Abstract
Azaspiracids are a class of recently discovered algae-derived shellfish toxins. Their distribution globally is on the increase with mussels being most widely implicated in azaspiracid-related food poisoning events. Evidence that these toxins were bound to proteins in contaminated mussels has been shown recently. In the present study characterization of these proteins in blue mussels, Mytilus edulis, was achieved using a range of advanced proteomics tools. Four proteins present only in the hepatopancreas of toxin-contaminated mussels sharing identity or homology with cathepsin D, superoxide dismutase, glutathione S-transferase Pi, and a bacterial flagellar protein have been characterized. Several of the proteins are known to be involved in self-defense mechanisms against xenobiotics or up-regulated in the presence of carcinogenic agents. These findings would suggest that azaspiracids should now be considered and evaluated as potential tumorigenic compounds. The presence of a bacterial protein only in contaminated mussels was an unexpected finding and requires further investigation. The proteins identified in this study should assist with development of urgently required processes for the rapid depuration of azaspiracid-contaminated shellfish. Moreover they may serve as early warning indicators of shellfish exposed to this family of toxins.
Azaspiracids (AZAs)1 are a group of recently discovered algae-derived toxins following a shellfish poisoning event in 1995 in The Netherlands from consumption of Irish mussels (Mytilus edulis) (1). Initially the dinoflagellate Protoperidinium crassipes was proposed to be the organism producing AZAs (2); however, recent research has identified a new dinoflagellate, provisionally designated strain 3D9, as the source (3). Since the first AZA poisoning event in 1995 AZA incidents have been widely reported throughout Europe (4–6) and more recently in Morocco and eastern Canada (7, 8). AZA distribution thus appears to be on the increase and has become a public health concern and poses severe problems for the aquaculture industry. A regulatory limit of 160 μg of AZA/kg of shellfish in flesh has been proposed (9, 10) by the European Commission based on current information relating to the risks of consumption of contaminated shellfish.
The most widely implicated species in AZA-associated food poisoning is mussels (7, 11). The blue mussel, M. edulis, has been widely used as a sentinel species for monitoring coastal environments and environmental pollution (12–14). Thus the recent appearance of AZAs could be considered as an indication of environmental changes that we do not as yet understand. A number of biochemical markers are known to be a good guide of the level of environmental stress to which living organisms have been subjected. It is also recognized that mussels produce proteins that can act as biomarkers to environmental contamination. Proliferating cell nuclear antigen and multixenobiotic resistance polyglycoprotein were revealed as biomarkers for genotoxic stress derived from benzo[a]pyrene in Baltic Sea blue mussels (15). Cu,Zn-superoxide dismutase (SOD), GSTs, and catalase are also well established biomarkers for the assessment of environmental stress in mussels following organic pollution and heavy metal exposure (16–21).
Proteomics has proven to be a powerful technique for characterizing proteins expressed in specific tissues for many factors ranging from species differences to exposure to stress. For instance, López et al. (22) used proteomics to expand their understanding of the molecular differentiation between the mussels M. edulis and Mytilus galloprovincialis, whereas Apraiz et al. (23) identified the proteomic signatures in mussels exposed to marine pollutants.
In the current study a range of advanced proteomics tools was used to further study the different protein profiles we recently observed between AZA-contaminated and non-contaminated mussels (24). Their identification and characterization may provide information toward identifying the mode of action of the toxins, which is currently unknown, and provide an indication as to why the AZA phenomenon has arisen so recently. If as recently suggested (24) prolonged AZA retention in shellfish is due to their association with proteins, then suitable processes could be developed to speed up the unusually low rates of depuration, which can take up to 8 months (25). A further important rationale for the work would be the identification of biomarkers that may serve as early warning indicators of AZA contamination in shellfish.
MATERIALS AND METHODS
Reagents and Consumables
All solvents used in this study of HPLC or analytical grade quality were obtained from Sigma-Aldrich. Bovine serum albumin, trypsin, Triton® X-100 (polyethylene glycol tert-octylphenyl ether), and protease inhibitor were also purchased from Sigma-Aldrich; Tris base, glycine, disodium hydrogen orthophosphate 12-hydrate, and sodium dihydrogen orthophosphate anhydrous were sourced from BDH. Water was deionized and purified using an Elga Purelab system. The protein assay kit and (Precision Plus ProteinTM) KaleidoscopeTM prestained standards were purchased from Bio-Rad. Novex® 4–20% Tris-glycine gels, Novex native Tris-glycine sample buffer, See Blue® Plus 2, SimplyBlueTM SafeStain, NuPAGE® 10% bis-Tris gels, NuPAGE sample reducing agent, NuPAGE LDS sample buffer, NuPAGE antioxidant, and MOPS-SDS running buffer were provided by Invitrogen. AZA-1 and AZA-2 standards had been purified previously from the same mussels that were used for this study (26).
Mussel Sampling
Mussels belonging to the species M. edulis were collected in 2006 off the north coast of Ireland during a toxin outbreak. The samples were transported to the laboratory within 12 h of harvesting and immediately stored at −20 °C until analysis. These marine bivalve molluscs were found to contain AZA-1, AZA-2, and AZA-3 by LC-MS/MS (24). Digestive glands dissected from the mussels were maintained at −20 °C prior to further extraction and analysis.
Sample Preparation
For the current study, extracts were freshly prepared from frozen stored intact mussel samples. AZA toxins and proteins were co-extracted from homogenized mussel digestive glands (10 g). Briefly the digestive glands were successively blended with 5 ml of water containing protease inhibitor (six times), water containing protease inhibitor and 1% Triton X-100 (three times), and water containing protease inhibitor and 10% propanol-2 (twice). Combined supernatants were further subjected to IEF via the Rotofor® preparative cell, and size exclusion chromatography (SEC) was performed on a BioSep-SEC-S 2000 polyetheretherketone column (300 × 7.50 mm; Phenomenex, Macclesfield, Cheshire, UK) using 0.1 m sodium phosphate buffer at pH 6.8 as the mobile phase. SEC pools were labeled A, B, and C and were kept at −80 °C prior to gel electrophoresis. As in the 2008 study (24), by mass spectrometry pool B was found to contain the highest levels of AZAs.
Protein Concentration
The protein content of each pool (A, B, and C) was determined with a protein assay kit (Bio-Rad) using bovine serum albumin as standard.
