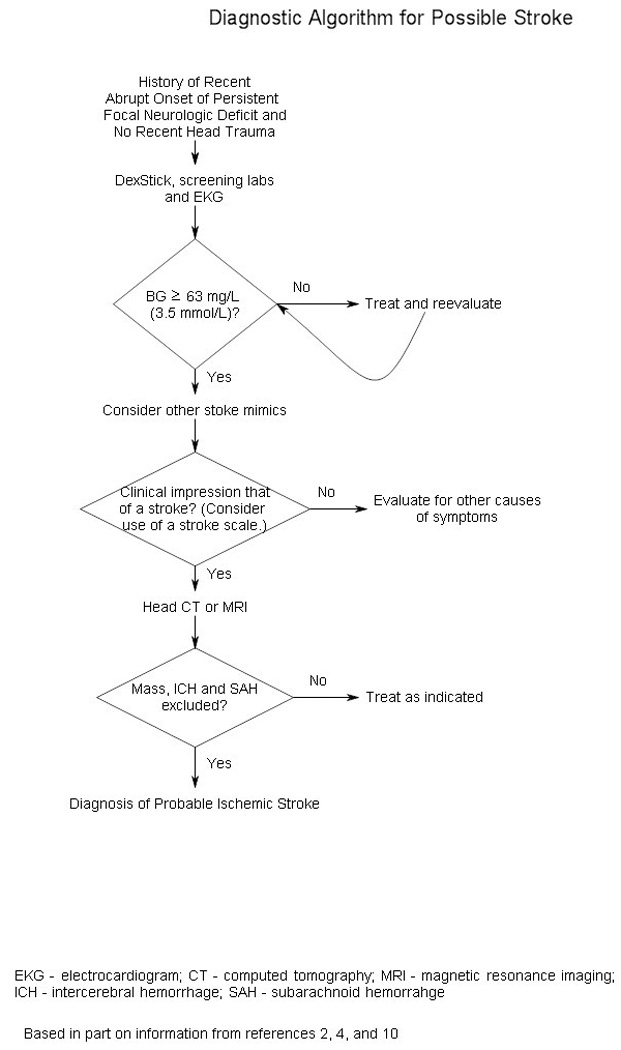
Algorithm for the diagnosis of acute stroke.
Table 5.
Immediate Diagnostic Studies: Evaluation of Suspected Acute Ischemic Stroke
| All patients |
|---|
| Noncontrast brain CT or brain MRI |
| Blood glucose |
| Serum electrolytes and renal function tests |
| Electrocardiograph |
| Markers of cardiac ischemia |
| Complete blood count, including platelet count* |
| Prothrombin time/international normalized ratio* |
| Activated partial thromboplastin time* |
| Oxygen saturation |
| Selected patients |
| Hepatic function tests |
| Toxicology screen |
| Blood alcohol level |
| Pregnancy test |
| Arterial blood gas (if hypoxemia suspected) |
| Chest radiography (if lung disease suspected) |
| Lumbar puncture (if subarachnoid hemorrhage suspected and head CT negative for blood) |
| Electroencephalogram (if seizure suspected) |
CT = computed tomography; MRI = magnetic resonance imaging.
Although it is desirable to know the results of these tests before giving recombinant tissue plasminogen activator, thrombolytic therapy should not be delayed while awaiting the results unless there is clinical suspicion of a bleeding abnormality or thrombocytopenia, the patient has received heparin or warfarin, or the use of anticoagulants is not known.
Adapted with permission from Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists [published corrections appear in Stroke. 2007;38(6):e38, and Stroke. 2007;38(9):e96]. Stroke. 2007;38(5):1665.
