Summary
Increased deimination and peptidyl arginine deiminase type 2 (PAD2) expression has been observed in age-related neurodegenerative diseases without discrimination between their aging and disease component. Here, we describe reduced levels of deimination commensurate with reduced protein, mRNA and activity of peptidylarginine deiminase type 2 in the retina, optic nerve and plasma of aged rats when compared to young rats. The decrease was significant in the ganglion cell layer, inner plexiform layer and inner nuclear layer. Because our observations suggest reduced deimination is a consequence of aging, we conclude that increased deimination must be a consequence of disease. Our findings are important to understand late-onset and progressive diseases such as glaucoma, pseudoexfoliation syndrome, age-related macular degeneration and Oguchi’s disease.
Keywords: aging, deimination, neurodegenerative diseases, optic nerve, peptidylarginine deiminase type 2, retina
Posttranslational modifications (PTMs) to proteins are fundamental steps required in the regulation of many cellular processes. Proteins may undergo hundreds of PTMs. In aging, the most frequently discussed modifications include, but are not limited to, the oxidation of amino acid side chains (especially, side chains of prolyl, arginyl, lysyl and histidinyl residues) by mixed-function oxidation systems; the deamidation of asparaginyl and glutaminyl residues; the racemization and isomerization of aspartyl, asparaginyl and prolyl residues; the oxidation of cysteine sulfhydryl groups; and spontaneous changes in protein conformation that are apparently unlinked to changes in amino acid composition (Stadtman, 1988). Protein deimination is a PTM that is carried out by peptidyl arginine deiminases (PADs) and involves conversion of protein-bound arginine into citrulline (Vossenaar et al., 2003). Mammalian cells possess five protein deiminases, PAD1–4 and 6 (Vossenaar et al., 2003). Protein deimination is known to occur in epidermal, muscle and neuronal tissues. PAD1, 3 catalyze deimination in the skin; PAD2, the major PAD in the eye and brain; and PAD4 is nuclear and ubiquitous (Asaga & Ishigami, 2001; Vossenaar et al., 2003; Bhattacharya et al., 2006b). PAD4 activation was suggested to result in transcriptional repression (Wang et al., 2004). Elevated levels of PAD2 and protein deimination have been found in rheumatoid arthritis (Scofield, 2004), and in several human neurological diseases such as multiple sclerosis (Moscarello et al., 2002), autoimmune encephalomyelitis (Nicholas et al., 2005), Alzheimer’s (Maruyama et al., 2005; Louw et al., 2007), amyotrophic lateral sclerosis (Chou et al., 1996) and glaucoma (Bhattacharya et al., 2006a, b). Using proteomic mass spectrometry, PAD2 was recently identified in the optic nerve of glaucomatous donors but not in normal controls (Bhattacharya et al., 2006b). Only a handful of proteins: keratin, myelin basic protein (MBP), glial fibrillary acidic protein, vimentin, trichohyalin, histones (H2A, H3 and H4), filaggrin and fibrinogen are currently known to undergo deimination (Algeciras & Bhattacharya, 2007). Modulation in levels of deimination has not been ascribed to a specific physiological condition as yet. Moreover, it remains unknown whether protein deimination is associated with the process of aging, a specific phenotype of aging or is likely to be mechanistically related to a disease process (Schoneich, 2006). It is therefore important to establish the cause of the observed increased deimination in glaucoma and multiple sclerosis. Here, we have investigated changes in deiminated proteins systemically, in the retina and in the optic nerve associated with the process of normal aging utilizing the F1 hybrid between Fischer 344 and Brown Norway rats (F344BN).