Native PAGE
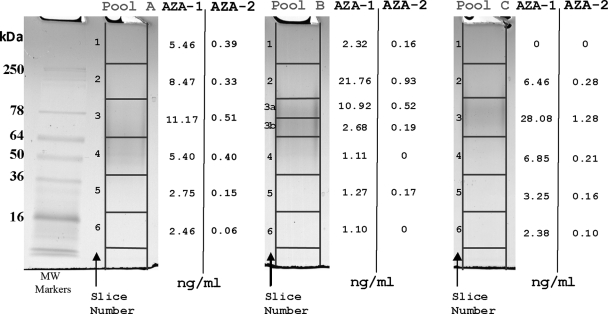
Samples from pools A, B, and C were thawed, Vortex-mixed, and run on two Novex 4–20% Tris-glycine gels simultaneously. Samples A, B, and C (40 μl; containing 9.5, 13.7, and 6.2 μg of protein, respectively) were mixed with Novex native Tris-glycine sample buffer (20 μl) and loaded onto each gel cassette. An aliquot (10 μl) of See Blue Plus 2 was used as a molecular weight marker on each gel. Gels were bathed in 25 mm Tris base and 192 mm glycine buffer (pH 8.3). Proteins were resolved at a constant voltage of 150 V until the dye front reached the bottom of the gel (∼1.5h). Following electrophoresis, gels were removed from the cassettes, and one was stained with SimplyBlue SafeStain. Stained gel bands were scanned using a GS-800 densitometer (Bio-Rad), and this image was used as a template to locate protein bands on the unstained gel. Both stained and unstained gels were sliced (see Fig. 1), and the stained slices were placed in Eppendorf tubes and stored at 4 °C. Unstained gel slices were placed in Eppendorf tubes containing methanol (150 μl) to dissolve any AZA present. To aid this extraction, gel slices were pierced with a clean needle, Vortex-mixed, and kept overnight at 4 °C. The methanol extracts were then subjected to LC-MS/MS analysis to determine the location in the gel with the highest density of AZA toxins. Based on this information, protein bands in slices 2, 3a, and 3b from pool B of the stained gel were excised and digested (see “In-gel Digestion”) for analysis by nano-LC-ESI-MS/MS.
Fig. 1.
AZA-1 and AZA-2 distribution in native gel slices. The gel was cut into 1-cm squares except for slice 3 in Pool B that was further cut in two. Slices B2 and B3a were further subjected to in-gel tryptic digest followed by nano-LC-ESI-MS/MS for protein identification.
SDS-PAGE
Samples from pools A, B, and C were also subjected to gel electrophoresis under denaturing conditions. All samples were heated for 5 min at 95 °C, Vortex-mixed, and applied to NuPAGE 10% bis-Tris gels. Following this, two further gels were run simultaneously under reducing and non-reducing conditions. Both gels were prerun for 5 min before sample loading. An aliquot of sample from pool B (65 μl containing 22.3 μg of protein) was mixed with NuPAGE LDS sample buffer (25 μl) and NuPAGE sample reducing agent or double distilled water (10 μl) and loaded onto each gel cassette. Samples from pools A and C (22 μl containing 5.2 and 3.4 μg of protein, respectively) were mixed with NuPAGE LDS sample buffer (8 μl) and NuPAGE Sample reducing agent or double distilled water (3 μl) and loaded onto the cassette. An aliquot (10 μl) of (Precision Plus ProteinTM) Kaleidoscope prestained standards was used as a molecular weight marker on each gel. Gels were bathed in MOPS-SDS running buffer, and NuPAGE antioxidant (500 μl) was added to the top electrode chamber. Protein resolution and staining of gels were carried out as described earlier. Following electrophoresis gels were removed from cassettes, and protein bands of interest were excised, placed into Eppendorf tubes, and stored at 4 °C prior to in-gel digestion and mass spectrometric analysis for protein identification. Protein bands were analyzed by MALDI-TOF/TOF and nano-LC-ESI-MS/MS.
In-gel Digestion
The gel bands were excised and cut into 1-cm portions. These were then subjected to in-gel digestion using a ProGest Investigator in-gel digestion robot (Genomic Solutions, Ann Arbor, MI) using standard protocols (27). Briefly the gel cubes were destained by washing with acetonitrile and subjected to reduction and alkylation before digestion with trypsin at 37 °C. For ESI-MS/MS analyses, the peptides were extracted with 10% formic acid and concentrated (to 20 μl) using a SpeedVac (ThermoSavant). In the case of MALDI-TOF/TOF analyses, the digest solution (0.5 μl) was applied to the MALDI target along with α-cyano-4-hydroxycinnamic acid matrix (0.5 μl; 10 mg/ml in 50:50 acetonitrile, 0.1% TFA) and allowed to dry.
MS for AZA Monitoring
LC-MS/MS was used to assess AZA concentrations in this study. MS was carried out using ESI in positive ion mode, and monitoring transitions were: 842.4 → 672.3, 856.4 → 672.3, and 828.6 → 658.6 for AZA-1, AZA-2, and AZA-3, respectively. Full details of the technique are provided in Nzoughet et al. (24).
Mass Spectrometry for Protein Identification
Proteins were identified by peptide mass fingerprinting and MS/MS analysis (using two different mass spectrometry applications: nano-LC-ESI-MS/MS and MALDI-TOF/TOF).
Nano-LC-ESI-MS/MS
For ESI-MS/MS applications, resulting samples from in-gel digestion were separated using an UltiMate nanoLC system (LC Packings, Amsterdam, The Netherlands) equipped with a PepMap C18 trap and column using a 60-min gradient of increasing acetonitrile concentration consisting of 0.1% formic acid (5–35% acetonitrile in 35 min, respectively, and 35–50% in a further 20 min followed by 95% acetonitrile to clean the column). The eluent was sprayed into a QSTAR Pulsar XL tandem mass spectrometer (Applied Biosystems, Foster City, CA) and analyzed in information-dependent acquisition mode, performing 1 s of MS followed by 3-s MS/MS analyses of the two most intense peaks seen by MS. These masses were then excluded from analysis for the next 60 s. MS/MS data for doubly and triply charged precursor ions were converted to centroid data without smoothing using the Analyst QS1.1 mascot.dll data import filter with default settings. The data were also subjected to MSBLAST searches using the Paracel and EMBL search algorithms (ProBLAST, Applied Biosystems).
MALDI-TOF/TOF
Samples from in-gel digestion were also processed by MALDI-TOF/TOF. MALDI MS was acquired using a 4800 MALDI TOF/TOF Analyzer (Applied Biosystems) equipped with a neodymium-doped yttrium aluminium garnet (Nd:YAG) 355 nm laser and calibrated using a mixture of peptides. The most intense peptides (up to 15) were selected for MS/MS analysis, and the combined MS and MS/MS data were analyzed using Global Proteome Server Explorer (Applied Biosystems).
De Novo Sequencing
De novo sequencing followed the same procedure as for the in-gel digestion and the MS and MS/MS analysis. However, instead of submitting them to a search program, MS/MS spectra were analyzed and manually interpreted in the case of the ESI data with the help of the BioAnalyst 1.1 software (Applied Biosystems) and used for similarities searches.
Database Searches
Peak lists were submitted to the Mascot search algorithm (Matrix Science, London, UK) for identification of the corresponding protein. The MS/MS peak lists (nano-LC-ESI MS/MS) generated were analyzed using the Mascot 2.1 search engine against the UniRef100 database (January 2008) containing 5,241,852 sequences, NCBInr database (January 2008) containing 5,869,058 sequences, and mass spectrometry-driven BLAST database (MSBLAST EMBL with default databases nrdb95 and MSBLAST Paracel with UniRef100). No species restriction was applied. The data were searched with tolerances of 0.2 Da for the precursor and fragment ions, trypsin as the cleavage enzyme, one missed cleavage, carbamidomethyl modification of cysteines as a fixed modification, and methionine oxidation selected as a variable modification. The combined MS and MS/MS data from MALDI-TOF/TOF application was analyzed using Global Proteome Server Explorer (Applied Biosystems) to interface with the Mascot 2.2 search engine (Matrix Science) against the UniRef100 database (January 2008) and NCBInr database (January 2008). No species restriction was applied. The data were searched with tolerances of 100 ppm for the precursor ions and 0.5 Da for the fragment ions, trypsin as the cleavage enzyme, assuming up to one missed cleavage, carbamidomethyl modification of cysteines as a fixed modification, and methionine oxidation selected as a variable modification. Identifications were accepted if they included a peptide ion score above the Mascot identity threshold (95% confidence).