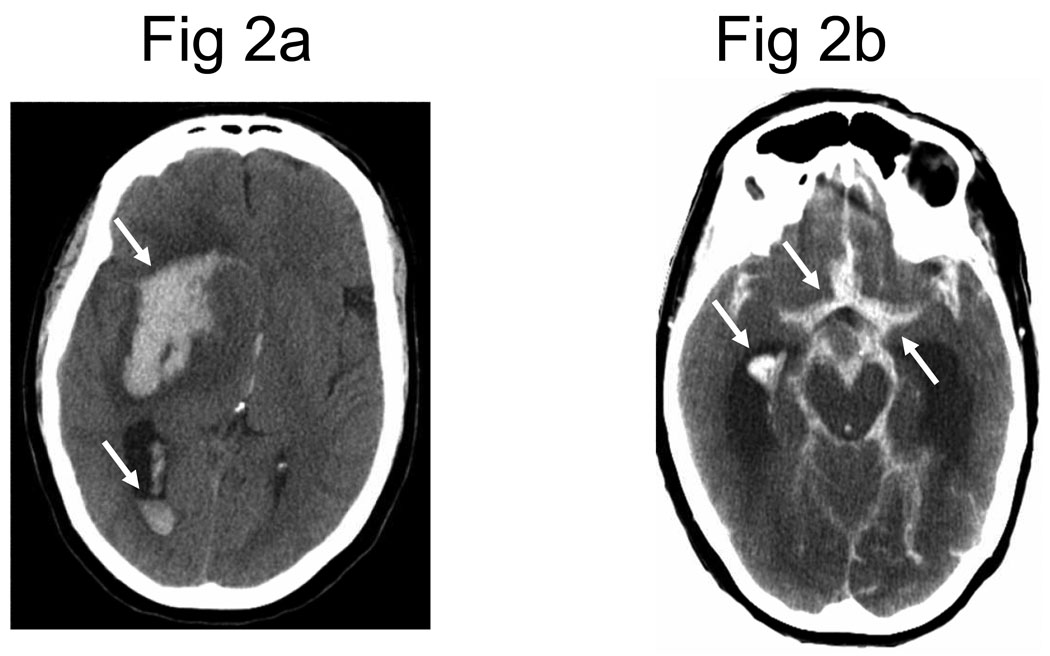
The primary purpose of neuroimaging in a patient with suspected ischemic stroke is to rule out the presence of other types of central nervous system lesions and to distinguish between ischemic and hemorrhagic stroke. Figure 2 shows examples of intracerebral hemorrhages on computed tomography (CT) scans. CT scans are considered sufficiently sensitive for detecting mass lesions, such as a brain mass or abscess, as well as detecting acute hemorrhage. However, CT scans may not be sensitive enough to detect an ischemic stroke, especially if it is small, acute, or in the posterior fossa (i.e., brainstem and cerebellum areas).27 The purpose of a CT scan is to rule out certain stroke mimics and detect hemorrhage, not necessarily to rule in the diagnosis of ischemic stroke. In other words, a normal CT scan does not rule out the diagnosis of ischemic stroke.
Figure 2.
Head computed tomography (CT) scans showing (A) an intracerebral hemorrhage and (B) subarachnoid hemorrhage. Note that acute hemorrhage appears hyperdense (white) on a CT scan.
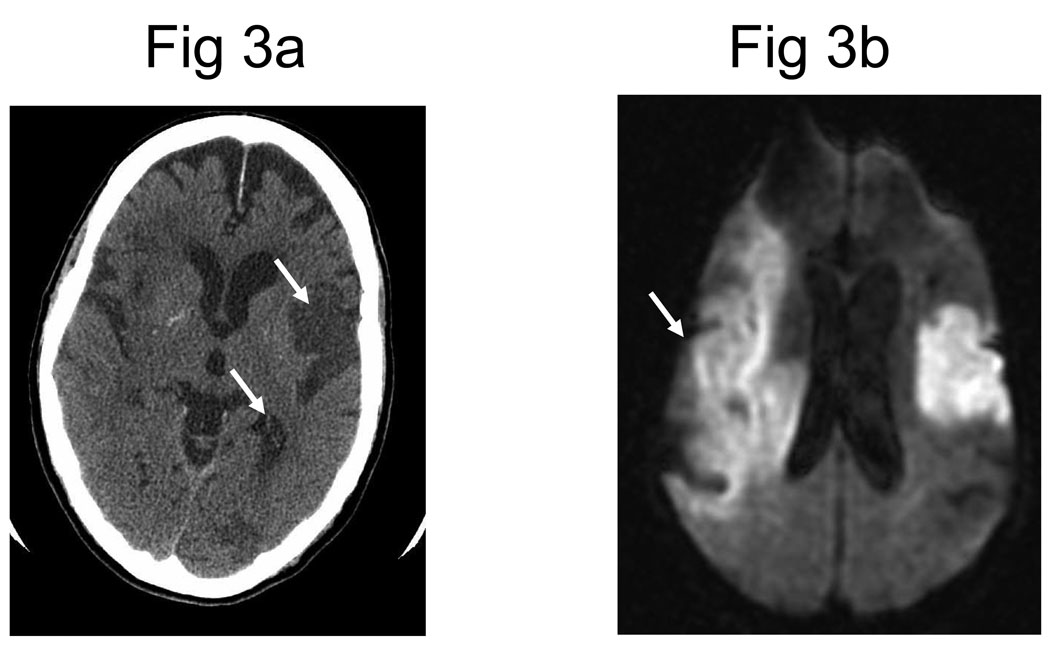
Multimodal magnetic resonance imaging (MRI) sequences, particularly diffusion-weighted imaging, have better resolution than CT; therefore, they have a greater sensitivity for detecting acute ischemic stroke28 and can diagnose about one half of all cases of TIA. Recent studies also indicate that MRI sequences (particularly gradient-recalled echo and diffusion-weighted imaging sequences) are as sensitive as CT scans for detecting intracerebral hemorrhagic stroke.29,30 Figure 3 shows the head CT and diffusion-weighted MRI images of a patient with a prior stroke and a new acute stroke. Figure 4 depicts the time course of resolution of ischemic changes on diffusion-weighted MRI.