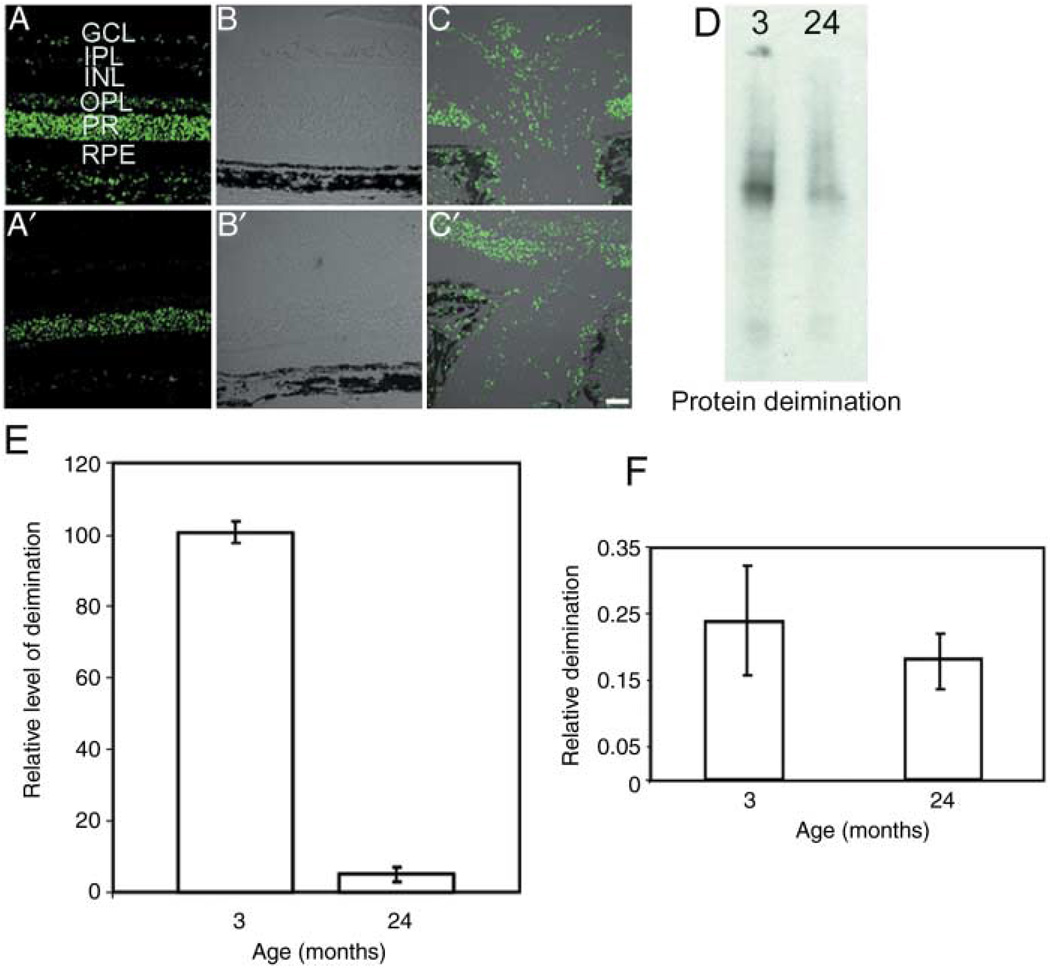
The presence of deiminated proteins was investigated in the retina and optic nerve of young (3-month-old to 4-month-old) and aged (24-month-old to 25-month-old) F344BN rats (Fig. 1). Immunohistochemistry of cryosections showed that the retina (Fig. 1A,B,A’, B’) and the optic nerve (Fig. 1C,C’) of the F344BN rats shows decreased levels of deiminated proteins in the aged animals when compared to the young ones. The decrease was significant in the ganglion cell layer, inner plexiform layer and inner nuclear layer (Fig. 1A,A’). Immunoblot quantification (Fig. 1D,E) of retinal extracts corroborated the immunohistochemical observation. Moreover, enzyme-linked immunosorbent assay analysis of the blood serum showed a decreased level of citrullinated proteins (Fig. 1F), suggesting that protein deimination was also reduced systemically in aged animals. Our observations in aged retina are consistent with findings of heavily deiminated MBP in infants and significant reduction in adults (Moscarello et al., 1994).
Fig. 1.
Decreased deiminated proteins in aged rats. (A–C) Immunohistochemical analyses. The retina (A–A’ and B–B’) and the optic nerve (C’–C’) of F344BN rats probed with anti-citrulline and a secondary Alexa 488 antibody. GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; OPL, outer plexiform layer; PR, photoreceptor; RPE, retinal pigment epithelium. Upper and lower panels correspond to 3 months and 24 months rat tissue, respectively. (B–C) Merged images of fluorescence and differential interference contrast microscopy. (D) Representative Western analysis for protein-bound citrulline. (E) The densitometric immunoblot quantification of deiminated proteins (relative units) from F344BN retina; age in months are as indicated. Standard deviation from three independent experiments has been shown. (F) Relative quantification of deiminated proteins in blood serum by enzyme-linked immunosorbent assay.
