Abstract
To infect their mammalian hosts, Fasciola hepatica larvae must penetrate and traverse the intestinal wall of the duodenum, move through the peritoneum, and penetrate the liver. After migrating through and feeding on the liver, causing extensive tissue damage, the parasites move to their final niche in the bile ducts where they mature and produce eggs. Here we integrated a transcriptomics and proteomics approach to profile Fasciola secretory proteins that are involved in host-pathogen interactions and to correlate changes in their expression with the migration of the parasite. Prediction of F. hepatica secretory proteins from 14,031 expressed sequence tags (ESTs) available from the Wellcome Trust Sanger Centre using the semiautomated EST2Secretome pipeline showed that the major components of adult parasite secretions are proteolytic enzymes including cathepsin L, cathepsin B, and asparaginyl endopeptidase cysteine proteases as well as novel trypsin-like serine proteases and carboxypeptidases. Proteomics analysis of proteins secreted by infective larvae, immature flukes, and adult F. hepatica showed that these proteases are developmentally regulated and correlate with the passage of the parasite through host tissues and its encounters with different host macromolecules. Proteases such as FhCL3 and cathepsin B have specific functions in larvae activation and intestinal wall penetration, whereas FhCL1, FhCL2, and FhCL5 are required for liver penetration and tissue and blood feeding. Besides proteases, the parasites secrete an array of antioxidants that are also highly regulated according to their migration through host tissues. However, whereas the proteases of F. hepatica are secreted into the parasite gut via a classical endoplasmic reticulum/Golgi pathway, we speculate that the antioxidants, which all lack a signal sequence, are released via a non-classical trans-tegumental pathway.
Fasciola hepatica is a helminth (worm) parasite with a worldwide distribution. Although traditionally regarded as a parasite of livestock, particularly sheep and cattle, that results in a large economic loss to the agricultural community it has recently emerged as an important human infection in many regions of the world, including South America, Iran, Egypt, and mainland South-East Asia (1). Dormant larvae contained within cysts adhere to vegetation and emerge as infective juveniles (newly excysted juveniles (NEJs))1 in the duodenum following ingestion by animals or humans. They infect their hosts by rapidly penetrating the intestinal wall and entering the peritoneal cavity where they break through the liver capsule. After 8–12 weeks of consistent burrowing, feeding, and growth within the liver parenchyma they move to their final destination within the bile ducts where they mature and produce enormous numbers of eggs (2). The two distinct clinical phases of fasciolosis are directly related to the migration of the parasites: acute fasciolosis, which manifests as fever, abdominal pain, weight loss, and hepatomegaly, is associated with liver tissue damage and inflammation caused by the migrating immature parasites, whereas chronic fasciolosis (usually subclinical) is coupled with the presence of the mature adult flukes in the bile ducts (1, 3).
These invasive helminth parasites undergo complex changes as they migrate within their definitive mammalian hosts. The developing parasites encounter different host tissues and macromolecules and have to contend with a continually changing physiological microenvironment (such as pH and oxygen availability) and a mounting humoral and cellular host immune response. Morphological and ultrastructural studies clearly show major alterations of the parasite surface and gastrodermis, the two host-parasite interfaces, as they migrate and grow (4). However, we are only beginning to understand the molecular and biochemical interactions that occur between host and parasite and how these adjust as the development of the parasite progresses. A deeper knowledge of such host-pathogen interplay should provide data on novel targets for anthelmintic compounds and potential antiparasite vaccine candidates.
Increasingly proteomics analysis is being used as a means to investigate the interaction of helminth parasites and their hosts particularly in cases where obtaining pathogen material is difficult (5–9). For some helminths these studies have been facilitated by the availability of large transcriptomics data sets (10–12). Unfortunately the Fasciola nucleotide sequences available in GenBankTM are relatively few and highly redundant (298 for F. hepatica and 142 for Fasciola gigantica as of January 15, 2009), and the adult F. hepatica ESTs currently available from the Wellcome Trust Sanger Centre (14,031 entries) are unannotated (therefore the current identification of peptides of interest requires manual BLAST analysis using specific query sequences). Accordingly in this study we used the semiautomated EST2Secretome pipeline to analyze all available F. hepatica ESTs for secretory proteins potentially involved in host-pathogen interactions (13). EST2Secretome was developed in our laboratory by optimizing signal peptide-mediated secreted protein prediction from our earlier predictions of parasitic nematode ESTs (14, 15). We integrated these transcriptomics data with a proteomics analysis of the molecules secreted by adult F. hepatica with a particular emphasis on proteolytic enzymes. Furthermore we also analyzed the somatic and secreted molecules of the infective NEJ parasites and compared these with the secretome of immature and adult parasites taken from liver tissues. In doing so we produced a comprehensive view of how F. hepatica differentially and developmentally express and secrete proteolytic enzymes and other molecules according to the specific challenges faced in the intestine, liver, and bile ducts. F. hepatica cathepsin Bs and cathepsin L (FhCL3) are stored as zymogens within the infective larvae ready to be trans-activated by specific asparaginyl endopeptidases and released to perform the highly specific function of host tissue invasion (intestinal epithelium and liver capsule). By contrast, cysteine proteases belonging to the phylogenetic clades FhCL1, FhCL2, and FhCL5 are expressed during the later stages within the liver and bile duct and function in tissue degradation and feeding alongside the cell lytic protein saposin and a newly described prolylcarboxypeptidase. Several novel developmentally regulated cathepsin L and cathepsin B cysteine proteases and members of two serine protease families, namely carboxypeptidase and trypsin-like serine proteases, which may also have important roles in host-parasite interplay, were also identified. Finally our observations led us to propose that whereas the major proteases of F. hepatica are secreted into the parasite gut via a classical ER/Golgi pathway, an array of abundant and highly regulated antioxidants are released via a non-classical trans-tegumental pathway.
EXPERIMENTAL PROCEDURES
Analysis of the F. hepatica ESTs Using EST2Secretome
Transcriptome analysis for secretory proteins from 14,031 Fasciola EST sequences, available from the Sanger Centre, UK, was performed using the semiautomated EST2Secretome pipeline recently reported by Nagaraj et al. (13). The EST2Secretome pipeline integrates a number of high quality programs for EST cleaning (SeqClean and RepeatMasker), assembly into contigs and singletons (CAP3), conceptual translation (ESTSCAN), and prediction of secreted proteins based on the presence of N-terminal secretory signal sequences (SignalP) and follows with the elimination of membrane proteins using transmembrane hidden Markov models (TMHMM). This approach has proven to be very reliable in identifying secretory proteins from a number of helminths including the bovine lungworm Dictyocaulus viviparus (16), gastrointestinal worm Trichostrongylus vitrinus (15), and the hookworm Ancylostoma caninum (17). The predicted secretory proteins are extensively annotated for functional protein families and motifs (InterProScan), gene ontologies (BLAST2GO) pathways KEGG orthology-based annotation system (KOBAS), protein-protein interactions (comparison with IntAct), mapping to Caenorhabditis elegans proteins (from WormPep), and subsequent correlation to RNAi phenotype data. The SignalP threshold value for secretory signal peptide prediction was set at 0.5 as determined for the large scale secretory protein prediction from helminth parasite ESTs (13).
Independently unannotated Fasciola ESTs that matched MS/MS data were used as queries for BLASTn (18) searches of all nucleotide sequences in the GenBank and European Molecular Biology Laboratory (EMBL) databases. Searches were performed using the National Center for Biotechnology Information (NCBI) server. Open reading frames were constructed from the Fasciola EST sequences (guided by their best BLASTn hits), and hypothetical proteins derived from their conceptual translation were submitted to the InterProScan algorithm (19) to detect conserved domains and motifs to help assign putative protein identifications.
Proteomics Analysis of Parasite Somatic and Secreted Proteins: Gel Electrophoresis and Mass Spectrometry
The profile of proteins secreted by adult F. hepatica has been recently studied using two-dimensional electrophoresis (2-DE) by us and others (8, 20, 21). However, because of the paucity of material that can be obtained for the infective NEJ parasites and 21-day-old liver stage parasites we used 1-DE in the present study. Nevertheless we validated this approach by subjecting adult parasite secretory proteins to 1-DE and comparing the data with those reported by Robinson et al. (8). Protein samples were analyzed by 1-DE using NuPAGE® Novex® 4–12% Bis-Tris gels (Invitrogen). NuPAGE lithium dodecyl sulfate sample buffer plus Sample Reducing Agent (Invitrogen) were added to the samples, and samples were heated at 95 °C for 5 min prior to electrophoresis. Gels were stained with colloidal Coomassie Blue G-250 (Sigma) and destained with 10% methanol (v/v) and 7% acetic acid (v/v). Following visualization, gel lanes were cut into six sections for analysis by mass spectrometry. Briefly the individual gel sections were cut into smaller pieces (∼1 mm2) and reduced and alkylated with 5 mm tributylphosphine and 20 mm acrylamide (Sigma) in 100 mm NH4HCO3 for 90 min. The excised sections were then in-gel digested with trypsin (Sigma proteomics grade), and the peptides were solubilized with 2% formic acid (Sigma) prior to analysis by nano-LC-ESI-MS/MS using a Tempo nano-LC system (Applied Biosystems) with a C18 column (Vydac) coupled to a QSTAR Elite QqTOF mass spectrometer running in information-dependent acquisition mode (Applied Biosystems). Peak list files generated by the Protein Pilot v1.0 software (Applied Biosystems) using default parameters were exported to local MASCOT v2.1.0 (Matrix Science) or PEAKS (Bioinformatics Solutions Inc.) search engines for database searching.
Database Searches
MS/MS data were used to search 3,239,079 entries in the MSDB (September 8, 2006) using MASCOT whereas PEAKS software was used to search the 14,031 F. hepatica EST sequences from the Wellcome Trust Sanger Institute. The enzyme specificity was set to trypsin, propionamide (acrylamide) modification of cysteines was used as a fixed parameter, and oxidation of methionines was set as a variable protein modification. The mass tolerance was set at 100 ppm for precursor ions and 0.2 Da for fragment ions. Only one missed cleavage was allowed. For MASCOT searches, matches achieving a molecular weight search (MOWSE) score >70 with at least two high scoring individual peptides were considered to be significant (6, 7). For PEAKS searches of the Fasciola EST database, at least two high scoring (>60%) matching peptides were required. However, other criteria were considered in assigning a positive identification including concordance between the calculated theoretical molecular mass of the protein and the observed position of the polypeptide by 1-DE. To account for matches to multiple members of the Fasciola cathepsin family, we searched for peptides specific to individual enzymes or clades (Ref. 8; see “Results”). The proteomics data and transcriptome analysis were integrated to give a more complete view of gene expression/secretion in adult F. hepatica. This integrated data set provided a framework for comparison with the infective NEJs and 21-day-old immature flukes at the subproteome (i.e. secretome) level.
Quantitation of Fasciola Proteins by Exponentially Modified Protein Abundance Index (emPAI)
We analyzed the emPAI provided in the output of the MASCOT MS/MS ion search to estimate the relative expression of proteases and antioxidants identified in the developmental stages of F. hepatica. The emPAI used by MASCOT is a modification of the formula developed by Ishihama et al. (22) and gives a label-free relative quantification of the proteins in a mixture. The raw emPAI values obtained represent the transformed ratio of the number of experimentally observed peptides (composed of unique precursor ions, including different charge states of the same peptide, that match or exceed the threshold level for homology or identity) to the total number of peptides that can theoretically be detected within the operating mass range and retention time range of the mass spectrometer (calculated by MASCOT based on the mass of the protein, the average amino acid composition of the database searched, and the enzyme specificity). For this analysis, the raw emPAI values (averaged from three separate gels) for all Fasciola proteases or antioxidants identified were added to give a figure representing total expression for each within a particular developmental stage. The raw emPAI values for each individual protease or antioxidant were then converted to a percentage of this total to estimate their relative expression levels (see Figs. 2A and 3A). As the method used by MASCOT to calculate the number of observable peptides differs from that originally described by Ishihama et al. (22) and is not freely available, it was decided not to calculate emPAI values manually for those proteins identified using the PEAKS software to avoid introducing errors into the subsequent analysis. To account for redundancy, molecules potentially containing shared sequences were grouped together (e.g. all cathepsin B variants were classed as FhCB, all cathepsin L1 variants were classed as FhCL1, etc.).