Figure 3.
(A) Noncontrast computed tomography (CT) showing two hypodense regions indicating old infarctions in the distribution of the left-middle cerebral (arrow) and posterior cerebral arteries (arrow). (B) Diffusion-weighted magnetic resonance imaging obtained shortly after the CT reveals a new extensive infarction (arrow) in the right-middle cerebral artery distribution not evident on the CT.
Reprinted with permission from Image source Wilford Hall Medical Center, as archived in MedPix.
Figure 4.
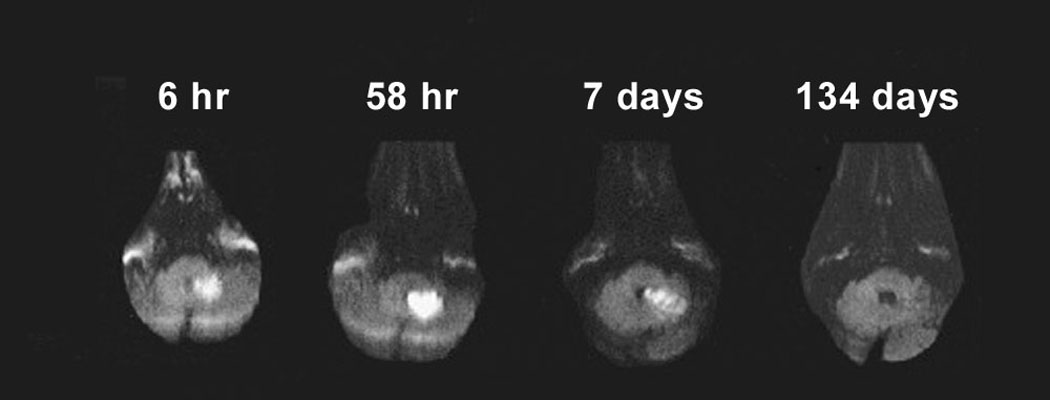
Time course of diffusion-weighted imaging abnormalities. Diffusion-weighted imaging can detect ischemic stroke in the very early period (six-hour image). Lesions typically reach maximum intensity three to five days after stroke onset (as shown in the 58-hour image), then typically fade over one to four weeks (abnormality still seen in seven-day image, but has disappeared in the 134-day image).
Although MRI scans have better resolution than CT scans, MRI scanners are less available and more expensive than CT scanners. Also, MRI scans cannot be performed on persons with certain types of implanted devices (e.g., pacemakers) or in persons with claustrophobia. If a patient is within the time window of acute stroke intervention, guidelines recommend that a MRI scan can be ordered if it can be obtained as quickly as a CT scan; if not, then CT is the recommended test because acute stroke treatments should not wait for detailed imaging when the history and physical is consistent for acute stroke.11 Table A compares CT and MRI in the setting of acute stroke.11,26 Guidelines recommend that whichever imaging modality is performed, it should be interpreted by a physician with expertise in reading brain imaging studies.