Next, we analyzed PAD2 expression, that is, the enzyme that carries out protein deimination. A decrease in PAD2 expression and mRNA levels (Fig. 2A) was found in the aged retinas compared to the young. The relative PAD2 immunoreactivity (hollow bars) and enzymatic activity levels (solid bars) are consistent with each other (Fig. 2B). Observed decreased immunoreactivity compared to enzymatic activity measured using benzoylarginine (Watanabe et al., 1988) is caused by immunoblot-processing-related losses. A decreased PAD2 immunoreactivity (Supplementary Fig. S1) and activity (not shown) was also observed in the optic nerve. In addition, immunohistochemistry for PAD2 (Fig. 2C,C’) was significantly decreased in aged rats. Labeling with ganglion cell marker Thy-1(Barnstable & Drager, 1984) showed a slight decrease in old (Fig. 2D’) F344BN retinas compared to the young (Fig. 2D). On the other hand, microtubule-associated protein 2, a marker for ganglion cell and inner plexiform layer (Okabe et al., 1989; Cristofanilli et al., 2004) displayed no significant change in its labeling between young (Fig. 2E) and aged F344BN retinas (Fig. 2E’). F344BN rats show retinal degeneration and optic nerve damage at very advanced ages (Supplementary Figs S2 and S3); however, the damage at corresponding ages is less significant when compared with Sprague-Dawley or Long-Evans RCS rats (Cheng et al., 2006). Therefore, the observed decrease in total deiminated proteins and PAD2 is not only because of loss of ganglion cells. Our results suggest that the elevated PAD2 and deiminated proteins in late onset and progressive ocular diseases such as glaucomas (Bhattacharya et al., 2006a,b) are likely because of the pathological process of disease and not age-associated changes. Open questions remaining include the physiological role of deimination, determination of PADs substrate specificity, whether deimination is related to neuronal remodeling and plasticity. Only further investigation can unravel the exact role and consequences of loss of deimination during aging. Future identification of specifically modified proteins will enhance our understanding about the biological functional pathways affected by protein deimination in the aging retina. The results presented here will encourage investigation for deciphering the role of deimination in the normal state, in the young, and the consequences of loss in older animals, as well as investigation into aberrant PAD2 activity in the pathogenic states.
Fig. 2.
Aged retina and peptidyl arginine deiminase type 2 (PAD2). Representative Western, Northern and enzymatic activity analysis for PAD2 in the retina and the optic nerve. (A) Representative Western for PAD2 (upper panel) and Northern analysis for PAD2 and GAPDH message. (B) Densitometric quantification of PAD2 Western (hollow bars) and enzymatic activity (solid bars) of 5 µg protein from young and aged retinas. (C, C’, D, D’, E, E’) Merged DAPI and antibody stained images. The retinal cryosections were probed with monoclonal anti-PAD2, anti-Thy1 and anti-MAP2 as indicated and detected with secondary antibodies coupled to Alexa 488. Upper and lower panels correspond to 3-month-old and 24-month-old F344 BN retina, respectively.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants P30 EY014801, EY017153, EY16112, EY015638, EY017153; Hope for Vision award; RPB Career Development Award (Sanjoy K. Bhattacharya) and Thomas R. Lee Award from American Health Assistance Foundation. We thank Drs H. Takahara and Vincent Monnier for providing the PAD2 antibody and for critical reading of the manuscript, respectively.
Footnotes
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1474-9726.2008.00376.x
(This link will take you to the article abstract).