De novo assigned sequences were used for similarity searches using the BLASTP algorithm, protein-protein BLAST (basic local alignment search tool) (28) by on-line submissions. Searches were performed against the NCBI/BLAST non-redundant protein sequence database (nrdb) with BLOSUM 62 as the search matrix and carried out using both standard settings and entering the organism name, M. edulis.
RESULTS
Mussels used in the study collected off the north coast of Ireland were naturally contaminated with AZA toxins. The digestive glands were found to contain 33.23 and 3.63 μg/g AZA-1 and AZA-2, respectively. This AZA concentration was very high, and if it were conservatively estimated that the glands only accounted for ∼10% of total organism weight this would be well above the European Commission threshold of 0.16 μg/g for AZAs. AZA3 was also detected in the samples by monitoring the appropriate transition, 828.6 → 658.6. However, because an analytical standard for this AZA analogue was not available it was not determined quantitatively.
AZA toxins and proteins were extracted from mussel contaminated digestive glands, and the resulting extract was examined by IEF and SEC as reported previously (24). Again as in our earlier study (24), SEC fractions identified as containing the highest concentrations of toxins were pooled (pools A, B, and C) and retained for further investigation. Pools A, B, and C were found to contain 3.0, 5.2, and 2.9 μg/ml AZA-1 and 0.2, 0.4, and 0.2 μg/ml AZA-2, respectively. Pool B, which had the highest toxin concentration, was selected for further investigation.
Detection of AZAs in Native PAGE Slices
Substantial quantities of AZA toxins in a number of the gel slices (notably A2, A3, B2, B3a, and C3) were detected (Fig. 1). The surprising detection of AZAs under native conditions gave a strong indication that they were coupled to, or somehow associated with, the proteins present in these fractions.
Protein Identification in Native PAGE Slices
Proteins in slices B2 and B3a were subjected to in-gel digestion and characterized by MS/MS analysis using an ESI-MS/MS analyzer. These data indicated that blue mussel SOD, bacteria murein lipoprotein (major outer membrane lipoprotein precursor), and a protein similar to cathepsin D by homology to Apis mellifera were the major proteins present (Table I).
Table I. Azaspiracid-up-regulated proteins from pool B separated by non-denaturing PAGE.
Underlined amino acids, identical amino acids found in the assigned protein; Oxid, oxidized methionine. The periods denote the site of trypsin cleavage.
| Assignment | Mascot score/number of unique peptides above homology threshold | Peptide sequence identified above homology cutoff and precursor ion mass and charge state for single peptide identifications | Ion scorea | Homology to protein | Method of identification |
|---|---|---|---|---|---|
| Slice B2 | |||||
| SOD | 108/2 | R.LACGVIGISKV.- | 53 | gi 34481600 (M. edulis)b | Nano-LC-ESI-MS/MS |
| K.LSLTGPQSIIGR.T | 55 | ||||
| Murein lipoprotein (major outer membrane lipoprotein) | 59/1 | K.IDQLSSDVQTLNAK.V | 59 | gi 127525b | Nano-LC-ESI-MS/MS |
| 766.39, 2+ | |||||
| Slice B3a | |||||
| SOD | 238/3 | R.LACGVIGISKV.- | 55 | gi 34481600 (M. edulis)b | Nano-LC-ESI-MS/MS |
| K.LSLTGPQSIIGR.T | 67 | ||||
| R.TVVVHADIDDLGK.G | 70 | ||||
| Cathepsin D | 55/2 | K. ISVDGVTPVFFYNMVK.Q | 55 | gi 66560290 (A. mellifera)b | Nano-LC-ESI-MS/MS |
| 834.93, 2+ | |||||
| K. ISVDGVTPVFFYNMVK.Q | 42 | ||||
| Oxid, 842.93, 2+ |
a Score greater than 51 indicates identity, and score greater than 32 indicates homology.
b NCBInr database.
Protein Identification in SDS-PAGE Protein Bands
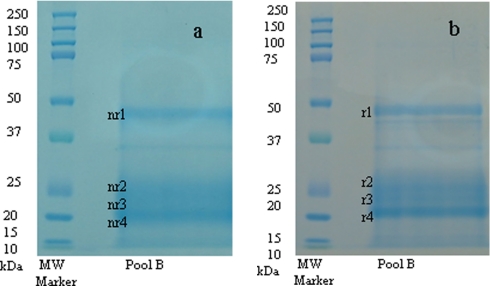
Pools A, B, and C were subjected to SDS-PAGE under both reducing and non-reducing conditions. Data are only shown for pool B and are presented in Fig. 2. SDS-PAGE under both conditions showed expression of the same four major protein bands with apparent molecular masses of 46, 26, 24, and 21 kDa, as observed previously (24). These bands were consecutively named band r1 to r4 and nr1 to nr4 for reduced and non-reduced conditions, respectively. Their identification was accomplished by use of either MALDI-TOF/TOF or nano-LC-ESI-MS/MS of peptides produced by proteolytic digestion of bands excised from the gels (Table II).
Fig. 2.
SDS-PAGE performed under non-reducing (a) and reducing (b) conditions. Shown from left to right are: lane 1, molecular mass marker; and lane 2, pool B. Bands nr/r1, nr/r2, nr/r3, and nr/r4 were further subjected to in-gel tryptic digest followed by either MALDI-MS/MS or nano-LC-ESI-MS/MS analyses for protein identification.
Table II. Azaspiracid-up-regulated proteins from Pool B separated by denaturing SDS-PAGE.
Underlined amino acids, identical amino acids found in the assigned protein; Oxid, oxidized methionine. The periods denote the site of trypsin cleavage.
| Assignment | Mascot score/number of unique peptides above homology threshold | Peptide sequence identified above homology cutoff and precursor ion mass and charge state, for single peptide identifications | Ion scorea or E value | Homology to protein | Method of identification |
|---|---|---|---|---|---|
| Band nr1 | |||||
| Cathepsin D | 68/1 | K.EGGELILGGSDPK.H | 68a | gi 46309251 (T. pacificus)b | Nano-LC-ESI-MS/MS |
| 636.32, 2+ | |||||
| 60/2 | K.ISVDGVTPVFFYNMVK.Q | 60a | gi 66560290 (A. mellifera)b | ||
| 834.92, 2+ | |||||
| K.ISVDGVTPVFFYNMVK.Q | 40a | ||||
| Oxid, 842.93, 2+ | |||||
| p53 family proteins (DeltaN p63/p73-like protein, p63/p73 like protein, p53 tumor suppressor like-protein) | KKMLLVDER | 6.7c | gb AAZ05996.1d | MALDI-TOF/TOF de novo | |
| novo | |||||
| FKGLLDFTDNTPR | 7.3c | gb AAZ05995.1d | ESI-MS/MS de novo | ||
| QVSDSATSTAVVEGR | 9.7c | gb AAT72301.1 (blue mussel M. edulis)d | |||
| Band nr2 | |||||
| SOD | 71/1 | K.LSLTGPGSIIGR.T | 43a | gi 34481600 (blue mussel M. edulis)b | Nano-LC-ESI-MS/MS |
| 621.37, 2+ | |||||
| Band r3 | |||||
| Glutathione S-transferase Pi | LALLTDWPTDLEATR | 13c | gb AAS60226.1 (blue mussel M. edulis)d | ESI-MS/MS de novo | |
| Multidrug resistance-associated protein | NLVELYNDDVDGK | 0.41c | gb AAK84397.1d | ESI-MS/MS de novo | |
| YQWEGGDTRQK | 18c | gb AAK84398 (blue mussel M. edulis)d | |||
| Band r4 | |||||
| Flagellar P-ring protein precursor | AALMVTADLPPYTPPG | 68% identity | A. tumefaciens, Swiss-Prot Q44340 FLGI_AGRT5e | Nano-LC-ESI-MS/MS | |
| CVTADLPPYPAPGK | 40c | M. populi, Ref YP_001923314.1d | ESI-MS/MS de novo | ||
| CVTADLPPYPA | 45c | ||||
| Lysozyme | TLLNDYSAGK | 5.5c | gb AAN16207.1 (blue mussel M. edulis)d | ESI-MS/MS de novo | |
| YGSFWDGHQVK | 13c |
a Ion score. Score greater than 51 indicates identity, and score greater than 30 indicates homology.