Table A.
Comparison of CT and MRI in the Setting of Acute Stroke
| Factors | CT | MRI |
|---|---|---|
| Availability | Typically more available and can be done more quickly than MRI; if patients are eligible for acute thrombolysis, CT is sufficient |
Typically less available and can be done less quickly than CT; if patients are eligible for acute thrombolysis, MRI should only be conducted if it is available as CT; treatment should not be delayed because of time needed to obtain an MRI |
| Resolution | Less resolution than MRI, but sufficient to assess for ischemic stroke mimics such as a mass, abscess, or hemorrhage (including subarachnoid); detects subacute strokes of at least moderate size |
Greater resolution than CT for all ischemic stroke mimics except for subarachnoid (not as well studied); diffusion-weighted imaging sequence detects acute, small strokes that may go undetected by CT, and can distinguish between acute and older strokes |
| Contraindications and risks | Scan uses radiography, so patients are exposed to radiation |
Contraindicated for anyone with metal in the body (e.g., pacemakers); may not be tolerated by persons with claustrophobia (motion in scanner degrades resolution) |
Unlike ischemic stroke and intracerebral hemorrhage, diagnosing subarachnoid hemorrhage requires a different diagnostic algorithm. The frequency of misdiagnosis for subarachnoid hemorrhage can be as high as 50 percent on initial presentation.13 Although MRI can detect subarachnoid hemorrhage, CT is still considered the imaging test of choice for persons suspected to have subarachnoid hemorrhage.31 CT scans have a 95 to 100 percent sensitivity of detecting subarachnoid blood in the first 12 hours; however, unlike ischemic stroke, sensitivity greatly decreases over time as the subarachnoid blood is cleared. The sensitivity of subarachnoid hemorrhage detection by CT drops to about 50 percent after one week, and is not detectable by CT after a period of about two to three weeks.13,31
Persons with suspected subarachnoid hemorrhage and a normal CT should undergo a lumbar puncture to detect bilirubin. Red blood cells can be found in a subarachnoid hemorrhage and a traumatic tap. Distinguishing between these two entities requires recognition that only within the human body do red blood cells break down into bilirubin. Red blood cells in cerebrospinal fluid collected from a traumatic tap will break down into oxyhemoglobin, but not into bilirubin. Because the breakdown of red blood cells can take up to 12 hours, guidelines recommend that the lumbar puncture should wait until 12 hours after the initial onset of symptoms.32 Bilirubin will turn fluid yellow (xanthochromia), but visual inspection alone is not considered sufficiently reliable.32 Therefore, all specimens should undergo spectrophotometry analysis to detect bilirubin, which can be detected as long as two weeks after the initial onset of symptoms. If subarachnoid hemorrhage is detected, the patient should immediately undergo angiography (CT angiography, MRI angiography, or catheter angiography) to look for an aneurysm.
Teaching Patients to Recognize Stroke Symptoms
Guidelines recommend that persons suffering an acute stroke activate the emergency medical system by calling 9-1-1. However, patients frequently do not activate the emergency medical system, or do not activate it immediately. Such behavior accounts for up to two thirds of the delay to hospital admission.33 Therefore, patients frequently present outside the time window for thrombolytic therapy. Patient and family sense of urgency for stroke symptoms is associated with greater use of emergency medical systems,34 which results in shorter times to evaluation and admission.35,36
Numerous surveys have shown that there is considerable room for improvement in knowledge of stroke in the general population. When persons are asked to answer “yes” or “no” as to whether a described symptom can be a sign of a stroke, they are correct about 60 to 80 percent of the time. However, when persons are asked open-ended questions to name stroke warning signs, most cannot name more than one warning sign.37
To improve public awareness of stroke warning signs, numerous organizations have embarked on public education campaigns. Family physicians are well placed to emphasize these messages in their practices.