Fig. 2.
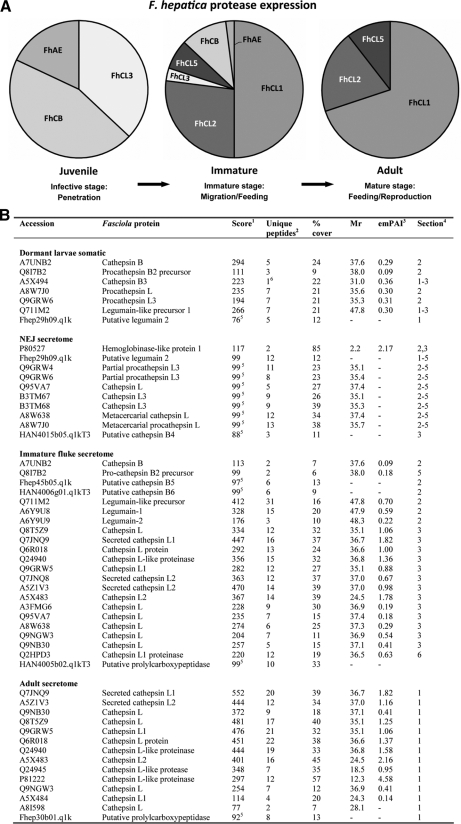
Expression of F. hepatica proteases. A, the expression levels of the major proteolytic enzymes used by F. hepatica during its mammalian life cycle. The label-free emPAI (22) derived from Fasciola MS/MS CID data was used to determine the abundance of the various cathepsin Ls (FhCL), cathepsin Bs (FhCB), and asparaginyl endopeptidases (FhAE) that are expressed by juvenile flukes (dormant larvae) and secreted by the immature (liver stage) flukes and adult (bile duct stage) parasites. B, identification of F. hepatica proteases by nano-LC-ESI-MS/MS. Dormant larvae somatic proteins and those secreted by NEJs, 21-day old immature flukes and adult F. hepatica were separated by 1-DE and analyzed by nano-LC-ESI-MS/MS. Mass spectrometry data were submitted to either the MSDB or a custom-made database composed of all F. hepatica ESTs (14,031 entries) currently available from the Wellcome Trust Sanger Centre using MASCOT and PEAKS software, respectively. Adult F. hepatica ESTs that matched with MS/MS data were submitted as queries to BLASTn (18) or conceptually translated and submitted to InterProScan (19) to detect conserved domains and motifs. Footnotes are as follows. 1, MASCOT scores are given for matches to F. hepatica cDNAs. 2, number of unique peptide matches. 3, raw emPAI values provided by the MASCOT search engine give an approximate quantification of the protein (22). These values were converted to a percentage of total protease expression within each Fasciola developmental stage (see “Experimental Procedures”) and were used to generate the pie charts shown in A. 4, section of the 1-D gel from which the peptides were identified. 5, PEAKS scores (expressed as a percentage) are given for matches to peptides encoded by F. hepatica ESTs. 6, single peptide-based identification (see supplemental Fig. 4).
Fig. 3.
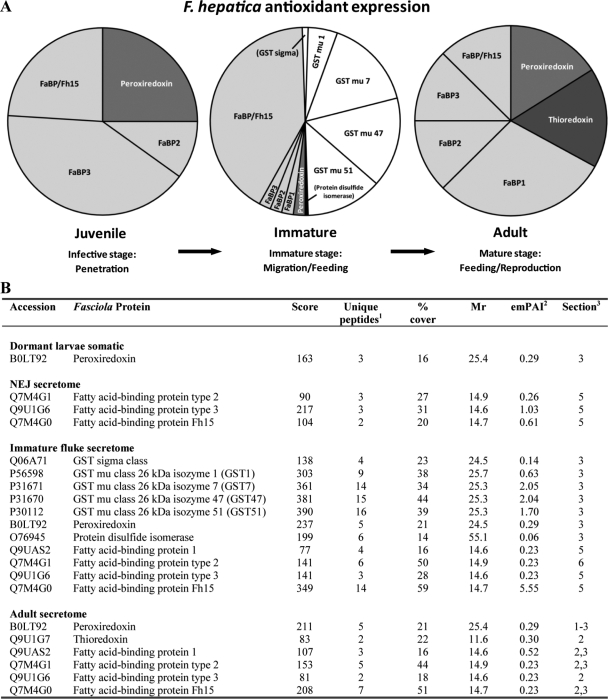
Expression of F. hepatica antioxidants. A, the expression levels of the major antioxidant molecules used by F. hepatica during its mammalian life cycle. The emPAI values (22) derived from Fasciola CID data were used to determine the abundance of the various antioxidants that are expressed and/or secreted by juvenile flukes (dormant larvae and NEJs) and secreted by the immature (liver stage) flukes and adult (bile duct stage) parasites. B, identification of F. hepatica antioxidants by nano-LC-ESI-MS/MS. Dormant larvae somatic proteins and those secreted by NEJs, 21-day-old immature flukes, and adult F. hepatica were separated by 1-DE and analyzed by nano-LC-ESI-MS/MS. MASCOT searches were performed against the MSDB. Footnotes are as follows. 1, number of unique peptide matches. 2, raw emPAI values provided by the MASCOT search engine give an approximate quantification of the protein (22). These values were converted to a percentage of total antioxidant expression within each Fasciola developmental stage (see “Experimental Procedures”) and were used to generate the pie charts shown in A. 3, section of the 1-D gel from which the peptides were identified.
Excystment of F. hepatica Metacercariae and Preparation of Somatic Larvae Proteins
The dormant cysts of F. hepatica metacercariae contain two layers, the outer of which can be contaminated with plant or other extraneous material. Here we describe a method for removing the outer cyst and adhered material so that somatic proteins of the dormant infective larvae can be analyzed. In addition, we describe a rapid method for activating the larvae and inducing them to emerge from the cysts so that in vitro secreted proteins can be isolated. Thus, F. hepatica metacercariae (Baldwin Aquatics Inc., Monmouth, OR) were vortexed for 10 s in 0.5% sodium hypochlorite and then incubated at room temperature for 20 min (this procedure dissolves the outer cyst layer). They were then washed three times in distilled water by centrifugation at 2000 × g for 2 min. The juvenile larvae, which were now only contained within a clear inner cyst layer, were used to prepare somatic protein extracts and secretory proteins. To prepare the somatic extracts the parasites were homogenized in RIPA buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm PMSF, 1 mm EDTA, 1% Triton X-100, 1% sodium deoxycholate (Sigma), 0.1% SDS, 1× Complete Mini protease inhibitor mixture (Roche Applied Science)) and placed on ice for 30 min. The protein extract was centrifuged at 13,000 rpm for 10 min to remove insoluble debris, and the supernatant was stored at −20 °C until use.
To prepare secreted proteins the washed parasites were resuspended and incubated in excystment medium (0.5% sodium bicarbonate, 0.4% sodium chloride, 0.2% sodium taurocholate, 0.07% concentrated HCl, 0.006% l-cysteine) for up to 3 h at 37 °C in 5% CO2. NEJs emerged from the cysts within 2–3 h and were transferred to prewarmed (37 °C) culture medium, RPMI 1640 medium (Invitrogen) containing 2 mm l-glutamine, 30 mm HEPES, 0.1% (w/v) glucose, and 2.5 μg/ml gentamycin, and cultured for 24 h.
To determine whether cysteine proteases were essential to cyst rupture, F. hepatica metacercariae (50 per treatment in triplicate) were either excysted with medium as described above or in medium lacking the 0.006% (w/v) l-cysteine or supplemented with the cysteine protease inhibitors trans-epoxysuccinyl-l-leucylamido(4-guanidino)butane (E-64, Sigma) or 1 mm carbobenzoxy-phenylalanyl-alanine-diazomethyl ketone (Z-Phe-Ala-CHN2, Bachem, St. Helens, UK) to a final concentration of 1 mm. The numbers of excysted parasites were counted after 3-h incubation at 37 °C in 5% CO2, and the data were analyzed using Student's t test. The experiments were repeated twice.
Preparation of Immature and Mature Adult F. hepatica Secretory Proteins
Immature F. hepatica flukes (21 days old) were recovered from the livers of female BALB/c mice (experimentally infected with 20 metacercariae), whereas adult parasites were recovered from the bile ducts of Merino sheep 16 weeks after an experimental infection with 200 metacercariae. Immature and adult parasites were washed in prewarmed (37 °C) PBS, pH 7.3, before transfer to culture medium (as described above) for 24 and 8 h, respectively. Secretory proteins were concentrated from the culture supernatants by precipitation with methanol/chloroform as described previously (6). Pellets were resuspended in 10 μl of RIPA buffer and stored at −20 °C prior to separation by electrophoresis.
RESULTS
Transcriptomics Profiling of Adult F. hepatica Secretory Proteins
Of the 14,031 adult F. hepatica raw EST sequences available, a total of 12,954 (92.3%) quality sequences were obtained (Table I). Cluster analysis of the 12,954 ESTs yielded 4236 representative ESTs (rESTs; 2749 contig and 1487 singleton sequences) of which 2960 (68.9%) had open reading frames. These preprocessed ESTs ranged from 60 to 2093 bp with a mean of 569 bp and S.D. of 268 bp. After clustering, the mean length of the contigs increased to 788 bp with S.D. of 358 bp. The G + C content of the coding sequences was 44.5%, which is similar to the figure of 43.5% reported for the adult bovine lungworm D. viviparus from EST analysis (16). This value is slightly higher than that reported for the related trematode Schistosoma mansoni (34%; Ref. 23) but consistent with those reported for nematodes (32–51%; Ref. 24), C. elegans (37%), and Caenorhabditis briggsae (38%) (25).
Table I. Summary analysis of 14,031 adult F. hepatica ESTs using EST2Secretome.
The contigs and singletons generated by preprocessing, overall rESTs, peptides from conceptual translation, and putative secretory proteins identified are shown.
| F. hepatica ESTs | Numbers |
|---|---|
| Raw sequences obtained | 14,031 |
| Cleaned sequences | 12,954 (92.3%) |
| Clusters of multiple sequences (contigs) | 2,749 (19.6%) |
| Clusters of singletons | 1,487 (10.6%) |
| Total rESTs | 4,236 (30.2%) |
| Putative peptides | 2,960 (68.9% rESTs) |
| Secreted proteins (SignalP cutoff, 0.5) | 160 (5.4% peptides) |
All rESTs were then subjected to analysis using our recently reported semiautomated bioinformatics platform (EST2Secretome; Ref. 13) to predict secretory proteins from the F. hepatica EST database. Using this approach, we identified a subset of parasite proteins likely to participate in the most significant interactions that occur between the adult stage of this parasite and its mammalian host. Thus, 173 Fasciola secretory proteins were predicted by the EST2Secretome pipeline with 160 true positives based on the EST2Secretome annotation mapping homology to proteins in a non-redundant secreted protein database (SecProtSearch) derived from the literature, the secreted protein database SPD (26), and the manually curated signal peptide database SPdb (27) as well the gene ontology annotations of subcellular localization of the top homologues identified by BLAST (92.5% accuracy) (Table I). Because ESTs are usually not full length and are often truncated, manual inspection of the final data set is required as it is possible that transmembrane sequences are erroneously identified as secreted proteins and thus elude the filtration step by TMHMM.
From the detailed annotations of the 160 adult secreted proteins the predominance of cathepsin L cysteine proteases is clearly evident as these are represented by a total of 66 (41.2%) proteins (Table II and supplemental Table 1). Robinson et al. (8) recently showed that the adult F. hepatica cathepsin L proteases separated into five distinct clades. Of these 66 adult rESTs encoding cathepsin Ls, 48 (72.5%) represented clade FhCL1 (38 subclade FhCL1A and 10 subclade FhCL1B), 11 (17%) encoded clade FhCL2, and two (3%) encoded clade FhCL5 cathepsins (8). Consistent with Robinson et al. (8) no cDNAs encoding clades FhCL3 or FhCL4 were detected in the adult ESTs because these proteases have been reported as specific for the infective NEJ (see below and Ref. 28). Interestingly five rESTs (7.5%) encoded cathepsin Ls that could not be placed into any of the five phylogenetic clades based on primary sequence alignment analysis.