b NCBInr database.
c E value.
d BLAST.
e MSBLAST EMBL database.
Protein Identification from ESI-MS/MS Spectra
ESI-MS/MS analysis was used to identify the protein present in band nr1 (Fig. 2 and Table II). Multiple hits were found for cathepsin D by homology to A. mellifera and Todarodes pacificus (Japanese fly) from the NCBInr and UniRef100 databases. This finding was consistent with the result obtained for native gel slice B3a (Table I).
Protein nr2 (Fig. 2 and Table II) was revealed by MS/MS analysis as being consistent with blue mussel SOD, although the individual scores are not above the identity threshold. One peptide found, using the NCBInr database, to share homology with mussel SOD was identical to that found in native gel slices B2 and B3a (Table I).
Protein band r4 (Fig. 2 and Table II) shows some homology to flagellar P-ring protein precursor (basal body P-ring protein) from Agrobacterium tumefaciens based on nano-ESI-MS/MS analysis followed by MSBLAST EMBL database identification. This assignment was also confirmed by de novo peptide sequencing. A. tumefaciens is a soil bacterium that causes tumors in plants (29, 30), and flagellar biosynthesis has been studied most thoroughly in Escherichia coli K-12 and Salmonella enterica serovar Typhimurium LT2.
De Novo Sequencing from ESI-MS/MS and MALDI-TOF/TOF Spectra
In addition to Mascot searches, de novo sequencing was also performed to mine more deeply into the mass spectra as the species of interest are so poorly represented in the protein databases. MS/MS spectra were interpreted manually using BioAnalyst in the case of ESI-MS/MS data or completely manually for the MALDI-TOF/TOF data. De novo amino acid assignments are presented in Figs. 3, 4, and 5 for protein bands 1, 3, and 4, respectively. The deduced peptides were submitted to a homology search for identification of the protein. Similarities found for protein band 1 were with DeltaN p63/p73, p63/p73, and p53 tumor suppressor-like proteins by homology to blue mussels as illustrated in Fig. 3 and Table II. These proteins, all members of the p53 tumor suppressor family (31), are implicated in many vertebrate cancers and may also be involved in invertebrate cancers (32). Protein r3 was identified as being related to glutathione S-transferase Pi and multidrug resistance-associated protein by homology to blue mussel protein (Fig. 4 and Table II). These proteins, which are known to participate in the defense mechanism against toxic compounds, have been detected in cancerous tissue.
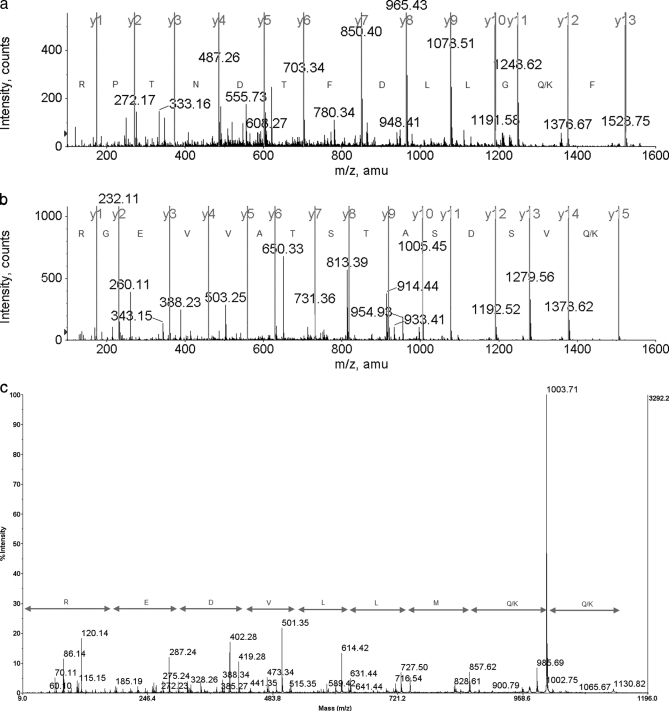
Fig. 3.
a, band nr1, peptide FKGLLDFTDNTPR, ESI-MS/MS de novo sequencing amino acid assignment. b, band nr1, peptide QVSDSATSTAVVEGR, ESI-MS/MS de novo sequencing amino acid assignment). c, band nr1, peptide KKMLLVDER, MALDI-TOF/TOF de novo sequencing amino acid assignment.
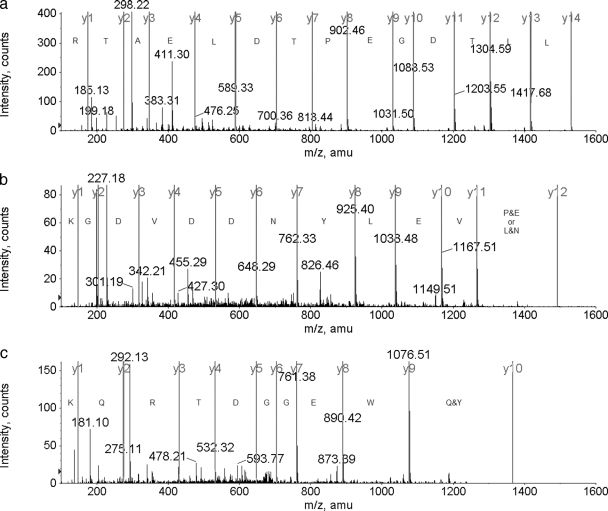
Fig. 4.
a, band r3, peptide LALLTDWPTDLEATR, ESI-MS/MS de novo sequencing amino acid assignment. b, band r3, peptide (EP/NL)VELYNDDVDGK, ESI-MS/MS de novo sequencing amino acid assignment. c, band r3, peptide YQWEGGDTRQK, ESI-MS/MS de novo sequencing amino acid assignment.
Fig. 5.
a, band r4, peptide CVTADLPPYPAPGK, ESI-MS/MS de novo sequencing amino acid assignment. b, band r4, peptide CVTADLPPYPA, ESI-MS/MS de novo sequencing amino acid assignment. c, band r4, peptide TLLNDYSAGK, ESI-MS/MS de novo sequencing amino acid assignment. d, band r4, peptide YGSFWDGHQVK, ESI-MS/MS de novo sequencing amino acid assignment.