Sort: Key Recommendations for Practice
| Clinical recommendation | Evidence rating |
References |
|---|---|---|
| Patients with an abrupt onset of a focal persistent neurologic deficit should be evaluated for stroke. |
C | 8, 9, 17, 19 |
| Stroke mimics should be excluded by history and diagnostic testing. |
C | 8, 11, 14, 20, 21, 23 |
| Diagnostic tools can aid in stroke diagnosis. | C | 8, 9 |
| All patients with stroke should have urgent neuroimaging with computed tomography or magnetic resonance imaging. |
C | 11 |
| Patients and family members should be educated about stroke symptoms and the need for urgent evaluation. |
C | 11 |
A = consistent, good-quality patient-oriented evidence; B = inconsistent or limited-quality patient-oriented evidence; C = consensus, disease-oriented evidence, usual practice, expert opinion, or case series. For information about the SORT evidence rating system, go to http://www.aafp.org/afpsort.xml.
Acknowledgments
The authors would like to thank Dr. Jack Tsao for providing Figure 2 and Dr. James Smirniotopoulos for his assistance with Figure 3.
Author disclosure: Dr. Cheng is supported by a career development award from The National Institute of Neurological Disorders and Stroke (K23NS058571).
Biographies
KENNETH S. YEW, CAPT, MC, USN, is an assistant professor in the department of family medicine at the Uniformed Services University of the Health Sciences in Bethesda, Md. Dr. Yew received his medical degree from Michigan State University College of Human Medicine, East Lansing. He completed an internal medicine internship at Naval Regional Medical Center, Oakland, Calif., a family medicine residency at Naval Hospital, Jacksonville, Fla., and a faculty development fellowship at the University of North Carolina, Chapel Hill.
ERIC CHENG, MD, MS, is an assistant professor in the department of neurology at the University of California, Los Angeles. Dr. Cheng received his medical degree from the University of Chicago (Ill.). He completed an internal medicine internship and a neurology residency at University of California, Los Angeles, where he also obtained a degree from the department of health services.
Footnotes
Reprints are not available from the authors.
The views expressed in this article are those of the authors and do not necessarily reflect the official position of the Department of the Navy, Department of Defense, Department of Veterans Affairs or the United States Government.
Patient information: A handout on stroke and transient ischemic attack, written by the authors of this article, is provided on page 000.
This clinical content conforms to AAFP criteria for evidence-based continuing medical education (EB CME).
The online version of this article includes supplemental content at http://aafp.org/afp.
Contributor Information
Kenneth S. Yew, Uniformed Services University of the Health Sciences, Bethesda, Maryland.
Eric Cheng, University of California, Los Angeles, Department of Neurology, Los Angeles, California.
REFERENCES
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. Medline. [DOI] [PubMed] [Google Scholar]
- 3.Kumar S, Caplan LR. Why identification of stroke syndromes is still important. Curr Opin Neurol. 2007;20(1):78–82. doi: 10.1097/WCO.0b013e328013e964. Medline. [DOI] [PubMed] [Google Scholar]
- 4.Hand PJ, Haisma JA, Kwan J, et al. Interobserver agreement for the bedside clinical assessment of suspected stroke. Stroke. 2006;37(3):776–780. doi: 10.1161/01.STR.0000204042.41695.a1. Medline. [DOI] [PubMed] [Google Scholar]
- 5.Kraaijeveld CL, van Gijn J, Schouten HJ, Staal A. Interobserver agreement for the diagnosis of transient ischemic attacks. Stroke. 1984;15(4):723–725. doi: 10.1161/01.str.15.4.723. [DOI] [PubMed] [Google Scholar]
- 6.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337(8756):1521–1526. doi: 10.1016/0140-6736(91)93206-o. Medline. [DOI] [PubMed] [Google Scholar]
- 7.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369(9558):306–318. doi: 10.1016/S0140-6736(07)60153-6. Medline. [DOI] [PubMed] [Google Scholar]
- 8.Nor AM, Davis J, Sen B, et al. The Recognition of Stroke in the Emergency Room (ROSIER) scale: development and validation of a stroke recognition instrument. Lancet Neurol. 2005;4(11):727–734. doi: 10.1016/S1474-4422(05)70201-5. Medline. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein LB, Simel DL. Is this patient having a stroke? JAMA. 2005;293(19):2391–2402. doi: 10.1001/jama.293.19.2391. Medline. [DOI] [PubMed] [Google Scholar]
- 10.Morgenstern LB, Lisabeth LD, Mecozzi AC, et al. A population-based study of acute stroke and TIA diagnosis. Neurology. 2004;62(6):895–900. doi: 10.1212/01.wnl.0000115103.49326.5e. Medline. [DOI] [PubMed] [Google Scholar]
- 11.Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists [published corrections appear in Stroke. 2007;38(6):e38, and Stroke. 2007;38(9):e96] Stroke. 2007;38(5):1655–1711. doi: 10.1161/STROKEAHA.107.181486. Medline. [DOI] [PubMed] [Google Scholar]
- 12.National Institute of Neurological Disorders and Stroke. NIH Stroke Scale. [Accessed February 13, 2009];2003 http://www.ninds.nih.gov/doctors/NIH_Stroke_Scale.pdf.