Table II. Secretory proteins predicted from adult F. hepatica rESTs.
Adult F. hepatica secretory proteins were predicted using the EST2Secretome pipeline (13) using the default SignalP threshold value of 0.5. Their putative functionality based on BLAST analysis and the presence of InterPro domains is shown. Supporting proteomics data from the three Fasciola life cycle stages and the RNAi phenotypes for their C. elegans homologues are also shown. Imm, 21-day-old immature fluke; Ad, adult fluke; Ste, sterile; Lvl, larval lethal; Lva, larval arrest; Emb, embryonic lethal; Gro, slow growth; Unc, locomotion abnormal; Daf, dauer formation abnormal; Pvl, protruding vulva; Dpy, dumpy; Slu, sluggish; Clr, clear; age, life span abnormal; Age, extended life span; Stp, sterile progeny; Rup, exploded through vulva; Ric, aldicarb-resistant; Cons, constipated; Mig, cell migration abnormal; Gom, gonad migration abnormal; Bmd, organism morphology abnormal. —, none.
| Description (top BLAST hit) | Organism | InterPro domains | rESTs | Proteomics data | RNAi phenotypes of C. elegans homologues |
|---|---|---|---|---|---|
| Cathepsin L | F. hepatica | Peptidase C1A | 66 | NEJ, Imm, Ad | Emb, Gro, Unc |
| Novel proteins (no significant hits) | — | — | 36 | — | — |
| Saposin-like protein 3 | F. hepatica | Saposin B | 13 | Imm, Ad | Emb, Gro, Unc, Ste, Pvl, Lva |
| Vitelline protein B1 | F. hepatica | Trematode eggshell synthesis | 9 | — | Daf |
| Cathepsin B endoprotease | F. hepatica | Peptidase C1A | 6 | NEJ, Imm | — |
| Legumain | Opisthorchis viverrini | Peptidase C13, legumain | 5 | NEJ, Imm | Emb |
| Cystatin | F. hepatica | Proteinase inhibitor I25, cystatin | 3 | — | Gro |
| Unknown proteins | C. sinensis | — | 3 | NEJ, Ada | Ste, Lvl, Lva, Emb |
| Uncharacterized proteinsb | S. japonicum | New Pfam domain | 2 | — | — |
| Protein-disulfide isomerase | F. hepatica | Disulfide isomerase | 2 | Imm | Gro, Lva, Bmd, Dpy, Emb, Unc, Slu, Clr |
| SJCHGC01895 protein | S. japonicum | Peptidase S1 and S6 | 2 | — | Emb |
| Cubulin | Canis familiaris | CUB | 2 | NEJ | — |
| Unnamed protein | Tetraodon nigroviridis | Deoxyribonuclease I | 1 | — | Emb, Lva |
| SJCHGC00967 protein | S. japonicum | Deoxyribonuclease II | 1 | — | age |
| Apoferritin-2 | S. japonicum | Ferritin | 1 | Imm | bar-1(ga80) |
| Hypothetical protein | Aedes aegypti | Phospholipase D | 1 | — | — |
| SJCHGC06223 protein | S. japonicum | Peptidase S10, serine carboxypeptidase | 1 | — | — |
| Cyclophilin B | Xenopus tropicalis | Peptidyl-prolyl cis-trans isomerase | 1 | NEJ, Ad | — |
| Unnamed protein | Kluyveromyces lactis | — | 1 | — | — |
| Peptidoglycan recognition protein | Argopecten irradians | Amidase 2 | 1 | — | Stp |
| Myoglobin 2 | P. westermani | Globin, globin-like | 1 | — | Cons, Gom, Emb, Rup, Bmd, Mig, Gro |
| SJCHGC09717 protein | S. japonicum | — | 1 | — | — |
| Gag-pol polyprotein | Strongylocentrotus purpuratus | — | 1 | — | Age, Ric |
a Identified in adult F. hepatica secretions following gel filtration (data not shown).
b New protein family assigned to this protein: PF11703.
The next most abundant secreted protein based on the number of ESTs identified was saposin-like protein 3 that has been reported as a secreted protein by Grams et al. (29) and suggested to play a role in red blood cell lysis (30). Other proteins of interest to our study include several novel cathepsin B cysteine endoproteases (designated cathepsins B4–B10 in the current study), four novel asparaginyl endopeptidases or legumains (designated legumains 4–7 in the current study), and a cysteine protease inhibitor, cystatin. Additionally three putative novel adult serine proteases were identified: a serine carboxypeptidase and two proteins with trypsin-like protease domains. Other ESTs encoded secreted vitelline protein B1 that is found in eggs produced by the adult parasite (31) (Table II).
A Fasciola protein-disulfide isomerase was also predicted that has previously been identified in the secretions of adult flukes (32). Protein-disulfide isomerases have roles in protein folding, and a Fasciola recombinant enzyme was shown to mediate the oxidative refolding of reduced RNase (32). A putative peptide with a number of cubulin domains was also predicted in the current analysis. Cubulin domains occur predominantly in extracellular proteins or plasma membrane-associated proteins with a range of functions including complement activation, tissue repair, cell signaling, and inflammation (33). Although the Fasciola peptide contains a predicted N-terminal transmembrane region, its molecular function remains unknown. We note that although orthologues are available for six proteins, including an uncharacterized secretory protein from Clonorchis sinensis, their function remains elusive. A total of 36 secreted proteins (21.9%) are novel, but no database matches exist at the present time (Table II).
Proteomics Profiling of Adult F. hepatica Secreted Proteins
We and others have previously characterized the major secretory proteins expressed by adult F. hepatica using 2-DE (8, 20, 21). To complement these earlier studies and to validate the use of 1-DE for proteomics analysis, we analyzed tryptic digests extracted from gel sections of adult F. hepatica secreted proteins (see Fig. 1) by mass spectrometry. Twenty-two different proteins secreted by adult F. hepatica were identified in this analysis: 19 matched to previously identified Fasciola cDNAs, and three corresponded to putative proteins encoded by novel ESTs identified by our present EST2Secretome analysis but were unidentified by Robinson et al. (8) (Figs. 2B and 3B and supplemental Tables 2 and 3).
Fig. 1.
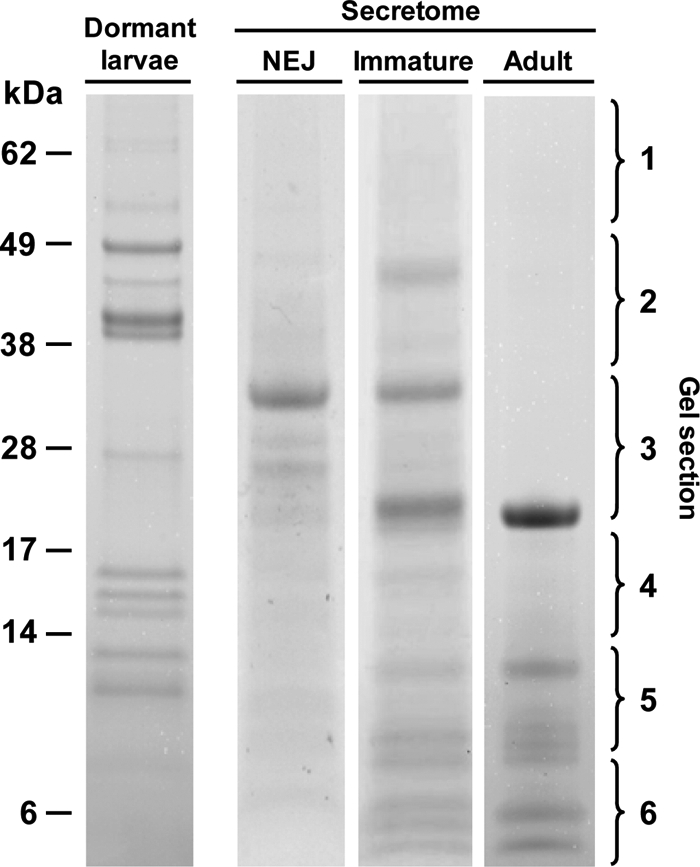
Analysis of F. hepatica somatic and secretory proteins by 1-DE. Shown is the typical 1-D profile of somatic proteins expressed by dormant F. hepatica larvae as well as proteins secreted by F. hepatica NEJs (NEJ), 21-day-old immature flukes (Immature), and adult parasites (Adult). Proteins (10 μg) were separated using NuPAGE Novex 4–12% Bis-Tris gels (Invitrogen) and stained with colloidal Coomassie Blue G-250. Following trypsin digests, peptides were extracted from gel sections 1–6 and analysed by mass spectrometry.
In accordance with the transcriptomics predictions, cathepsin L proteases were highly represented in adult fluke secretions. Matches to 13 cathepsin L sequences were observed and included clades FhCL1, FhCL2, and FhCL5 enzymes (FhCL4 or FhCL3 enzymes were not detected) (8). These identifications were based on the presence of clade-specific peptide matches such as NSWGLSWGER (ion m/z 596.30, +2) and VTGYYTVHSGSEVELK (ion m/z 590.32, +3) that are clade 1-specific peptides and three peptides, DYYYVTEVK (m/z 590.26, +2), VTGYYTVHSGDEIELK (m/z 604.32, +3), and LTHAVLAVGYGSQDGTDYWIVK (m/z 798.42, +3), characteristic of FhCL2 cathepsin Ls as well as the presence of peptides such as NSWGTWWGEDGYIR (m/z 863.90, +2) and FGLETESSYPYR (m/z 724.85, +2) that aid the identification of clade 5 cathepsin Ls (8). The three previously unidentified secreted proteins were saposin, a peptidyl-prolyl cis-trans isomerase, and a protein with homology to an uncharacterized C. sinensis secretory protein.
Several proteins that were predicted to be secreted by EST2Secretome were not identified by mass spectrometry of the adult parasite secretome both in this study and in the studies of Jefferies et al. (20), Morphew et al. (21), and Robinson et al. (8); these were cathepsin B, legumain, serine carboxypeptidase, two trypsin-like enzymes, and the eggshell vitelline protein B1. On the other hand, proteomics analysis detected four fatty acid-binding proteins (FaBP1, FaBP2, FaBP3, and Fh15) and two redox enzymes (peroxiredoxin and thioredoxin) that were not predicted by EST2Secretome analysis. A putative novel prolylcarboxypeptidase (also observed in 21-day-old immature parasites; see below) was also identified by mass spectrometry and yet was not predicted to be a secretory peptide. This is surprising because at least one adult fluke EST (Fhep06a01.q1k) encoding the N-terminal end of the enzyme contains a putative signal peptide. However, this EST was not retained as a singleton after repeat masking and truncation in the early stages of EST processing in EST2Secretome.
Identification of F. hepatica Dormant Larval Somatic Proteins and NEJ Secretory Proteins
By removing the outer cyst layer of the dormant metacercarial infective stage of the parasite we could extract somatic protein without contamination with extraneous proteins and analyze these by 1-DE. Approximately 14 protein bands could be visualized following Coomassie Blue-stained SDS-PAGE (Fig. 1) that on inspection appeared very similar to the banding pattern of Fasciola larval somatic proteins reported by Tkalcevic et al. (34). Proteins in these bands were in-gel digested with trypsin and analyzed by nano-LC-ESI-MS/MS, and the resulting CID data were used for database searching. A total of 26 different Fasciola dormant larvae proteins were identified: 12 matched to previously identified Fasciola cDNAs (or cDNAs from related trematode species), and 14 corresponded to proteins encoded by novel ESTs (Figs. 2B and 3B and supplemental Tables 2 and 3). A further five peptides encoded by Fasciola ESTs were also identified, but these lacked conserved protein domains and could not be assigned putative functions based on BLAST searches.