Protein r4 had multiple hits with flagellar P-ring protein precursor (Methylobacterium populi) and with blue mussel lysozyme (Fig. 5 and Table II). De novo sequencing indicated that this protein is flagellar P-ring protein with three peptides having homology to the M. populi and A. tumefaciens sequences. De novo sequencing provided a hit for M. edulis lysozyme with two peptides found to share homology (Fig. 5 and Table II).
DISCUSSION
In the present study, characterization of protein biomarkers in mussels (M. edulis) contaminated with AZA toxins was investigated. Mussel digestive gland (hepatopancreas) is a tissue known to both play a central role in organic xenobiotic metabolism (33) and also for concentration of AZA toxins (34). Therefore it represented a key organ for study not only of AZA accumulation but also for possible toxin-protein interactions and protein up-regulation.
In a recent study, it was found that three proteins expressed in the digestive glands of mussels contaminated with AZAs were up-regulated when compared with non-contaminated glands (24). Moreover that study also revealed the presence of a fourth protein only in highly contaminated mussel hepatopancreas. This work now confirms those findings with up-regulated proteins, bands 1, 2, and 3, and the protein found only in contaminated mussels, band 4, observed by SDS-PAGE under both reducing and non-reducing conditions (Fig. 2).
The expression of either cathepsin D or p53 family proteins (band nr1) would strongly suggest that mussels are pathologically affected by the presence of AZAs. Cathepsin D is a ubiquitous aspartyl endoproteinase distributed in lysosomes and plays an important role in protein degradation and generation of bioactive proteins (35). It has been shown to promote cancer cell proliferation (36, 37), to act as a proapoptotic mediator of apoptosis, and to activate the caspase family of proteases (38). It has also been proposed as a therapeutic target for cancer (36) and a biomarker for identifying patients with highly malignant tumor phenotypes (39). Mytilus p53, p63/p73, and DeltaN p63/p73 are identical in their core regions with variation limited to their C and N termini (31). The tumor suppressor p53, whose protein product is a key transcriptional regulator of genes involved in cell cycle arrest and apoptosis, has homology with p63/p73 (40). Proteins p63/p73 are reported to be capable of inducing p53-responsive genes, whereas DeltaN p73 protein species antagonized the activity of p53 (41–44). Their expression in mussel species has been the subject of intense research (45), and they have been proposed as potential markers for the assessment of aquatic environments. The involvement of cathepsin D and p53 family proteins in apoptosis thus indicates that exposure to AZAs could have consequences for both vertebrate and invertebrate animals.
Although evidence from both mass spectral data and their role within organisms would suggest either of the above proteins as suitable candidates for the protein observed in band 1, molecular weight determination would point toward only cathepsin D. The molecular mass of 46 kDa determined in the present study is in good agreement with that found in previous studies on cathepsin D (35, 46). Further evidence of the adverse health effects of AZAs is demonstrated in the present study by the increased expression of SOD (band nr2) in contaminated mussels. SODs, metalloenzymes characterized by redox-active metals at the catalytic sites (16), catalyze the dismutation of superoxide into molecular oxygen and hydrogen peroxide (47). As a result, SODs represent one important line of defense against reactive oxygen species and, in particular, the superoxide anion resulting from aerobic metabolism. Blue mussels have been shown to express elevated activities of SOD when threatened by contaminated sediments and pollutants such as chlorine, antifouling Mexel 432, polyamine, polycyclic aromatic hydrocarbons, and heavy metals such as copper, zinc, chromium, cadmium, etc. (16, 17, 48). From the results obtained in this study, it now appears that SOD is also up-regulated when mussels are exposed to elevated levels of AZAs. Although this enzyme may not be a useful early warning specific indicator of AZA contamination, its measurement may prove worthwhile to indicate the early presence of chemical contamination.
The overexpressed protein in band r3 that was found to have homology with GST Pi or MRP also has implications for animal health because these proteins are involved in defense systems and xenobiotic detoxication and are associated with carcinogenesis. The MRP family are organic anion transporters, i.e. they transport anionic drugs and neutral drugs conjugated to acidic ligands, such as glutathione (GSH), glucuronate, or sulfate (49). GSTs are a family of multifunctional isoenzymes that catalyze the nucleophilic addition of glutathione to a widely heterogeneous group of compounds (50). Various biological functions have been ascribed to this enzyme group including transport of lipophilic compounds such as drugs and xenobiotics (51). By far the most studied function of the GST enzymes is their role in cellular detoxification (52) primarily against oxygen free radicals and peroxides produced by physiological cellular processes and exogenous stimuli. GST Pi has implications for human health where overexpression has been associated with carcinogenesis and is used as a tumor marker for gastrointestinal malignancies (53–56).
The above observations would thus now indicate that AZAs could pose a considerable health risk to vertebrate and non-vertebrate animals following their consumption. Support of such risks are found in the work by Ito et al. (57) where research on AZA-1 toxicology highlighted lung tumor appearance, malignant lymphomas, and an increased weight of liver following administration of AZA-1 in a chronic study in mice.
Although the mass spectral data and their role within organisms would suggest either GST Pi or MRP as likely candidates for the protein observed in band 3, molecular weight determination would strongly indicate GST Pi as the protein present. The literature value of 23.7 kDa (58) (for mussel M. galloprovincialis GST Pi) is in good agreement with the apparent molecular mass of 24 kDa observed in this study.
Although our data indicate that proteins in bands 1, 2, and 3 are of mussel origin, the protein in band r4 showed partial homology with a bacterium protein, flagellar P-ring (M. populi), and with blue mussel lysozyme. Members of the genus Methylobacterium are ubiquitous in nature and can be isolated from almost any freshwater environment with dissolved oxygen. They are distributed among a wide variety of natural habitats, including soil, dust, air, and freshwater and aquatic sediments (59). Lysozyme, a small enzyme also widely distributed in organisms from bacteriophages to human, protects organisms from bacterial infection by hydrolyzing 1,4-linked glycoside bonds of bacterial cell wall peptidoglycans (60). Lysozyme-like activity in shellfish has been known for many years (61, 62) and thought to be involved in digestion (63) as well as in self-defense against pathogenic bacteria (64). Thus the presence of either flagellar P-ring or lysozyme proteins in AZA-contaminated mussels but absence in non-contaminated mussels (24) could be explained if the AZA-contaminated mussels had been subjected to bacterial infection. Interestingly our study would suggest the presence of the flagellar P-ring protein precursor rather than lysozyme as the latter protein has a molecular mass of 13 kDa in mussel M. edulis (65) as opposed to 21 kDa observed here. Additional evidence pointing toward the presence of the flagellar P-ring precursor comes from the positive identification by both peptide sequencing and peptide mapping. The bacteria flagellum is the most important organelle of motility in bacteria and plays a key role in many bacterial lifestyles, including virulence (66, 67).
The proteins identified in this study should assist with development of urgently required processes for the rapid depuration of AZA-contaminated shellfish. Their identity will assist with investigations into the mechanisms underlying the toxin-protein interactions. Knowledge of these interactions will enable development of suitable methods for release of the AZA toxins from their associated protein(s). Presently we envisage that treatments involving enzymes, temperature, or pH may be capable of releasing the toxins and that such procedures could be easily incorporated into industrial depuration processes.