- 13.Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354(4):387–396. doi: 10.1056/NEJMra052732. Medline. [DOI] [PubMed] [Google Scholar]
- 14.Hand PJ, Kwan J, Lindley RI, Dennis MS, Wardlaw JM. Distinguishing between stroke and mimic at the bedside: the brain attack study. Stroke. 2006;37(3):769–775. doi: 10.1161/01.STR.0000204041.13466.4c. Medline. [DOI] [PubMed] [Google Scholar]
- 15.Kothari RU, Pancioli A, Liu T, Brott T, Broderick J. Cincinnati Prehospital Stroke Scale: reproducibility and validity. Ann Emerg Med. 1999;33(4):373–378. doi: 10.1016/s0196-0644(99)70299-4. Medline. [DOI] [PubMed] [Google Scholar]
- 16.Harbison J, Hossain O, Jenkinson D, Davis J, Louw SJ, Ford GA. Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke. 2003;34(1):71–76. doi: 10.1161/01.str.0000044170.46643.5e. Medline. [DOI] [PubMed] [Google Scholar]
- 17.Kidwell CS, Starkman S, Eckstein M, Weems K, Saver JL. Identifying stroke in the field. Prospective validation of the Los Angeles prehospital stroke screen (LAPSS) Stroke. 2000;31(1):71–76. doi: 10.1161/01.str.31.1.71. Medline. [DOI] [PubMed] [Google Scholar]
- 18.Bray JE, Martin J, Cooper G, Barger B, Bernard S, Bladin C. Paramedic identification of stroke: community validation of the melbourne ambulance stroke screen. Cerebrovasc Dis. 2005;20(1):28–33. doi: 10.1159/000086201. Medline. [DOI] [PubMed] [Google Scholar]
- 19.von Arbin M, Britton M, de Faire U, Helmers C, Miah K, Murray V. Validation of admission criteria to a stroke unit. J Chronic Dis. 1980;33(4):215–220. doi: 10.1016/0021-9681(80)90066-1. Medline. [DOI] [PubMed] [Google Scholar]
- 20.Libman RB, Wirkowski E, Alvir J, Rao TH. Conditions that mimic stroke in the emergency department. Implications for acute stroke trials. Arch Neurol. 1995;52(11):1119–1122. doi: 10.1001/archneur.1995.00540350113023. Medline. [DOI] [PubMed] [Google Scholar]
- 21.Hemmen TM, Meyer BC, McClean TL, Lyden PD. Identification of nonischemic stroke mimics among 411 code strokes at the University of California, San Diego, Stroke Center. J Stroke Cerebrovasc Dis. 2008;17(1):23–25. doi: 10.1016/j.jstrokecerebrovasdis.2007.09.008. Medline. [DOI] [PubMed] [Google Scholar]
- 22.Qureshi AI, Ezzeddine MA, Nasar A, et al. Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med. 2007;25(1):32–38. doi: 10.1016/j.ajem.2006.07.008. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerber KA, Brown DL, Lisabeth LD, Smith MA, Morgenstern LB. Stroke among patients with dizziness, vertigo, and imbalance in the emergency department: a population-based study. Stroke. 2006;37(10):2484–2487. doi: 10.1161/01.STR.0000240329.48263.0d. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nazir FS, Lees KR, Bone I. Clinical features associated with medically unexplained stroke-like symptoms presenting to an acute stroke unit. Eur J Neurol. 2005;12(2):81–85. doi: 10.1111/j.1468-1331.2004.01010.x. Medline. [DOI] [PubMed] [Google Scholar]
- 25.Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack–proposal for a new definition. N Engl J Med. 2002;347(21):1713–1716. doi: 10.1056/NEJMsb020987. Medline. [DOI] [PubMed] [Google Scholar]