Of the 26 positively matched Fasciola proteins six were proteases including two cathepsin L3 proteases, three cathepsin B endopeptidases, and an asparaginyl endopeptidase-like precursor (discussed in detail below). Others included structural proteins related to muscle function such as actin, myosin-regulatory light chain, and a troponin C homologue (supplemental Tables 2 and 3). Four metabolic enzymes were also identified: pyruvate carboxylase (gluconeogenesis), malate dehydrogenase (tricarboxylic acid cycle), and aldolase and enolase (glycolysis). Mass spectrometry data also matched to a putative cullin protein (roles in protein degradation and ubiquitination), a cyclophilin-like peptidyl-prolyl cis-trans isomerase, and a tetraspanin membrane protein. Other notable peptide matches included the Fasciola antioxidant enzyme peroxiredoxin, two histone proteins, a heat shock protein 70, dynein light chain, a ribosome production factor, ribosomal protein L30, and a protein bearing a conserved RNA-binding motif (supplemental Tables 2 and 3).
By using an artificial medium that replicated the surfactant and reducing conditions in the duodenum we could activate the dormant infective larvae and induce them to excyst. Proteins secreted by F. hepatica NEJs during in vitro culture were isolated from the medium, separated by 1-DE, and analyzed by nano-LC-ESI-MS/MS (Fig. 1). Matches to 29 different proteins, of which 10 were proteases including seven cathepsin L3 proteases, one cathepsin B, and two asparaginyl endopeptidase-like proteases, were obtained from MS/MS data from Fasciola NEJ secreted proteins.
Other protein matches to Fasciola cDNA sequences included enolase, three fatty acid-binding proteins (Fh2, Fh3, and Fh15), and peroxiredoxin (Fig. 3B and supplemental Tables 2 and 3). A further 16 different putative peptides were identified following searches of the F. hepatica EST database including the metabolic enzymes fructose-bisphosphate aldolase, phosphoenolpyruvate carboxykinase, glyceraldehyde-3-phosphate dehydrogenase, malate dehydrogenase, and an ATPase. Other matches included putative structural proteins such as calponin, spermadhesin, histones (H2A, H2B, and H4), redox enzymes (thioredoxin and peptidyl-prolyl cis-trans isomerase), and an uncharacterized protein with predicted transmembrane regions. Finally molecules with roles in protein turnover such as ubiquitin and a putative serpin were also identified.
Identification of Immature F. hepatica Secretory Proteins
Parasites were removed from the livers of infected mice and maintained in vitro for collection of secreted proteins. The overall complexity of the secretory proteins of these 21-day-old immature F. hepatica was greater compared with that of the NEJs or adult parasites (Fig. 1) and yielded a total of 45 different protein identifications (Figs. 2B and 3B and supplemental Tables 2 and 3). Of these, 34 were matched to previously identified Fasciola cDNAs, and 11 corresponded to putative proteins encoded by novel ESTs. Mass spectrometry data also matched to peptides encoded by a further two F. hepatica EST sequences that lacked conserved protein domains and could not be assigned putative functions based on BLAST searches.
Of the 45 positively matched proteins 22 were proteases and included 14 cathepsin Ls, four cathepsin Bs, three asparaginyl endopeptidases (legumains), and a newly discovered prolylcarboxypeptidase. The remaining 23 proteins secreted by the immature liver stage parasites included a GST Sigma class enzyme, four isoforms of Mu class GSTs (GST1, GST7, GST47, and GST 51), four fatty acid-binding proteins (FaBP1, FaBP2, FaBP3, and Fh15), two saposin-like proteins (SAP1 and SAP3), two enzymes of glycolysis (enolase and triose-phosphate isomerase), and two enzymes involved in cell redox homeostasis (peroxiredoxin and protein-disulfide isomerase). Other significant peptide matches included annexin, ferritin, ubiquitin, a 14-3-3 protein, a multicystatin, and a putative ATP-binding cassette (ABC) transporter protein.
DISCUSSION
The database of F. hepatica ESTs available from the Wellcome Trust Sanger Centre is now sufficiently large to allow a significant transcriptomics analysis of this helminth pathogen that until now has been lacking in this field. We used our newly developed EST2Secretome pipeline to analyze these data sets with the view to identifying molecules secreted by the adult trematode F. hepatica unlike earlier applications to nematode parasites that focused on the complete transcriptome. Furthermore we integrated these results with data generated from proteomics analysis performed here and in previous reports (8, 20, 21) to build a picture of how the developing parasite sustains itself within the mammalian host with particular emphasis on proteases as virulence and tissue-damaging factors.
EST2Secretome analysis of the adult F. hepatica ESTs identified 160 cDNAs encoding secreted proteins, 41% of which encoded cathepsin L cysteine proteases. The abundance of adult cathepsin L sequences was noted in a previous analysis of entries in the public databases (8, 35). When these sequences were subjected to a phylogenetic investigation it was demonstrated that they could be separated into five clusters or clades: FhCL1, FhCL2, FhCL3, FhCL4, and FhCL5. Because of the high level of conservation between the clades it was not possible to study temporal patterns of specific cathepsin gene expression by conventional means (such as RT-PCR). Analysis of the >14,000 adult parasite EST sequences in the present study confirmed the expression of three of these distinct clades (FhCL1, FhCL2, and FhCL5) in this fully mature stage of the parasite. It also supported the lack of expression of clades FhCL3 and FhCL4 in adult parasites, although proteases encoding these genes are expressed by NEJs (see below and Ref. 28). We also identified five novel cathepsin L proteases that although encoded by partial nucleotide sequences (contigs 1553, 2626, and 1886 and singletons Fhep55b05.q1k and Fhep18b10.q1k) may represent a new phylogenetic cluster.
The F. hepatica cathepsin L proteases, the largest family of proteases known in any helminth pathogen, arose by a series of gene duplications. Members underwent selective functional diversity brought about by specific alterations within the active site that ultimately produced a repertoire of proteases with overlapping but distinct substrate specificity (8). Our proteomics investigation was consistent with the EST2Secretome analysis by demonstrating the predominance of these proteases in the adult F. hepatica secretome (∼80% of total protein secreted) and indicated a major role for the enzymes in supporting the existence of the parasites within the bile duct. Here the adult parasites are obligate blood feeders, and therefore the most obvious function for the cathepsin L enzymes is in the digestion of blood macromolecules. Recently Lowther et al. (36) showed that members of the most predominant cathepsin L clade, FhCL1, have evolved an active site with a strong preference for hydrophobic amino acids, such as Leu, Ala, Phe, and Val, that are particularly abundant in hemoglobin (42%) indicating a specific adaptation for digestion of this protein. Hemoglobin is the prime supplier of amino acids that the parasites use in the anabolism of egg proteins, a major task because they produce 30–50,000 eggs/day/worm (37). Members of the other major clade, FhCL2, have specific amino acid substitutions in the active site cleft that gives these proteases the ability to cleave substrates with Pro residues and facilitates the degradation of proline-rich interstitial collagen during the migration of the parasites through the tissues (38).
Consistent with the importance of assimilating nutrients from host red blood cells was the identification of ESTs encoding a saposin-like molecule as the next most abundant in the data set. This protein, which was termed FhSAP-2 because of its similarity to a saposin-like protein family and the amoebapore precursors of Entamoeba histolytica, has been implicated in the penetration and lysis of ingested host red blood cells not only in Fasciola (29, 30) but also in other helminths (39). Saposin-like molecules are likely secreted from granules within cells lining the parasite esophagus so that ingested red blood cells are lysed as soon as they enter the digestive track. Hydrolysis of released hemoglobin by the cathepsin L proteases then takes place in the acidic cecum (37).
Several Fasciola proteins predicted as components of the secretome by our EST2Secretome analysis were not identified by mass spectrometry in secretions of adult parasites maintained in culture (8, 20) or within the bile ducts (21). The most highly represented of these was the major eggshell protein vitelline protein B1 (vpB1), which is produced by mature vitelline cells to form the hard protective trematode eggshell (31). However, because this process occurs within the ootype and uterus of the adult fluke it is likely that vpB1 is retained within the eggshell and not secreted outside the parasite. Other examples are the transcripts encoding six novel cathepsin B cysteine proteases and five new asparaginyl endopeptidases. Although some isotypes of these proteases are secreted by the infective larvae and immature liver stage flukes (see below), they are absent from the secretome of adult F. hepatica or F. gigantica as previously reported using biochemical, proteomics, and immunoblotting methods (8, 40, 41). Considering these observations and the fact that cathepsin B and asparaginyl endopeptidases also function internally within cells (42, 43), it is likely that these newly discovered proteases have functions within the internal tissues of this complex multicellular parasite and are involved in generalized protein turnover, remodeling, and/or catabolism. Alternatively or additionally, because immunocytochemistry and in situ hybridization have localized cysteine proteases and transcripts to the reproductive structures of other trematodes (44, 45) these proteases may be involved in the process of egg production. Other proteins identified by EST2Secretome analysis but not by proteomics, including the previously uncharacterized proteins such as deoxyribonucleases, carboxypeptidase, and trypsin-like peptidases that have homologues in Schistosoma japonicum and C. sinensis, may also be confined to internal tissues of the parasite or be expressed and secreted at levels below the detection capacity of the proteomics methods so far used (cDNAs of these were poorly represented in the transcriptome of the adult parasite).
Conversely a number of F. hepatica proteins that have previously been described as major components of the adult parasite secretions by standard protein and proteomics methods were not predicted by the EST2Secretome pipeline. These included the fatty acid-binding proteins FaBP1, FaBP2, FaBP3, and Fh15 (20, 21) and two redox enzymes, thioredoxin and peroxiredoxin (20, 46–48). Primary sequence analysis using SignalP (49) confirmed that these proteins lacked predicted N-terminal signal peptides for secretion via the classical ER/Golgi pathway, and hence they must be released by alternate mechanisms. Morphew et al. (21) suggested that release of Fasciola FaBPs was due to shedding of the tegument as part of a stress response during in vitro culture of parasites because they were not detected in vivo. However, morphological studies have shown that blebbing or shedding of the F. hepatica surface tegument and renewal by proteins synthesized by highly metabolic subtegumental cells is a continuous property that may be an immunodefensive strategy (4, 50). This non-classical mechanism for protein secretion may be compared with those reported for some leaderless eukaryotic proteins, for example the ER/Golgi-independent secretion of leaderless peptides including interleukin (IL)-1α, IL-1β, and fibroblast growth factor-2 (51). In the case of IL-1β, which is processed and activated by caspase-1, it has been proposed that both molecules are packaged together into plasma membrane blebs (by ABC transporters) and are rapidly released as microvesicles following phospholipase-mediated fusion with the plasma membrane (52–54). The identification of a phospholipase in the transcriptome of adult flukes (Table II) together with the presence of ABC transporters within the tegument (55) supports the possibility of a similar mechanism for the ER/Golgi-independent secretion pathway in Fasciola.
In summary, our EST2Secretome pipeline was successful in identifying the major secreted proteins of adult F. hepatica. Integration of this analysis with proteomics data is important for the study of helminth host-pathogen relationships to distinguish proteins that are secreted extracorporeally from those secreted within the internal tissues of the parasites. Additionally this integrated approach identified major helminth secreted proteins that may reach the exterior by novel or non-classical secretory pathways.