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Algeciras ME, Bhattacharya SK. Targeting optic nerve citrullination in glaucoma: a role for protein-arginine deiminase 2 (PAD2) inhibitors. Drugs Future. 2007;32:999–1006. [Google Scholar]
- Asaga H, Ishigami A. Protein deimination in the rat brain after kainate administration: citrulline-containing proteins as a novel marker of neurodegeneration. Neurosci. Lett. 2001;299:5–8. doi: 10.1016/s0304-3940(00)01735-3. [DOI] [PubMed] [Google Scholar]
- Barnstable CJ, Drager UC. Thy-1 antigen: a ganglion cell specific marker in rodent retina. Neuroscience. 1984;11:847–855. doi: 10.1016/0306-4522(84)90195-7. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK, Bhat MB, Takahara H. Modulation of peptidyl arginine deiminase 2 and implication for neurodegeneration. Curr. Eye Res. 2006a;31:1063–1071. doi: 10.1080/02713680600991437. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK, Crabb JS, Bonilha VL, Gu X, Takahara H, Crabb JW. Proteomics implicates peptidyl arginine deiminase 2 and optic nerve citrullination in glaucoma pathogenesis. Invest. Ophthalmol. Vis. Sci. 2006b;47:2508–2514. doi: 10.1167/iovs.05-1499. [DOI] [PubMed] [Google Scholar]
- Cheng H, Nair G, Walker TA, Kim MK, Pardue MT, Thule PM, Olson DE, Duong TQ. Structural and functional MRI reveals multiple retinal layers. Proc. Natl. Acad. Sci. USA. 2006;103:17525–17530. doi: 10.1073/pnas.0605790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SM, Wang HS, Taniguchi A. Role of SOD-1 and nitric oxide/cyclic GMP cascade on neurofilament aggregation in ALS/MND. J. Neurol. Sci. 1996;139:16–26. doi: 10.1016/0022-510x(96)00090-1. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Thanos S, Brosius J, Kindler S, Tiedge H. Neuronal MAP2 mRNA: species-dependent differential dendritic targeting competence. J. Mol. Biol. 2004;341:927–934. doi: 10.1016/j.jmb.2004.06.045. [DOI] [PubMed] [Google Scholar]
- Louw C, Gordon A, Johnston N, Mollatt C, Bradley G, Whiteley CG. Arginine deiminases: therapeutic tools in the etiology and pathogenesis of Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2007;22:121–126. doi: 10.1080/14756360600990829. [DOI] [PubMed] [Google Scholar]
- Maruyama N, Ishigami A, Kondo Y. [Molecular abnormality in aging: its contribution to clinical pathology] Rinsho Byori. 2005;53:728–734. [PubMed] [Google Scholar]
- Moscarello MA, Wood DD, Ackerley C, Boulias C. Myelin in multiple sclerosis is developmentally immature. J. Clin. Invest. 1994;94:146–154. doi: 10.1172/JCI117300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscarello MA, Pritzker L, Mastronardi FG, Wood DD. Peptidylarginine deiminase: a candidate factor in demyelinating disease. J. Neurochem. 2002;81:335–343. doi: 10.1046/j.1471-4159.2002.00834.x. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Sambandam T, Echols JD, Barnum SR. Expression of citrullinated proteins in murine experimental autoimmune encephalomyelitis. J. Comp. Neurol. 2005;486:254–266. doi: 10.1002/cne.20527. [DOI] [PubMed] [Google Scholar]
- Okabe S, Shiomura Y, Hirokawa N. Immunocytochemical localization of microtubule-associated proteins 1A and 2 in the rat retina. Brain Res. 1989;483:335–346. doi: 10.1016/0006-8993(89)90178-9. [DOI] [PubMed] [Google Scholar]
- Schoneich C. Protein modification in aging: an update. Exp. Gerontol. 2006;41:807–812. doi: 10.1016/j.exger.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Scofield RH. Autoantibodies as predictors of disease. Lancet. 2004;363:1544–1546. doi: 10.1016/S0140-6736(04)16154-0. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Protein modification in aging. J. Gerontol. 1988;43:B112–B120. doi: 10.1093/geronj/43.5.b112. [DOI] [PubMed] [Google Scholar]
- Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003;25:1106–1118. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wysocka J, Sayegh J, Lee YH, Perlin JR, Leonelli L, Sonbuchner LS, McDonald CH, Cook RG, Dou Y, Roeder RG, Clarke S, Stallcup MR, Allis CD, Coonrod SA. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306:279–283. doi: 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Akiyama K, Hikichi K, Ohtsuka R, Okuyama A, Senshu T. Combined biochemical and immunochemical comparison of peptidylarginine deiminases present in various tissues. Biochim. Biophys. Acta. 1988;966:375–383. doi: 10.1016/0304-4165(88)90088-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.