- 26.Marler JR, Tilley BC, Lu M, et al. Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology. 2000;55(11):1649–1655. doi: 10.1212/wnl.55.11.1649. Medline. [DOI] [PubMed] [Google Scholar]
- 27.Mullins ME, Schaefer PW, Sorensen AG, et al. CT and conventional and diffusion-weighted MR imaging in acute stroke: study in 691 patients at presentation to the emergency department. Radiology. 2002;224(2):353–360. doi: 10.1148/radiol.2242010873. Medline. [DOI] [PubMed] [Google Scholar]
- 28.Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology. 2000;217(2):331–345. doi: 10.1148/radiology.217.2.r00nv24331. Medline. [DOI] [PubMed] [Google Scholar]
- 29.Kidwell CS, Chalela JA, Saver JL, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2004;292(15):1823–1830. doi: 10.1001/jama.292.15.1823. Medline. [DOI] [PubMed] [Google Scholar]
- 30.Fiebach JB, Schellinger PD, Gass A, et al. Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging. Stroke. 2004;35(2):502–506. doi: 10.1161/01.STR.0000114203.75678.88. Medline. [DOI] [PubMed] [Google Scholar]
- 31.Masdeu JC, Irimia P, Asenbaum S, et al. EFNS guideline on neuroimaging in acute stroke. Report of an EFNS task force. Eur J Neurol. 2006;13(12):1271–1283. doi: 10.1111/j.1468-1331.2006.01507.x. Medline. [DOI] [PubMed] [Google Scholar]
- 32.Cruickshank A, Auld P, Beetham R, et al. Revised national guidelines for analysis of cerebrospinal fluid for bilirubin in suspected subarachnoid haemorrhage. Ann Clin Biochem. 2008;45(pt 3):238–244. doi: 10.1258/acb.2008.007257. Medline. [DOI] [PubMed] [Google Scholar]
- 33.Wester P, Radberg J, Lundgren B, Peltonen M. Factors associated with delayed admission to hospital and in-hospital delays in acute stroke and TIA: a prospective, multicenter study.Seek-Medical-Attention-in-Time Study Group. Stroke. 1999;30(1):40–48. doi: 10.1161/01.str.30.1.40. Medline. [DOI] [PubMed] [Google Scholar]
- 34.Schroeder EB, Rosamond WD, Morris DL, Evenson KR, Hinn AR. Determinants of use of emergency medical services in a population with stroke symptoms: the Second Delay in Accessing Stroke Healthcare (DASH II) Study. Stroke. 2000;31(11):2591–2596. doi: 10.1161/01.str.31.11.2591. Medline. [DOI] [PubMed] [Google Scholar]
- 35.Agyeman O, Nedeltchev K, Arnold M, et al. Time to admission in acute ischemic stroke and transient ischemic attack. Stroke. 2006;37(4):963–966. doi: 10.1161/01.STR.0000206546.76860.6b. Medline. [DOI] [PubMed] [Google Scholar]
- 36.Rossnagel K, Jungehulsing GJ, Nolte CH, et al. Out-of-hospital delays in patients with acute stroke. Ann Emerg Med. 2004;44(5):476–483. doi: 10.1016/j.annemergmed.2004.06.019. Medline. [DOI] [PubMed] [Google Scholar]
- 37.Nicol MB, Thrift AG. Knowledge of risk factors and warning signs of stroke. Vasc Health Risk Manag. 2005;1(2):137–147. doi: 10.2147/vhrm.1.2.137.64085. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A.Scott PA, Silbergleit R. Misdiagnosis of stroke in tissue plasminogen activator-treated patients: characteristics and outcomes. Ann Emerg Med. 2003;42(5):611–618. doi: 10.1016/s0196-0644(03)00443-8. Medline. [DOI] [PubMed] [Google Scholar]