Proteomics Analysis of the Dormant and Infective Stage Larvae
Having collated the secretome data for adult F. hepatica we performed a comparative analysis of adult F. hepatica with the infective larvae that invade their host by penetrating the intestinal wall. The larvae of F. hepatica (<1 mm in length) are released by the intermediate snail host (Galba truncatula) and encyst on vegetation that is consumed by the host. For research purposes these are produced in an aquarium, and the larvae are allowed to encyst on cellophane. However, because under these conditions the encysted metacercariae can become contaminated with extraneous material we used a method of washing in 2% hypochlorite to remove this and the outer cyst wall so that a proteomics study of the dormant larvae could be performed. We also used a medium containing bile duct surfactants to activate the larvae and induce them to excyst so that their secretome could be analyzed. We found that the level of Fasciola excystment was significantly reduced when the excystment medium excluded the reducing agent l-cysteine (91% reduction, p = 0.0008; not shown) compared with medium containing l-cysteine. The requirement for reducing conditions implied a role for cysteine proteases in the excystment process because the activity of these thiol-dependent proteases is enhanced in the presence of reducing agents. We therefore proved this point by showing that the level of excystment was significantly reduced when the broad range cysteine protease inhibitor E-64 (98% reduction, p = 0.0433) or the cathepsin-specific inhibitor Z-Phe-Ala-CHN2 (99% reduction, p = 0.0205) were added to the excystment medium. Proteomics analysis of soluble extracts of dormant larvae and the secretions of the NEJs identified 26 and 29 proteins, respectively (Figs. 2B and 3B and supplemental Tables 2 and 3). Significantly 23% of all the somatic larval proteins and 31% of the secreted proteins were cysteine proteases consisting of cathepsin L (37%), cathepsin Bs (45%), and asparaginyl endopeptidases (18%). Because the former two classes are potently inhibited by E-64 and Z-Phe-Ala-CHN2 but the latter class is not, the results implicate cathepsin Ls and/or cathepsin B in the process of cyst rupture.
Phylogenetics studies showed that cDNA clones generated from transcripts of Fasciola larvae, designated clade FhCL3, encoded a cathepsin L protease that was specific to this infective stage (8, 35); the cDNA encoding this protease, FhCL3_nl22, was originally described by Harmsen et al. (56). Earlier studies by Tkalcevic et al. (34) using N-terminal sequencing to identify proteins expressed by larvae reported a sequence of a cathepsin L cysteine protease that we now know matches exactly with the predicted N terminus of FhCL3_nl22 (56). More recently, Cancela et al. (28) confirmed the restricted expression of FhCL3 and another protease, FhCL4, to infective larvae by isolating cDNAs encoding these enzymes from a F. hepatica larvae-specific cDNA library and subsequently by using RT-PCR expression analysis. No FhCL4 peptides were identified in the present analysis suggesting that this enzyme may be expressed at low levels, performs specific intracellular functions, and is not secreted by F. hepatica. However, we confirmed the presence of FhCL3 in NEJ by identifying several FhCL3-specific peptides with matches to FhCL3_nl22. It is noteworthy that the mass of the dormant larvae somatic cathepsin L3, which was found in a protein band migrating at ∼37 kDa, is consistent with that of an inactive cathepsin L precursor, or zymogen, which is supported by the presence of mass ion m/z 644.28, +2 that matched to a peptide (SNDVSWHEWK) found only within the N-terminal prosegment region of the protease. No peptides matching with cathepsin Ls were identified in protein bands in the region corresponding to the fully processed and active mature enzymes, i.e. mass ∼24 kDa (Fig. 1, NEJ secretome gel section 3). By contrast, although a few cathepsin L3 peptides were present at ∼37 kDa in the secretome of NEJ (including the FhCL3 prosegment peptides LGLNQFTDLTFEEFK (m/z 901.49, +2) and SNDVSWHEWK (m/z 644.28, +2)), the most robust peptide matches were obtained from gel sections at the molecular mass of ∼24 kDa corresponding to the size of a fully activated mature enzyme (Fig. 1, NEJ secretome gel section 3). Collectively these observations show that the dormant larvae of F. hepatica express a specific cathepsin L protease, FhCL3, stored as an inactive zymogen that is rapidly secreted and activated following emergence from their cysts to become infective larvae.
Fasciola cathepsin B-like cysteine proteases were also identified in somatic extracts of larvae with peptides matching to proteins encoded by three different cDNAs that were previously designated cathepsin B1 (UniProt accession number A7UNB2),2 cathepsin B2 (UniProt accession number Q8I7B2),3 and cathepsin B3 (UniProt accession number A5X494; Ref. 28). All three cathepsin B proteases were identified in intense protein bands that migrated in reducing SDS-PAGE at molecular masses of ∼36–50 kDa and ∼50–70 kDa (Fig. 1, dormant larvae gel sections 1 and 2) suggesting that like FhCL3 these may represent stored zymogens. The presence of peptides FINIEHFK (m/z 524.28, +2) and QHLGLLEETPEER (m/z 775.89, +2) confirmed the existence of cathepsin B2 as an unprocessed zymogen whereas peptides QNLGVLEETPEDR (m/z 750.36, +2) and YSVSENDLPESFDAR (m/z 864.90, +2; which spans the juncture between the prosegment and the mature domain) indicate that cathepsins B1 and B3 are also stored as zymogens by Fasciola NEJs.
However, unlike the FhCL3, we also found some cathepsin Bs at ∼20–36 kDa (Fig. 1, NEJ secretome gel section 3), which is consistent with the occurrence of processed active enzymes within the dormant larvae (which have a predicted molecular mass of 29.6 kDa; Ref. 57). Although each of the three cathepsin Bs were also found as fully active enzymes in the secretions of larvae, in the region of ∼20–36 kDa, we also discovered a novel family member, encoded by a Fasciola EST HAN4015b05.q1kT3 (here designated cathepsin B4) (Fig. 2B and Table III).
Table III. Database of the repertoire of proteases expressed by F. hepatica in the mammalian host.
Somatic proteases expressed by dormant larvae and those secreted by NEJs, immature flukes, and adult F. hepatica identified by mass spectrometry are shown.
| Accession no. | Fasciola protein | Dormant larvae | NEJ secretome | Immature secretome | Adult secretome |
|---|---|---|---|---|---|
| FhCL1A | |||||
| Q8T5Z9 | Cathepsin L | − | − | + | + |
| Q7JNQ9 | Cathepsin L1 | − | − | + | + |
| Q6R018 | Cathepsin L protein | − | − | + | + |
| Q2HPD3 | Cathepsin L1 proteinase | − | − | + | + |
| Q24940 | Cathepsin L-like proteinase | − | − | + | + |
| FhCL1B | |||||
| Q9GRW5 | Cathepsin L1 | − | − | + | + |
| FgCL1C | |||||
| Q8MUT6 | Cathepsin L | − | − | − | − |
| FhCL2 | |||||
| Q7JNQ8 | Secreted cathepsin L2 | − | − | + | + |
| A5Z1V3 | Secreted cathepsin L2 | − | − | + | + |
| A5X483 | Cathepsin L2 | − | − | + | + |
| A3FMG6 | Cathepsin L | − | − | + | − |
| FhCL3 | |||||
| Q95VA7 | Cathepsin L | + | + | + | − |
| A8W638 | Cathepsin L | + | + | + | − |
| A8W7J0 | Metacercarial procathepsin L | − | + | − | − |
| Q9GRW4 | Partial procathepsin L3 | − | + | − | − |
| Q9GRW6 | Partial procathepsin L3 | − | + | − | − |
| B3TM67 | Cathepsin L3 | − | + | − | − |
| B3TM68 | Cathepsin L3 | − | + | − | − |
| FhCL4 | |||||
| Fhep55b05.q1k | Putative cathepsin L | − | − | − | − |
| FhCL5 | |||||
| Q9NGW3 | Cathepsin L | − | − | + | + |
| Q9NB30 | Cathepsin L | − | − | + | + |
| Cathepsin B | |||||
| A7UNB2 | Cathepsin B1 | + | − | + | − |
| Q8I7B2 | Cathepsin B2 | + | − | + | − |
| A5X494 | Cathepsin B3 | + | − | − | − |
| HAN4015b05.q1kT3 | Putative cathepsin B4 | − | + | − | − |
| Fhep45b05.q1k | Putative cathepsin B5 | − | − | + | − |
| HAN4006g01.q1kT3 | Putative cathepsin B6 | − | − | + | +a |
| Fhep44e10.q1k | Putative cathepsin B7 | − | − | − | +a |
| Fhep11b02.q1k | Putative cathepsin B8 | − | − | − | +a |
| FhContig1639 | Putative cathepsin B9 | − | − | − | +a |
| FhContig2164 | Putative cathepsin B10 | − | − | − | +a |
| Asparaginyl endopeptidases | |||||
| Q711M2 | Legumain-like precursor 1 | + | + | + | − |
| Fhep29h09.q1k | Putative legumain 2 | + | + | − | − |
| A6Y9U8 | Legumain 3 | − | − | + | +a |
| A6Y9U9 | Legumain 4 | − | − | + | +a |
| Fhep21f02.q1k | Putative legumain 5 | − | − | − | +a |
| FhContig1272 | Putative legumain 6 | − | − | − | +a |
| FhContig2292 | Putative legumain 7 | − | − | − | +a |
| Prolylcarboxypeptidase (s28) | |||||
| Fhep30b01.q1k | Putative prolylcarboxypeptidase | − | − | + | + |
| Serine carboxypeptidase (s10) | |||||
| FhContig542 | Putative serine carboxypeptidase | − | − | − | +a |
| Trypsin-like serine proteases | |||||
| FhContig492 | Putative peptidase S1/S6 | − | − | − | +a |
| FhContig2453 | Putative trypsin-like Ser/Cys | − | − | − | +a |
a Expression data only available at the transcript level.
Cysteine proteases are produced as inactive zymogens consisting of a prosegment and mature domain (58). The prosegment lies along the active site groove (in reverse to the direction of protein substrates) preventing unwanted hydrolysis during trafficking and storage of the protease within the cell. Removal of the prosegment from the mature domain exposes the active site of the hydrolase to entry of macromolecular protein substrates. Dalton et al. (59) proposed that this activation step is mediated by proteolytic clipping at a “protease-susceptible” region between the prosegment and mature enzyme and that this event may be performed by the same or another protease molecule. Dalton and Brindley (60) and Dalton et al. (59) proposed that in F. hepatica and S. mansoni an asparaginyl endopeptidase (otherwise known as legumain), which cleaves peptide bonds C-terminal to Asn residues, is involved in the trans-processing and activation of cathepsin L and B cysteine proteases. In support of this argument all members of these classes of cysteine protease in both helminth parasites possess an Asn residue in the vicinity of the prosegment/mature juncture (8, 60, 61). Furthermore, Sajid et al. (62) and Beckham et al. (57) provided experimental evidence by showing that a recombinant asparaginyl endopeptidase could trans-process and activate recombinant cathepsin B of F. hepatica and S. mansoni. In the present study, we identified two asparaginyl endopeptidases in the somatic proteins and secretome of Fasciola NEJs. One of the identified enzymes (legumain-like precursor, TrEMBL accession number Q711M2; termed legumain 1) matched exactly with an N-terminal sequence for asparaginyl endopeptidase obtained from NEJ somatic proteins by Tkalcevic et al. (34). Although this enzyme retains the His158 of the conserved catalytic dyad residues, the Cys200 is replaced by a serine residue, and therefore this enzyme may display an altered substrate specificity compared with the normal asparaginyl endopeptidases. However, the enzyme does contain a conserved asparagine residue (Asn323) that is required for C-terminal processing to an active mature enzyme (63). The second legumain-like protease identified in the NEJ somatic protein extracts is encoded by a Fasciola EST (identifier Fhep29h09.q1k; termed legumain 2), but as this EST is incomplete at the 5′-end, it is not possible to determine whether the enzyme encoded by this sequence retains the conserved active site His158/Cys200 dyad. Interestingly the enzyme encoded by the legumain 2 EST lacks the conserved Asn323 required for autoactivation. The overlapping primary sequence of the two enzymes exhibits 51% identity, and therefore individual enzymes were easily differentiated on the basis of their tryptic peptides. Notwithstanding the anomalies within the sequence of these two enzymes, the data suggest that the NEJ possesses the enzymatic machinery to rapidly process and activate the stored zymogens of cathepsins L and B. Recently Morita et al. (64) reported that purified bovine asparaginyl endopeptidase can degrade fibronectin, a major component of the extracellular matrix. If they possess similar biochemical properties, a second tangible role for the asparaginyl endopeptidases secreted by the infective larvae could be to facilitate penetration of the host intestine.