In conclusion, a range of proteomics tools was used to characterize a number of biomarkers present in AZA-contaminated mussels. Three proteins were identified that suggest mussels treat AZAs as harmful to their health. These three up-regulated proteins are interlinked in relation to their biological significance as all are involved in self-defense system mechanisms and associated with carcinogenesis. The presence of these proteins provides new evidence for AZAs being potential tumorigenic compounds or a tumor promoter. This finding has important implications for the consumers of foods contaminated with these toxins. The presence of a fourth protein from an apparent bacterial source could open up an interesting debate as to whether bacteria may somehow be involved in the production of algal AZA toxins themselves. Previous studies have highlighted the role of bacteria in the production of paralytic shellfish toxins by dinoflagellated algae and amnesic shellfish toxins. It is thought that bacteria may produce precursors that are used directly in toxin production or indirectly as “elicitors” of toxin production, or they may regenerate nitrogenous nutrients required for toxin production (68–71). The exact role of the bacteria in the production of AZA toxins is unclear, but the unexpected discovery of the bacterial protein warrants further investigation.
Whether any of the four proteins identified in this study could be used as a tracking or early warning device of a toxin outbreak will require further investigation and thus is presently highly speculative. Although there are excellent monitoring procedures to detect AZA contamination based on mass spectrometry there are no portable/field-based rapid detection techniques for AZAs. This has been due to the great difficulty in raising antibodies to the toxin to enable development of lateral flow-based devices commonly used to rapidly detect other forms of natural toxic episodes in farming (72). Future research will focus on determining whether the pattern of protein up-regulation noted in the present study is consistent in samples of contaminated mussels from other locations and whether the up-regulation occurs during different stages of an outbreak.
Acknowledgments
The Wellcome Trust is acknowledged for instrument funding (ESI-QSTAR Pulsar XL and 4800 MALDI TOF/TOF Analyzer, both from Applied Biosystems). We thank Stewart Floyd for technical support in the project.
Footnotes
* This work was supported by the Department of Agriculture and Rural Development for Northern Ireland.
1 The abbreviations used are:
- AZA
- azaspiracid
- SOD
- superoxide dismutase
- SEC
- size exclusion chromatography
- BLAST
- basic local alignment search tool
- MRP
- multidrug resistance-associated protein
- bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- LDS
- lithium dodecyl sulfate
- EMBL
- European Molecular Biology Laboratory
- NCBInr
- National Center for Biotechnology Information non-redundant.
REFERENCES
- 1.McMahon T., Silke J. ( 1996) Winter toxicity of unknown aetiology in mussels. Harmful Algae News 14, 2 [Google Scholar]
- 2.James K. J., Moroney C., Roden C., Satake M., Yasumoto T., Lehane M., Furey A. ( 2003) Ubiquitous ‘benign’ alga emerges as the cause of shellfish contamination responsible for the human toxic syndrome, azaspiracid poisoning. Toxicon 41, 145– 151 [DOI] [PubMed] [Google Scholar]
- 3.Krock B., Tillmann U., John U., Cembella A. D. ( 2008) Characterization of azaspiracids in plankton size-fractions and isolation of an azaspiracid-producing dinoflagellate from the North Sea. Harmful Algae 8, 254– 263 [Google Scholar]
- 4.Satake M., Ofuji K., Naoki H., James K. J., Furey A., McMahon T., Silke J., Yasumoto T. ( 1998) Azaspiracid, a new marine toxin having unique spiro ring assemblies, isolated from Irish mussels, Mytilus edulis. J. Am. Chem. Soc. 120, 9967– 9968 [Google Scholar]
- 5.James K. J., Furey A., Lehane M., Ramstad H., Aune T., Hovgaard P., Morris S., Higman W., Satake M., Yasumoto T. ( 2002) First evidence of an extensive northern European distribution of azaspiracid poisoning (AZP) toxins in shellfish. Toxicon 40, 909– 915 [DOI] [PubMed] [Google Scholar]
- 6.Magdalena A. B., Lehane M., Krys S., Fernández M. L., Furey A., James K. J. ( 2003) The first identification of azaspiracids in shellfish from France and Spain. Toxicon 42, 105– 108 [DOI] [PubMed] [Google Scholar]
- 7.Taleb H., Vale P., Amanhir R., Benhadouch A., Sagou R., Chafik A. ( 2006) First detection of azaspiracids in mussels in North West Africa. J. Shellfish Res. 25, 1067– 1070 [Google Scholar]
- 8.Twiner M. J, Rehmann N., Hess P., Doucette G. J. ( 2008) Azaspiracid shellfish poisoning: a review on the chemistry, ecology, and toxicology with an emphasis on human health impacts. Mar. Drugs 6, 39– 72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anonymous ( 2004) Regulation (EC) No 853/2004 of 29 April 2004, laying down specific hygiene rules for the hygiene of foodstuffs. Off. J. Eur. Communities L139, 55ff [Google Scholar]
- 10.Anonymous ( 2002) Commission Decision 225/2002/EEC, laying down detailed rules for the implementation of Council Directive 91/492/EEC as regards the maximum levels and the methods of analysis of certain marine biotoxins in bivalve molluscs, echinoderms, tunicates and marine gastropods (16.3.2002). Off. J. Eur. Communities L75, 62– 64 [Google Scholar]
- 11.Anonymous ( 2006) Risk Assessment of Azaspiracids (AZAs) in Shellfish, August 2006: a Report of the Scientific Committee of the Food Safety Authority of Ireland (FSAI), Food Safety Authority of Ireland (FSAI), Dublin, Ireland, UK [Google Scholar]
- 12.Nelson W. G. ( 1990) in Aquatic Toxicology and Risk Assessment ( Landis W. G., van der Schalie W. H. eds) Vol. 13, pp. 167– 175, American Society for Testing and Materials, Philadelphia, PA [Google Scholar]
- 13.Goldberg E. D. ( 1975) The mussel watch: a first step in global marine monitoring. Mar. Poll. Bull. 6, 111– 132 [Google Scholar]
- 14.Phillips D. J., Rainbow P. S. ( 1989) Strategies of metal sequestration in aquatic organisms. Mar. Environ. Res. 28, 207– 210 [Google Scholar]
- 15.Prevodnik A., Lilja K., Bollner T. ( 2007) Benzo[a]pyrene up-regulates the expression of the proliferating cell nuclear antigen (PCNA) and multixenobiotic resistance polyglycoprotein (P-gp) in Baltic Sea blue mussels (Mytilus edulis L.). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 145, 265– 274 [DOI] [PubMed] [Google Scholar]