Proteomics Analysis of the Migrating Liver Stage Parasite
The developmental stage of F. hepatica that migrates through the liver tissue is responsible for the clinical manifestations associated with acute animal and human fasciolosis. The migrating parasite causes extensive physical tissue destruction, tunneling, and hemorrhaging and induces immunologically related inflammatory damage (65). To gain insight into the variety of molecules produced by this parasite stage that may induce this pathology 21-day-old immature parasites were removed from the liver of infected mice and maintained in culture. A proteomics analysis of the medium revealed that these parasites secreted a greater range of proteins than the infective and adult stage flukes. In particular, they secreted the greatest range of cathepsin L cysteine proteases, including the FhCL3 that we identified in the NEJ (this was secreted solely as a mature active form of ∼24 kDa). Members of cathepsin L clades FhCL1, FhCL2, and FhCL5 originally identified in the adult parasite were also secreted by the immature flukes (these could be distinguished on the basis of the clade-/subclade-specific peptide matches described above and reported previously by Robinson et al. (8)).
Fasciola cathepsins B1 and B2 were also secreted by 21-day-old immature flukes, whereas cathepsin B3 was not detected. However, mass spectrometry data matched with two novel putative cathepsin B enzymes that were discovered in the EST2Secretome of adult ESTs. The enzyme encoded by EST Fhep45b05.q1k (designated cathepsin B5) was identified based on matches with the sequence-specific peptides NIMYEIMK (m/z 521.25, +2), LLGWGVEDGEK (m/z 601.79, +2), and FYAISSYNVYGGEK (m/z 799.36, +2) whereas the other, EST HAN4006g01.q1kT3 (designated cathepsin B6), was differentiated due to specific matches with the peptides FSTPK (m/z 579.29, +1), HTTGALLGGHAIR (m/z 652.35, +2), and TSYNLLHNEETIMK (m/z 846.90, +2).
Three asparaginyl endopeptidases were identified within the secretory proteins of the immature 21-day-old flukes. These included the NEJ legumain-like precursor legumain 1 with the altered Cys200/Ser200 active site residue but did not include the NEJ legumain 2. However, two asparaginyl endopeptidases (designated legumains 3 and 4) appeared to be specific to the liver migrating stage and, like their homologues that were originally identified in F. gigantica (41), retained the conserved catalytic His158/Cys200 dyad and Asn323 that is required for C-terminal processing (63). Unlike the asparaginyl endopeptidases of NEJs, those secreted by the immature flukes all migrated at ∼36 kDa suggesting that they had undergone some processing events to lead to fully active enzymes. It is possible that besides functioning in the trans-processing of cathepsins L and B the asparaginyl endopeptidases of the immature parasites participate in host tissue degradation.
Mass spectrometry data from the immature 21-day-old F. hepatica (and subsequently found in the adult parasite secretome) matched with several ESTs encoding a putative prolylcarboxypeptidase (Fig. 2B and Table III). The exopeptidase contains a predicted N-terminal signal peptide and a conserved catalytic triad (Ser161, Asp413, and His438) that is characteristic of the s28 family of serine peptidases including prolylcarboxypeptidase. Although this is the first report of a putative prolylcarboxypeptidase from a trematode pathogen, orthologues have been identified in parasitic nematodes including the gastrointestinal worm Haemonchus contortus where the enzymes act as anticoagulants to facilitate a blood-feeding lifestyle (66). It is likely that the Fasciola prolylcarboxypeptidase performs a similar role, acting alongside the saposin-like proteins to prevent blood coagulation to ensure effective lysis of ingested host red blood cells. A role for the putative prolylcarboxypeptidase in Fasciola nutrition is supported by the absence of the enzyme in the NEJ, which does not possess a functional gut.
Relative Expression Levels of Fasciola Proteases and Relationship to Virulence and Tissue Invasion
For F. hepatica NEJs, the emPAI data indicated that cathepsin L3 and cathepsin B enzymes were expressed at similar high levels (accounting for 37 and 45% of total proteases, respectively) whereas asparaginyl endopeptidases represented 18% of total proteases detected. By using inhibitors of cathepsin-like proteases we showed that excystment of the infective larvae is dependent on the cathepsin L3 and cathepsin B proteases. Asparaginyl endopeptidases are likely to be essential for the rapid trans-processing and activation of the stored zymogens of cathepsins L and B, and hence it is probable that one of the first steps in activation of the dormant larvae is the switching on of the genes encoding these enzymes. A role for cathepsin L and B proteases in the penetration of the host by NEJ was recently demonstrated using RNA interference methods by McGonigle et al. (67). Knockdown of both cathepsin L and cathepsin B transcripts in NEJ parasites blocked the ability of the larvae to penetrate and traverse the intestinal wall of a rodent host. Therefore, the two types of cysteine proteases, working together, must be responsible for degrading the intestinal tissue macromolecules to enable the rapid penetration of the host intestine. Cysteine proteases are also essential for infectivity of the closely related schistosome parasites (S. mansoni and Trichobilharzia regenti) that enter their hosts via the skin (68) and for the cyst emergence and infectivity of larvae of the trematode Paragonimus westermani (69–71).
Correlating with the migration of the parasites into the liver tissue and the development of the gut apparatus (beginning at ∼10 days; Ref. 36) the expression pattern of proteases becomes more complex. For the migrating parasite, the secretion of FhCL3 and cathepsin B becomes less important as these proteases account for only 3 and 2% of total proteases, respectively. In contrast, members of the other cathepsin L clades become more highly represented in the secretome: FhCL1 (50%), FhCL2 (27%), and FhCL5 (7%) (Fig. 2A). This striking shift in protease expression indicates a requirement for a new set of proteases for liver migration and for the degradation of tissue and blood macromolecules. As discussed above FhCL1 proteases have a particular adaptation to hemoglobin digestion whereas FhCL2 proteases exhibit unique collagenase-like activities (FhCL5 appears to be more closely related to FhCL1; Ref. 72). Asparaginyl endopeptidase secretion remains relatively high, accounting for 11% of total proteases secreted by the immature flukes, that may be required for activation of the cathepsin L proteases and possibly for directly engaging in tissue degradation. The expression of the prolylcarboxypeptidase and saposin at this stage signals the beginning of blood feeding. Working together this profile of molecules is an effective tissue-destroying machinery essential to parasite migration and growth but, at the same time, responsible for the pathogenesis associated with acute animal and human fasciolosis.
After the parasite has entered the bile duct it completes its maturation and becomes an obligate blood feeder. Blood provides all the necessary nutrients (macro- and micromolecules) needed for production of an enormous numbers of eggs, the principle function of the adult parasite (37). The total protease secretion is assumed completely by the various cathepsin L proteases, clades FhCL1 (69%), FhCL2 (22%), and FhCL5 (9%), which can degrade macromolecules such as hemoglobin to small peptides that are absorbed by the parasite for further digestion to free amino acids within its tissues (36, 37). The emPAI values determined for adult parasite proteases are similar to expression data obtained using 2-DE and densitometry (67, 27, and 5%, respectively; Ref. 8) and validate the use of emPAI for estimating Fasciola secretory protein levels in the other developmental stages. Within the bile duct the parasite can exist for years (cattle) or even decades (sheep) causing relatively little pathology apart from anemia and bile duct hyperplasia.
F. hepatica Secretes a Battery of Antioxidant Molecules
It is worth highlighting that for all three developmental stages of F. hepatica investigated in this study an array of antioxidant molecules was secreted in an abundance second only to the proteases. These included peroxiredoxin, thioredoxin, protein-disulfide isomerase, four fatty acid-binding proteins (FaBP1–3 and Fh15), and five GSTs (Sigma and Mu classes) (20, 21, 32, 46–48, 73). These molecules have been implicated in fluke immune avoidance mechanisms; for example, secreted isoforms of Fasciola GSTs were shown to decrease the proliferative response of rat spleen cells and diminish nitric oxide production by macrophages (73) whereas peroxiredoxin plays a key role driving host Th2 immune responses via the recruitment and alternative activation of peritoneal macrophages (74, 75). Fasciola peroxiredoxin, thioredoxin, and protein-disulfide isomerase may also protect the parasites from harmful reactive oxygen species released by host immune cells (32, 47, 48, 76).
Analysis of the emPAI values for these various antioxidants indicates that, like the proteases, their secretion is highly regulated during the migration of the parasite (Fig. 3A). The profile of secretory antioxidants is similar for the NEJs and adult flukes; both secrete several FaBPs together with peroxiredoxin (although thioredoxin is also secreted by the adult worms). However, the diversity of the antioxidant molecules secreted by the immature liver stage parasites is notably different from those secreted by the other two stages with the dramatic production of GSTs, including Sigma class GST and four isoforms of Mu class GSTs (GST1, GST7, GST47, and GST 51); together these account for 50% of total antioxidants expressed at this stage. GST may provide a particular defense for the fluke against a mounting host cellular immune response as the immature parasite is in direct contact with the immune system as it migrates through the liver parenchyma. At this stage also the parasite has moved from an aerobic to an anaerobic environment, and therefore significant changes take place in its metabolism, particularly in the tegument, which is the primary host-parasite interface (77). Clearly the combined action of FaBPs, peroxiredoxin, and thioredoxin offers sufficient protection for the adult flukes residing within the immunologically privileged bile ducts.
It was also noted that each of the aforementioned antioxidant classes found in the secretome of F. hepatica does not possess a signal sequence for secretion (with the exception of protein-disulfide isomerase that also has a putative ER retention signal and may act as a chaperone to ensure proper folding of classically secreted Fasciola peptides). This suggests that they are secreted via non-classical mechanisms, possibly shedding or blebbing of the tegument (see above). Accordingly we propose that two distinct mechanisms for protein export operate for the most abundant proteins in Fasciola: (a) a classical secretion of proteases and other molecules via the ER/Golgi from specialized gastrodermal cells of the gut as reported previously (78) and (b) a non-classical secretion of leaderless Fasciola antioxidants via a trans-tegumental route. This has clear implications for the development of novel anti-F. hepatica chemotherapy and could explain the additive effects of two of the most potent flukicides, triclabendazole and clorsulon, which act upon the tegument and gastrodermal cells, respectively (79–81).
Conclusion
Our integrated transcriptomics and proteomics analysis has provided an in-depth overview of protein secretion by the animal and human pathogen F. hepatica that represents a significant step toward a comprehensive understanding of the host-parasite interactions in fasciolosis. Of vital importance now is the completion of transcriptome data sets for both the infective NEJ larvae and immature liver stage parasites so that a comparative analysis of secretory proteins can be performed. Nevertheless we have described the secretion of the major molecules of this parasite and correlated their expression with critical steps in its migration and development within the mammalian host. This will allow a more strategic and rational approach to future anthelmintic or vaccine development programs. It is interesting that several of the major components of Fasciola secretions identified here are currently leading candidates for development as first generation antifluke vaccines (including cathepsins, peroxiredoxin, glutathione S-transferase, and fatty acid-binding proteins; Ref. 82) or are promising targets for novel flukicidal drugs (cathepsins; Ref. 83) demonstrating the value of our integrated approach for the future identification of new targets for therapeutic intervention.
Acknowledgments
We thank Matt Padula, Proteomics Technology Centre of Expertise, University of Technology Sydney, for assistance with the mass spectrometry and Matt Berriman of the Wellcome Trust Sanger Centre.
Footnotes
* The bioinformatics analysis was supported in part by Australian Research Council Grant LP0667795.
 The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
2 E. Ljunggren, M. Kozak, and L. Jedlina-Panasiuk, unpublished data.
3 E. Khaznadji, M. Peloille, and N. Moire, unpublished data.
1 The abbreviations used are:
- NEJ
- newly excysted juvenile
- EST
- expressed sequence tag
- rEST
- representative expressed sequence tag
- BLAST
- basic local alignment search tool
- ER
- endoplasmic reticulum
- 2-DE
- two-dimensional electrophoresis
- 1-DE
- one-dimensional electrophoresis
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- MSDB
- mass spectrometry protein sequence database
- emPAI
- exponentially modified protein abundance index
- E-64
- trans-epoxysuccinyl-l-leucylamido(4-guanidino)butane
- Z-Phe-Ala-CHN2
- carbobenzoxy-phenylalanyl-alanine-diazomethyl ketone
- FaBP
- fatty acid-binding protein
- ABC
- ATP-binding cassette
- vpB1
- vitelline protein B1
- IL
- interleukin
- RNAi
- RNA interference
- 1-D
- one-dimensional.
REFERENCES
- 1.Garcia H. H., Moro P. L., Schantz P. M. ( 2007) Zoonotic helminth infections of humans: echinococcosis, cysticercosis and fascioliasis. Curr. Opin. Infect. Dis. 20, 489– 494 [DOI] [PubMed] [Google Scholar]
- 2.Andrews S. J. ( 1999) The life-cycle of Fasciola hepatica, in Fasciolosis ( Dalton J. P. ed) pp. 1– 29, CAB International, Oxford [Google Scholar]
- 3.Mas-Coma S., Bargues M. D., Esteban J. G. ( 1999) Human fasciolosis, in Fasciolosis ( Dalton J. P. ed) pp. 411– 434, CAB International, Oxford [Google Scholar]
- 4.Fairweather I., Threadgold L. T., Hanna R. E. ( 1999) Development of Fasciola hepatica in the mammalian host, in Fasciolosis ( Dalton J. P. ed) pp. 47– 111, CAB International, Oxford [Google Scholar]
- 5.Hewitson J. P., Harcus Y. M., Curwen R. S., Dowle A. A., Atmadja A. K., Ashton P. D., Wilson A., Maizels R. M. ( 2008) The secretome of the filarial parasite, Brugia malayi: proteomic profile of adult excretory-secretory products. Mol. Biochem. Parasitol. 160, 8– 21 [DOI] [PubMed] [Google Scholar]
- 6.Robinson M. W., Connolly B. ( 2005) Proteomic analysis of the excretory-secretory proteins of the Trichinella spiralis L1 larva, a nematode parasite of skeletal muscle. Proteomics 5, 4525– 4532 [DOI] [PubMed] [Google Scholar]
- 7.Robinson M. W, Greig R., Beattie K. A., Lamont D. J., Connolly B. ( 2007) Comparative analysis of the excretory-secretory proteome of the muscle larva of Trichinella pseudospiralis and Trichinella spiralis. Int. J. Parasitol. 37, 139– 148 [DOI] [PubMed] [Google Scholar]
- 8.Robinson M. W., Tort J. F., Lowther J., Donnelly S. M., Wong E., Xu W., Stack C. M., Padula M., Herbert B., Dalton J. P. ( 2008) Proteomics and phylogenetic analysis of the cathepsin L protease family of the helminth pathogen, Fasciola hepatica: expansion of a repertoire of virulence-associated factors. Mol. Cell. Proteomics 7, 1111– 1123 [DOI] [PubMed] [Google Scholar]
- 9.Knudsen G. M., Medzihradszky K. F., Lim K. C., Hansell E., McKerrow J. H. ( 2005) Proteomic analysis of Schistosoma mansoni cercarial secretions. Mol. Cell. Proteomics 4, 1862– 1875 [DOI] [PubMed] [Google Scholar]
- 10.Ojopi E. P., Oliveira P. S., Nunes D. N., Paquola A., DeMarco R., Gregório S. P., Aires K. A., Menck C. F., Leite L. C., Verjovski-Almeida S., Dias-Neto E. ( 2007) A quantitative view of the transcriptome of Schistosoma mansoni adult-worms using SAGE. BMC Genomics 8, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliveira G. ( 2007) The Schistosoma mansoni transcriptome: an update. Exp. Parasitol. 117, 229– 235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laha T., Pinlaor P., Mulvenna J., Sripa B., Sripa M., Smout M. J., Gasser R. B., Brindley P. J., Loukas A. ( 2007) Gene discovery for the carcinogenic human liver fluke, Opisthorchis viverrini. BMC Genomics 8, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagaraj S. H., Gasser R. B., Ranganathan S. ( 2008) Needles in the EST haystack: large-scale identification and analysis of excretory-secretory (ES) proteins in parasitic nematodes using expressed sequence tags (ESTs). PLoS Negl. Trop. Dis. 2, e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagaraj S. H., Deshpande N., Gasser R. B., Ranganathan S. ( 2007) ESTExplorer: an expressed sequence tag (EST) assembly and annotation platform. Nucleic Acids Res. 35, W143– 147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagaraj S. H., Gasser R. B., Nisbet A. J., Ranganathan S. ( 2008) In silico analysis of expressed sequence tags from Trichostrongylus vitrinus (Nematoda): comparison of the automated ESTExplorer workflow platform with conventional database searches. BMC Bioinformatics 9, Suppl. 1, S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranganathan S., Nagaraj S. H., Hu M., Strube C., Schnieder T., Gasser R. B. ( 2007) A transcriptomic analysis of the adult stage of the bovine lungworm, Dictyocaulus viviparus. BMC Genomics 8, 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datu B. J., Gasser R. B., Nagaraj S. H., Ong E. K., O'Donoghue P., McInnes R., Ranganathan S., Loukas A. ( 2008) Transcriptional changes in the hookworm, Ancylostoma caninum, during the transition from a free-living to a parasitic larva. PLoS Negl. Trop. Dis. 2, e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. ( 1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389– 3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zdobnov E. M, Apweiler R. ( 2001) InterProScan: an integration platform for the signature-recognition methods in InterPro. Bioinformatics 17, 847– 848 [DOI] [PubMed] [Google Scholar]
- 20.Jefferies J. R., Campbell A. M., van Rossum A. J., Barrett J., Brophy P. M. ( 2001) Proteomic analysis of Fasciola hepatica excretory-secretory products. Proteomics 1, 1128– 1132 [DOI] [PubMed] [Google Scholar]
- 21.Morphew R. M., Wright H. A., LaCourse E. J., Woods D. J., Brophy P. M. ( 2007) Comparative proteomics of excretory-secretory proteins released by the liver fluke Fasciola hepatica in sheep host bile and during in vitro culture ex host. Mol. Cell. Proteomics 6, 963– 972 [DOI] [PubMed] [Google Scholar]
- 22.Ishihama Y., Oda Y., Tabata T., Sato T., Nagasu T., Rappsilber J., Mann M. ( 2005) Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteomics 4, 1265– 1272 [DOI] [PubMed] [Google Scholar]
- 23.El-Sayed N. M., Bartholomeu D., Ivens A., Johnston D. A., LoVerde P. T. ( 2004) Advances in schistosome genomics. Trends Parasitol. 20, 154– 157 [DOI] [PubMed] [Google Scholar]
- 24.Mitreva M., Zarlenga D. S., McCarter J. P., Jasmer D. P. ( 2007) Parasitic nematodes: from genomes to control. Vet. Parasitol. 148, 31– 42 [DOI] [PubMed] [Google Scholar]
- 25.Stein L. D., Bao Z., Blasiar D., Blumenthal T., Brent M. R., Chen N., Chinwalla A., Clarke L., Clee C., Coghlan A., Coulson A., D'Eustachio P., Fitch D. H., Fulton L. A., Fulton R. E., Griffiths-Jones S., Harris T. W., Hillier L. W., Kamath R., Kuwabara P. E., Mardis E. R., Marra M. A., Miner T. L., Minx P., Mullikin J. C., Plumb R. W., Rogers J., Schein J. E., Sohrmann M., Spieth J., Stajich J. E., Wei C., Willey D., Wilson R. K., Durbin R., Waterston R. H. ( 2003) The genome sequence of Caenorhabditis briggsae: a platform for comparative genomics. PLoS Biol. 1, E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y., Zhang Y., Yin Y., Gao G., Li S., Jiang Y., Gu X., Luo J. ( 2005) SPD—a web-based secreted protein database. Nucleic Acids Res. 33, D169– 173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choo K. H., Tan T. W., Ranganathan S. ( 2005) SPdb—a signal peptide database. BMC Bioinformatics 6, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancela M., Acosta D., Rinaldi G., Silva E., Durán R., Roche L., Zaha A., Carmona C., Tort J. F. ( 2008) A distinctive repertoire of cathepsins is expressed by juvenile invasive Fasciola hepatica. Biochimie 90, 1461– 1475 [DOI] [PubMed] [Google Scholar]
- 29.Grams R., Adisakwattana P., Ritthisunthorn N., Eursitthichai V., Vichasri-Grams S., Viyanant V. ( 2006) The saposin-like proteins 1, 2, and 3 of Fasciola gigantica. Mol. Biochem. Parasitol. 148, 133– 143 [DOI] [PubMed] [Google Scholar]
- 30.Espino A. M., Hillyer G. V. ( 2003) Molecular cloning of a member of the Fasciola hepatica saposin-like protein family. J. Parasitol. 89, 545– 552 [DOI] [PubMed] [Google Scholar]
- 31.Robinson M. W., Colhoun L. M., Fairweather I., Brennan G. P., Waite J. H. ( 2001) Development of the vitellaria of the liver fluke, Fasciola hepatica in the rat host. Parasitology 123, 509– 518 [DOI] [PubMed] [Google Scholar]
- 32.Salazar-Calderón M., Martín-Alonso J. M., Castro A. M., Parra F. ( 2003) Cloning, heterologous expression in Escherichia coli and characterization of a protein disulfide isomerase from Fasciola hepatica. Mol. Biochem. Parasitol. 126, 15– 23 [DOI] [PubMed] [Google Scholar]
- 33.Bork P., Beckmann G. ( 1993) The CUB domain. A widespread module in developmentally regulated proteins. J. Mol. Biol. 231, 539– 545 [DOI] [PubMed] [Google Scholar]
- 34.Tkalcevic J., Ashman K., Meeusen E. ( 1995) Fasciola hepatica: rapid identification of newly excysted juvenile proteins. Biochem. Biophys. Res. Commun. 213, 169– 174 [DOI] [PubMed] [Google Scholar]
- 35.Irving J. A., Spithill T. W., Pike R. N., Whisstock J. C., Smooker P. M. ( 2003) The evolution of enzyme specificity in Fasciola spp. J. Mol. Evol. 57, 1– 15 [DOI] [PubMed] [Google Scholar]
- 36.Lowther J., Robinson M. W., Donnelly S. M., Xu W., Stack C. M., Matthews J. M., Dalton J. P. ( 2009) The importance of pH in regulating the function of Fasciola hepatica cathepsin L1 cysteine protease. PLoS. Negl. Trop. Dis. 3, e369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalton J. P., Caffrey C. R., Sajid M., Stack C., Donnelly S., Loukas A., Don T., McKerrow J., Halton D. W., Brindley P. J. ( 2006) Proteases in trematode biology, in Parasitic Flatworms: Molecular Biology, Biochemistry, Immunology and Physiology ( Maule A. G., Marks N. J. eds) pp. 348– 368, CAB International, Oxford [Google Scholar]
- 38.Stack C. M., Caffrey C. R., Donnelly S. M., Seshaadri A., Lowther J., Tort J. F., Collins P. R., Robinson M. W., Xu W., McKerrow J. H., Craik C. S., Geiger S. R., Marion R., Brinen L. S., Dalton J. P. ( 2008) Structural and functional relationships in the virulence-associated cathepsin L proteases of the parasitic liver fluke, Fasciola hepatica. J. Biol. Chem. 283, 9896– 9908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Don T. A., Oksov Y., Lustigman S., Loukas A. ( 2007) Saposin-like proteins from the intestine of the blood-feeding hookworm, Ancylostoma caninum. Parasitology 134, 427– 436 [DOI] [PubMed] [Google Scholar]
- 40.Law R. H., Smooker P. M., Irving J. A., Piedrafita D., Ponting R., Kennedy N. J., Whisstock J. C., Pike R. N., Spithill T. W. ( 2003) Cloning and expression of the major secreted cathepsin B-like protein from juvenile Fasciola hepatica and analysis of immunogenicity following liver fluke infection. Infect. Immun. 71, 6921– 6932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adisakwattana P., Viyanant V., Chaicumpa W., Vichasri-Grams S., Hofmann A., Korge G., Sobhon P., Grams R. ( 2007) Comparative molecular analysis of two asparaginyl endopeptidases and encoding genes from Fasciola gigantica. Mol. Biochem. Parasitol. 156, 102– 116 [DOI] [PubMed] [Google Scholar]