- 16.Manduzio H., Monsinjon T., Rocher B., Leboulenger F., Galap C. ( 2003) Characterization of an inducible isoform of the Cu/Zn superoxide dismutase in the blue mussel Mytilus edulis. Aquat. Toxicol. 64, 73– 83 [DOI] [PubMed] [Google Scholar]
- 17.Manduzio H., Monsinjon T., Galap C., Leboulenger F., Rocher B. ( 2004) Seasonal variations in antioxidant defences in blue mussels Mytilus edulis collected from a polluted area: major contributions in gills of an inducible isoform of Cu/Zn-superoxide dismutase and of glutathione S-transferase. Aquat. Toxicol. 70, 83– 93 [DOI] [PubMed] [Google Scholar]
- 18.Regoli F., Principato G. ( 1995) Glutathione, glutathione-dependent and antioxidant enzymes in mussel, Mytilus galloprovincialis, exposed to metals under field and laboratory conditions: implications for the use of biochemical biomarkers. Aquat. Toxicol. 31, 143– 164 [Google Scholar]
- 19.Power A., Sheehan D. ( 1996) Seasonal variation in the antioxidant defence systems of gill and digestive gland of the blue mussel Mytilus edulis. Comp. Biochem. Physiol. 114, 99– 103 [Google Scholar]
- 20.Fitzpatrick P. J., O'Halloran J., Sheehan D., Walsh A. R. ( 1997) Assessment of a glutathione S-transferase and related proteins in the gill and digestive gland of Mytilus edulis (L.) as potential organic pollution biomarkers. Biomarkers 2, 51– 56 [DOI] [PubMed] [Google Scholar]
- 21.Canesi L., Viarengo A., Leonzio C., Filippelli, Gallo G. ( 1999) Heavy metals and glutathione metabolism in mussel tissues. Aquat. Toxicol. 46, 67– 76 [Google Scholar]
- 22.López J. L., Mosquera E., Fuentes J., Marina A., Vázquez J., Alvarez G. ( 2001) Two-dimensional gel electrophoresis of Mytilus galloprovincialis: differences in protein expression between intertidal and cultured mussels. Mar. Ecol. Prog. Ser. 224, 149– 156 [Google Scholar]
- 23.Apraiz I., Mi J., Cristobal S. ( 2006) Identification of proteomic signatures of exposure to marine pollutants in mussels (Mytilus edulis). Mol. Cell. Proteomics 5, 1274– 1285 [DOI] [PubMed] [Google Scholar]
- 24.Nzoughet K. J., Hamilton J. T., Floyd S. D., Douglas A., Nelson J., Devine L., Elliott C. T. ( 2008) Azaspiracid: first evidence of protein binding in shellfish. Toxicon 51, 1255– 1263 [DOI] [PubMed] [Google Scholar]
- 25.James K. J., Lehane M., Moroney C., Fernandez-Puente P., Satake M., Yasumoto T., Furey A. ( 2002) Azaspiracid shellfish poisoning: unusual toxin dynamics in shellfish and the increased risk of acute human intoxications. Food Addit. Contam. 19, 555– 561 [DOI] [PubMed] [Google Scholar]
- 26.Alfonso C., Alfonso A., Otero P., Rodríguez P., Vieytes M. R., Elliot C., Higgins C., Botana L. M. ( 2008) Purification of five azaspiracids from mussel samples contaminated with DSP toxins and azaspiracids. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 865, 133– 140 [DOI] [PubMed] [Google Scholar]
- 27.Shevchenko A., Wilm M., Vorm O., Mann M. ( 1996) Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 68, 850– 858 [DOI] [PubMed] [Google Scholar]
- 28.Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. ( 1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389– 3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cubero J., Martínez M. C., Llop P., López M. M. ( 1999) A simple and efficient PCR method for the detection of Agrobacterium tumefaciens in plant tumours. J. Appl. Microbiol. 86, 591– 602 [DOI] [PubMed] [Google Scholar]
- 30.Hoekema A., Hirsch P. R., Hooykaas P. J., Schilperoort R. A. ( 1983) A binary plant vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303, 179– 180 [Google Scholar]
- 31.Muttray A. F., Cox R. L., Reinisch C. L., Baldwin S. A. ( 2007) Identification of DeltaN isoform and polyadenylation site choice variants in molluscan p63/p73-like homologues. Mar. Biotechnol. 9, 217– 230 [DOI] [PubMed] [Google Scholar]
- 32.Ryan K. M., Phillips A. C., Vousden K. H. ( 2001) Regulation and function of the p53 tumor suppressor protein. Curr. Opin. Cell Biol. 13, 332– 337 [DOI] [PubMed] [Google Scholar]
- 33.Birmelin C., Mitchelmore C. L., Goldfarb P. S., Livingstone D. R. ( 1998) Characterisation of biotransformation enzyme activities and DNA integrity in isolated cells of the digestive gland of the common mussel, Mytilus edulis L. Comp. Biochem. Physiol. A 120, 51– 56 [Google Scholar]
- 34.Hess P., Nguyen L., Aasen J., Keogh M., Kilcoyne J., McCarron P., Aune T. ( 2005) Tissue distribution, effects of cooking and parameters affecting the extraction of azaspiracids from mussels, Mytilus edulis, prior to analysis by liquid chromatography coupled to mass spectrometry. Toxicon 46, 62– 71 [DOI] [PubMed] [Google Scholar]
- 35.Barrett A. J. ( 1970) Purification of isoenzymes from human and chicken liver. Biochem. J. 117, 601– 607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berchem G., Glondu M., Gleizes M., Brouillet J. P., Vignon F., Garcia M., Liaudet-Coopman E. ( 2002) Cathepsin-D affects multiple tumor progression steps in vivo: proliferation, angiogenesis and apoptosis. Oncogene 21, 5951– 5955 [DOI] [PubMed] [Google Scholar]
- 37.Fusek M., Vetvicka V. ( 2005) Dual role of cathepsin D: ligand and protease. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 149, 43– 50 [DOI] [PubMed] [Google Scholar]
- 38.Kågedal K., Johansson U., Ollinger K. ( 2001) The lysosomal protease cathepsin D mediates apoptosis induced by oxidative stress. FASEB J. 15, 1592– 1594 [DOI] [PubMed] [Google Scholar]
- 39.Leto G., Tumminello F. M., Crescimanno M., Flandina C., Gebbia N. ( 2004) Cathepsin D expression levels in nongynecological solid tumors: clinical and therapeutic implications. Clin. Exp. Metastasis 21, 91– 106 [DOI] [PubMed] [Google Scholar]
- 40.Chi S. W., Ayed A., Arrowsmith C. H. ( 1999) Solution structure of a conserved C-terminal domain of p73 with structural homology to the SAM domain. EMBO J. 18, 4438– 4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jost C. A., Marin M. C., Kaelin W. G., Jr. ( 1997) p73 is a human p53-related protein that can induce apoptosis. Nature 389, 191– 194 [DOI] [PubMed] [Google Scholar]
- 42.Kaghad M., Bonnet H., Yang A., Creancier L., Biscan J. C., Valent A., Minty A., Chalon P., Lelias J. M., Dumont X., Ferrara P., McKeon F., Caput D. ( 1997) Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell 90, 809– 819 [DOI] [PubMed] [Google Scholar]