- 42.Turk D., Guncar G. ( 2003) Lysosomal cysteine proteases (cathepsins): promising drug targets. Acta Crystallogr. D Biol. Crystallogr. 59, 203– 213 [DOI] [PubMed] [Google Scholar]
- 43.Chen J. M., Dando P. M., Stevens R. A., Fortunato M., Barrett A. J. ( 1998) Cloning and expression of mouse legumain, a lysosomal endopeptidase. Biochem. J. 335, 111– 117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michel A., Ghoneim H., Resto M., Klinkert M. Q., Kunz W. ( 1995) Sequence, characterization and localization of a cysteine proteinase cathepsin L in Schistosoma mansoni. Mol. Biochem. Parasitol. 73, 7– 18 [DOI] [PubMed] [Google Scholar]
- 45.Meemon K., Grams R., Vichasri-Grams S., Hofmann A., Korge G., Viyanant V., Upatham E. S., Habe S., Sobhon P. ( 2004) Molecular cloning and analysis of stage and tissue-specific expression of cathepsin B encoding genes from Fasciola gigantica. Mol. Biochem. Parasitol. 136, 1– 10 [DOI] [PubMed] [Google Scholar]
- 46.Gourbal B. E., Guillou F., Mitta G., Sibille P., Thèron A., Pointier J. P., Coustau C. ( 2008) Excretory-secretory products of larval Fasciola hepatica investigated using a two-dimensional proteomic approach. Mol. Biochem. Parasitol. 161, 63– 66 [DOI] [PubMed] [Google Scholar]
- 47.Salazar-Calderón M., Martín-Alonso J. M., Ruiz de Eguino A. D., Parra F. ( 2001) Heterologous expression and functional characterization of thioredoxin from Fasciola hepatica. Parasitol Res. 87, 390– 395 [DOI] [PubMed] [Google Scholar]
- 48.Sekiya M., Mulcahy G., Irwin J. A., Stack C. M., Donnelly S. M., Xu W., Collins P., Dalton J. P. ( 2006) Biochemical characterisation of the recombinant peroxiredoxin (FhePrx) of the liver fluke, Fasciola hepatica. FEBS Lett. 580, 5016– 5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emanuelsson O., Brunak S., von Heijne G., Nielsen H. ( 2007) Locating proteins in the cell using TargetP, SignalP, and related tools. Nat. Protoc. 2, 953– 971 [DOI] [PubMed] [Google Scholar]
- 50.Dalton J. P., Skelly P., Halton D. W. ( 2004) Role of the tegument and gut in nutrient uptake by parasitic platyhelminths. Can. J. Zool. 82, 211– 232 [Google Scholar]
- 51.Keller M., Rüegg A., Werner S., Beer H. D. ( 2008) Active caspase-1 is a regulator of unconventional protein secretion. Cell 132, 818– 831 [DOI] [PubMed] [Google Scholar]
- 52.MacKenzie A., Wilson H. L., Kiss-Toth E., Dower S. K., North R. A., Surprenant A. ( 2001) Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity 15, 825– 835 [DOI] [PubMed] [Google Scholar]
- 53.Andrei C., Margiocco P., Poggi A., Lotti L. V., Torrisi M. R., Rubartelli A. ( 2004) Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: implications for inflammatory processes. Proc. Natl. Acad. Sci. U.S.A. 101, 9745– 9750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mambula S. S., Stevenson M. A., Ogawa K., Calderwood S. K. ( 2007) Mechanisms for Hsp70 secretion: crossing membranes without a leader. Methods 43, 168– 175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumkate S., Chunchob S., Janvilisri T. ( 2008) Expression of ATP-binding cassette multidrug transporters in the giant liver fluke Fasciola gigantica and their possible involvement in the transport of bile salts and anthelmintics. Mol. Cell. Biochem. 317, 77– 84 [DOI] [PubMed] [Google Scholar]
- 56.Harmsen M. M., Cornelissen J. B., Buijs H. E., Boersma W. J., Jeurissen S. H., van Milligen F. J. ( 2004) Identification of a novel Fasciola hepatica cathepsin L protease containing protective epitopes within the propeptide. Int. J. Parasitol. 34, 675– 682 [DOI] [PubMed] [Google Scholar]
- 57.Beckham S. A., Law R. H., Smooker P. M., Quinsey N. S., Caffrey C. R., McKerrow J. H., Pike R. N., Spithill T. W. ( 2006) Production and processing of a recombinant Fasciola hepatica cathepsin B-like enzyme (FhcatB1) reveals potential processing mechanisms in the parasite. Biol. Chem. 387, 1053– 1061 [DOI] [PubMed] [Google Scholar]
- 58.Coulombe R., Grochulski P., Sivaraman J., Ménard R., Mort J. S., Cygler M. ( 1996) Structure of human procathepsin L reveals the molecular basis of inhibition by the prosegment. EMBO J. 15, 5492– 5503 [PMC free article] [PubMed] [Google Scholar]
- 59.Dalton J. P., Brindley P. J., Donnelly S., Robinson M. W. ( 2009) The enigmatic asparaginyl endopeptidase of helminth parasites. Trends Parasitol. 25, 59– 61 [DOI] [PubMed] [Google Scholar]
- 60.Dalton J. P., Brindley P. J. ( 1996) Schistosome asparaginyl endopeptidase Sm 32 in hemoglobin digestion. Parasitol. Today 12, 125. [DOI] [PubMed] [Google Scholar]
- 61.Robinson M. W., Dalton J. P., Donnelly S. ( 2008) Helminth pathogen cathepsin proteases: it's a family affair. Trends Biochem. Sci. 33, 601– 608 [DOI] [PubMed] [Google Scholar]
- 62.Sajid M., McKerrow J. H., Hansell E., Mathieu M. A., Lucas K. D., Hsieh I., Greenbaum D., Bogyo M., Salter J. P., Lim K. C., Franklin C., Kim J. H., Caffrey C. R. ( 2003) Functional expression and characterization of Schistosoma mansoni cathepsin B and its trans-activation by an endogenous asparaginyl endopeptidase. Mol. Biochem. Parasitol. 131, 65– 75 [DOI] [PubMed] [Google Scholar]
- 63.Chen J. M., Fortunato M., Barrett A. J. ( 2000) Activation of human prolegumain by cleavage at a C-terminal asparagine residue. Biochem. J. 352, 327– 334 [PMC free article] [PubMed] [Google Scholar]
- 64.Morita Y., Araki H., Sugimoto T., Takeuchi K., Yamane T., Maeda T., Yamamoto Y., Nishi K., Asano M., Shirahama-Noda K., Nishimura M., Uzu T., Hara-Nishimura I., Koya D., Kashiwagi A., Ohkubo I. ( 2007) Legumain/asparaginyl endopeptidase controls extracellular matrix remodeling through the degradation of fibronectin in mouse renal proximal tubular cells. FEBS Lett. 581, 1417– 1424 [DOI] [PubMed] [Google Scholar]
- 65.Behm C. A., Sangster N. C. ( 1999) Pathology, pathophysiology and clinical aspects, in Fasciolosis ( Dalton J. P. ed) pp. 185– 224, CAB International, Oxford [Google Scholar]
- 66.Geldhof P., Knox D. ( 2008) The intestinal contortin structure in Haemonchus contortus: an immobilised anticoagulant? Int. J. Parasitol. 38, 1579– 1588 [DOI] [PubMed] [Google Scholar]
- 67.McGonigle L., Mousley A., Marks N. J., Brennan G. P., Dalton J. P., Spithill T. W., Day T. A., Maule A. G. ( 2008) The silencing of cysteine proteases in Fasciola hepatica newly excysted juveniles using RNA interference reduces gut penetration. Int. J. Parasitol. 38, 149– 155 [DOI] [PubMed] [Google Scholar]
- 68.Kasny M., Dalton J. P., Mikes L., Horák P. ( 2007) Comparison of cysteine and serine peptidase activities in Trichobilharzia regenti and Schistosoma mansoni cercariae. Parasitology 134, 1599– 1609 [DOI] [PubMed] [Google Scholar]
- 69.Ikeda T. ( 2003) Involvement of cysteine proteinases in excystment of Paragonimus ohirai metacercariae induced by sodium cholate and A23187. J. Helminthol. 77, 21– 26 [DOI] [PubMed] [Google Scholar]
- 70.Chung Y. B., Kim T. S., Yang H. J. ( 2005) Early cysteine protease activity in excretory bladder triggers metacercaria excystment of Paragonimus westermani. J. Parasitol. 91, 953– 954 [DOI] [PubMed] [Google Scholar]
- 71.Na B. K., Kim S. H., Lee E. G., Kim T. S., Bae Y. A., Kang I., Yu J. R., Sohn W. M., Cho S. Y., Kong Y. ( 2006) Critical roles for excretory-secretory cysteine proteases during tissue invasion of Paragonimus westermani newly excysted metacercariae. Cell. Microbiol. 8, 1034– 1046 [DOI] [PubMed] [Google Scholar]
- 72.Smooker P. M., Whisstock J. C., Irving J. A., Siyaguna S., Spithill T. W., Pike R. N. ( 2000) A single amino acid substitution affects substrate specificity in cysteine proteinases from Fasciola hepatica. Protein Sci. 9, 2567– 2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cervi L., Rossi G., Masih D. T. ( 1999) Potential role for excretory-secretory forms of glutathione-S-transferase (GST) in Fasciola hepatica. Parasitology 119, 627– 633 [DOI] [PubMed] [Google Scholar]
- 74.Donnelly S., O'Neill S. M., Sekiya M., Mulcahy G., Dalton J. P. ( 2005) Thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages. Infect. Immun. 73, 166– 173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Donnelly S., Stack C. M., O'Neill S. M., Sayed A. A., Williams D. L., Dalton J. P. ( 2008) Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages. FASEB J. 22, 4022– 4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGonigle S., Curley G. P., Dalton J. P. ( 1997) Cloning of peroxiredoxin, a novel antioxidant enzyme, from the helminth parasite Fasciola hepatica. Parasitology 115, 101– 104 [DOI] [PubMed] [Google Scholar]
- 77.Tielens G. M. ( 1999) Metabolism, in Fasciolosis ( Dalton J. P. ed) pp. 277– 306, CAB International, Oxford [Google Scholar]
- 78.Collins P. R., Stack C. M., O'Neill S. M., Doyle S., Ryan T., Brennan G. P., Mousley A., Stewart M., Maule A. G., Dalton J. P., Donnelly S. ( 2004) Cathepsin L1, the major protease involved in liver fluke (Fasciola hepatica) virulence: propeptide cleavage sites and autoactivation of the zymogen secreted from gastrodermal cells. J. Biol. Chem. 279, 17038– 17046 [DOI] [PubMed] [Google Scholar]
- 79.Robinson M. W., Trudgett A., Hoey E. M., Fairweather I. ( 2002) Triclabendazole-resistant Fasciola hepatica: β-tubulin and response to in vitro treatment with triclabendazole. Parasitology 124, 325– 338 [DOI] [PubMed] [Google Scholar]
- 80.Meaney M., Allister J., McKinstry B., McLaughlin K., Brennan G. P., Forbes A. B., Fairweather I. ( 2007) Fasciola hepatica: ultrastructural effects of a combination of triclabendazole and clorsulon against mature fluke. Parasitol. Res. 100, 1091– 1104 [DOI] [PubMed] [Google Scholar]
- 81.Meaney M., Haughey S., Brennan G. P., Fairweather I. ( 2005) Ultrastructural observations on oral ingestion and trans-tegumental uptake of clorsulon by the liver fluke, Fasciola hepatica. Parasitol. Res. 95, 201– 212 [DOI] [PubMed] [Google Scholar]
- 82.McManus D. P., Dalton J. P. ( 2006) Vaccines against the zoonotic trematodes Schistosoma japonicum, Fasciola hepatica and Fasciola gigantica. Parasitology 133, S43– 61 [DOI] [PubMed] [Google Scholar]
- 83.Alcala-Canto Y., Ibarra-Velarde F., Sumano-Lopez H., Gracia-Mora J., Alberti-Navarro A. ( 2007) Effect of a cysteine protease inhibitor on Fasciola hepatica (liver fluke) fecundity, egg viability, parasite burden, and size in experimentally infected sheep. Parasitol. Res. 100, 461– 465 [DOI] [PubMed] [Google Scholar]