- 43.Grob T. J., Novak U., Maisse C., Barcaroli D., Lüthi A. U., Pirnia F., Hügli B., Graber H. U., De Laurenzi V., Fey M. F., Melino G., Tobler A. ( 2001) Human ΔNp73 regulates a dominant negative feedback loop for TAp73 and p53. Cell Death Differ. 8, 1213– 1223 [DOI] [PubMed] [Google Scholar]
- 44.Kartasheva N. N., Contente A., Lenz-Stöppler C., Roth J., Dobbelstein M. ( 2002) p53 induces the expression of its antagonist p73DN, establishing an autoregulatory feedback loop. Oncogene 21, 4715– 4727 [DOI] [PubMed] [Google Scholar]
- 45.Muttray A. F., Cox R. L., St-Jean S., van Poppelen P., Reinisch C. L., Baldwin S. A. ( 2005) Identification and phylogenetic comparison of p53 in two distinct mussel species (Mytilus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 140, 237– 250 [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto K., Katsuda N., Himeno M., Kato K. ( 1979) Cathepsin D of rat spleen Affinity Purification and Properties of two types of cathepsin D. Eur. J. Biochem. 95, 459– 467 [DOI] [PubMed] [Google Scholar]
- 47.Fridovich I. ( 1995) Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 64, 97– 112 [DOI] [PubMed] [Google Scholar]
- 48.Eertman R. H., Groenink C. L., Sandee B., Hummel H. ( 1995) Response of blue mussels Mytilus edulis L. following exposure to PAHs or contaminated sediment. Mar. Environ. Res. 39, 169– 173 [Google Scholar]
- 49.Borst P., Evers R., Kool M., Wijnholds J. ( 2000) A family of drugs transporters: the multidrug resistance-associated proteins. J. Natl. Cancer Inst. 92, 1295– 1302 [DOI] [PubMed] [Google Scholar]
- 50.Campbell J. A., Corrigall A. V., Guy A., Kirsch R. E. ( 1991) Immunohistologic localization of alpha, mu, and pi class glutathione S-transferases in human tissues. Cancer 67, 1608– 1613 [DOI] [PubMed] [Google Scholar]
- 51.Murray G. I., Taylor V. E., McKay J. A., Weaver R. J., Ewen S. W., Melvin W. T., Burke M. D. ( 1995) The immunohistochemical localization of drug-metabolizing enzymes in prostate cancer. J. Pathol. 177, 147– 152 [DOI] [PubMed] [Google Scholar]
- 52.Ripple M., Mulcahy R. T., Wilding G. ( 1993) Characteristics of the glutathione/glutathione-S-transferase detoxification system in melphalan resistant human prostate cancer cells. J. Urol. 150, 209– 214 [DOI] [PubMed] [Google Scholar]
- 53.Toffoli G., Viel A., Tumiotto L., Giannini F., Volpe R., Quaia M., Boiocchi M. ( 1992) Expression of glutathione-S-transferase-pi in human tumours. Eur. J. Cancer 28A, 1441– 1446 [DOI] [PubMed] [Google Scholar]
- 54.Niitsu Y., Takahashi Y., Saito T., Hirata Y., Arisato N., Maruyama H., Kohgo Y., Listowsky I. ( 1989) Serum glutathione-S-transferase-pi as a tumor marker for gastrointestinal malignancies. Cancer 63, 317– 323 [DOI] [PubMed] [Google Scholar]
- 55.Soma Y., Satoh K., Sato K. ( 1986) Purification and subunit-structural and immunological characterization of five glutathione S-transferase in human liver, and the acidic form as a hepatic tumor marker. Biochim. Biophys. Acta 869, 247– 258 [DOI] [PubMed] [Google Scholar]
- 56.Henderson C. J., McLaren A. W., Moffat G. J., Bacon E. J., Wolf C. R. ( 1998) Pi-class glutathione S-transferase: regulation and function. Chem. Biol. Interact. 111–112, 69– 82 [DOI] [PubMed] [Google Scholar]
- 57.Ito E., Satake M., Ofuji K., Higashi M., Harigaya K., McMahon T., Yasumoto T. ( 2002) Chronic effects in mice caused by oral administration of sublethal doses of azaspiracid, a new marine toxin isolated from mussels. Toxicon 40, 193– 203 [DOI] [PubMed] [Google Scholar]
- 58.Hoarau P., Damiens G., Roméo M., Gnassia-Barelli M., Bebianno M. J. ( 2006) Cloning and expression of a GST-pi gene in Mytilus galloprovincialis. Attempt to use the GST-pi transcript as a biomarker of pollution. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 143, 196– 203 [DOI] [PubMed] [Google Scholar]
- 59.Gallego V., García M. T., Ventosa A. ( 2005) Methylobacterium hispanicum sp. nov. and Methylobacterium aquaticum sp. nov., isolated from drinking water. Int. J. Syst. Evol. Microbiol. 55, 281– 287 [DOI] [PubMed] [Google Scholar]
- 60.Jolles P. ( 1996) Lysozymes: Model Enzymes in Biochemistry and Biology, Birkhauser, Basel [Google Scholar]
- 61.McHenery J. G., Birkbeck T. H. ( 1982) Characterization of the lysozyme of Mytilus edulis (L). Comp. Biochem. Physiol. B 71, 583– 589 [DOI] [PubMed] [Google Scholar]
- 62.Myrnes B., Johansen A. ( 1994) Recovery of lysozyme from scallop waste. Prep. Biochem. 24, 69– 80 [DOI] [PubMed] [Google Scholar]
- 63.McHenery J. G., Birkbeck T. H. ( 1979) Lysozyme of the mussel, Mytilus edulis (L). Mar. Biol. Lett. 1, 111– 119 [Google Scholar]
- 64.Allam B., Paillard P. ( 1998) Defence factors in clam extrapallial fluids. Dis. Aquat. Org. 33, 123– 128 [Google Scholar]
- 65.Olsen Ø. M., Nilsen I. W., Sletten K., Myrnes B. ( 2003) Multiple invertebrate lysozymes in blue mussel (Mytilus edulis). Comp. Biochem. Physiol. B 136, 107– 115 [DOI] [PubMed] [Google Scholar]
- 66.Bardy S. L., Ng S. Y., Jarrell K. F. ( 2003) Prokaryotic motility structures. Microbiology 149, 295– 304 [DOI] [PubMed] [Google Scholar]
- 67.Josenhans C., Suerbaum S. ( 2002) The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 291, 605– 614 [DOI] [PubMed] [Google Scholar]
- 68.Kodama M., Ogata T., Sato S. ( 1988) Bacterial production of saxitoxin. Agric. Biol. Chem. 52, 1075– 1077 [Google Scholar]
- 69.Kodama M., Ogata T., Sakamoto S., Sato S., Honda T., Miwatani T. ( 1990) Production of paralytic shellfish toxins by a bacterium Moraxella sp. isolated from Protogonyaulax tamarensis. Toxicon 28, 707– 714 [DOI] [PubMed] [Google Scholar]
- 70.Ogata T., Kodama M., Komura K., Sakamoto S., Sato S., Simidu U. ( 1990) Production of paralytic shellfish toxins by bacteria isolated from toxic dinoflagellates, in Toxic Marine Phytoplankton. Proceedings of the Fourth International Conference on Toxic Marine Phytoplankton, Held June 26–30, 1989, in Lund Sweden ( Granelli E., Sundsrom B., Edler L., Anderson D. M. eds) pp. 311– 315, Elsevier, New York [Google Scholar]
- 71.Stephen S. B. ( 1998) Ecophysiology and metabolism of ASP toxin production, in Physiological Ecology of Harmful Algal Blooms ( Anderson D. M., Cembella A. D., Hallegraeff G. M. eds) p. 405– 426, Springer-Verlag, Heidelberg, Germany [Google Scholar]
- 72.Maragos C. M. ( 2005) Recent developments in immunochemical methods, in Aflatoxin and Food Safety ( Abbas H. K. ed) 10th Ed., pp. 269– 290, CRC Press, Boca Raton, FL [Google Scholar]