Abstract
Two types of thalamic nuclei have been recognized: first order, which relay information from subcortical sources, and higher order, which may relay information from one cortical area to another. We have recently shown that muscarinic agonists depolarize all first order and most higher order relay cells but hyperpolarize a significant proportion of higher order relay cells. We now extend this result to serotonergic agonists, using rat thalamic brain slices and whole-cell, current- and voltage-clamp recordings from relay cells in various first order (the lateral geniculate nucleus, the ventral posterior nucleus, and the ventral portion of the medial geniculate body) and higher order nuclei (the lateral posterior, the posterior medial nucleus, and the dorsal portion of the medial geniculate body). Similar to the effects of muscarinic agonists, we found that first and most higher order relay cells were depolarized by serotonergic agonists, but 15% of higher order relay cells responded with hyperpolarization. Thus different subsets of higher order relay cells are hyperpolarized by these modulatory systems, which could have implications for the transfer of information between cortical areas.
Keywords: burst, first order, higher order, modulator, serotonin, tonic
Introduction
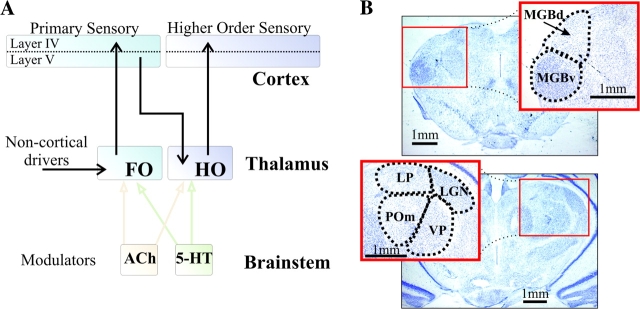
Thalamic relay nuclei have been divided into first and higher order (Guillery 1995; Fig. 1A). First order nuclei relay information from the periphery to cortex (e.g., the lateral geniculate nucleus, which relays retinal input), whereas higher order nuclei may relay information already in cortex from one area to another (e.g., the pulvinar, which appears to be part of a cortico-thalamo-cortical circuit emanating from layer 5 of the source cortical area). The first order nuclei include the lateral geniculate nucleus, the ventral posterior nucleus, and the ventral portion of the medial geniculate body; the higher order nuclei include the lateral posterior nucleus, the posterior medial nucleus, and the dorsal portion of the medial geniculate body. For details of these and other examples, see Guillery (1995) and Guillery and Sherman (2002).
Figure 1.
(A) Schematic representation of thalamocortical driver connections (black arrows) and 2 brainstem modulatory thalamic afferents (colored arrows) for first order (FO) and higher order (HO) thalamic relays. Brainstem cholinergic (ACh) and serotonergic (5-HT) centers are schematically indicated. (B) Nissl stain of typical coronal slices used in our experiments. Top, slice including auditory thalamus with expanded: MGBd, dorsal portion of the medial geniculate body; MGBv, ventral portion of the medial geniculate body. Bottom, slice including visual and somatosensory thalamus with expanded inset: LGN, (dorsal) lateral geniculate nucleus; LP, lateral posterior nucleus; POm, posterior medial nucleus; VP, ventral posterior nucleus.
In addition to the main input to be transmitted to cortex, relay cells in both first and higher order nuclei receive a large number of afferents known as modulators, which operate to affect the nature or amount of information relayed to cortex (Sherman and Guillery 1998, 2005). Examples of modulators are the glutamatergic layer 6 feedback from cortex, cholinergic, noradrenergic, and serotonergic inputs from brainstem, and histaminergic inputs from the hypothalamus (Guillery and Sherman 2002). Evidence of differences between first and higher order nuclei has been accumulating, and many of these relate to modulatory inputs. For instance, there is evidence that higher order nuclei receive a higher percentage of synapses from modulatory inputs than do first order nuclei (Wang et al. 2002; Van Horn and Sherman 2007), the zona incerta and anterior pretectal nucleus provide inputs using γ-aminobutyric acid (GABA) as a neurotransmitter to higher order relay cells but little or no innervation to first order relays (Barthó et al. 2002; Bokor et al. 2005), dopaminergic inputs appear to target higher order relays fairly selectively in the monkey (Sánchez-González et al. 2005), and activation of muscarinic receptors depolarizes all first order relay cells but hyperpolarizes a significant group of higher order relay cells (Mooney et al. 2004; Varela and Sherman 2007).
Interestingly, activation of serotonergic receptors in vitro has been reported to either hyperpolarize or depolarize relay cells in different thalamic nuclei, but the extent to which these results correlate with the first and higher order nature of the relays has not been explored. The serotonergic afferents to the thalamus originate in the medial and lateral divisions of the dorsal raphé nucleus (De Lima and Singer 1987; Vertes 1991; Gonzalo-Ruiz et al. 1995; Kirifides et al. 2001) and in the median raphé nucleus (Vertes et al. 1999; Gonzalo-Ruiz et al. 1995). The function of serotonergic projections to the thalamus is far from understood. Serotonergic centers have been implicated in a variety of functions, including a role in anxiety-related behaviors (Hornung 2003, Abrams et al. 2004) and, in coordination with other modulatory systems of the brainstem, they are important in generating the various stages of the sleep–wake cycle (Steriade and McCarley 2005).
The most extensive study to date on the effects of serotonin (5-HT) across thalamic nuclei suggests that higher order relays may be primarily hyperpolarized by 5-HT (Monckton and McCormick 2002; in ferrets), but the sample sizes were limited in first order nuclei. We sought to determine the effect of 5-HT in first and higher order thalamic nuclei and to compare it with the effect of muscarinic agonists.
Methods
Preparation of Slices
All of our procedures followed the animal care guidelines of The University of Chicago. Brain slices were prepared from Sprague–Dawley rats (Harlan Sprague–Dawley, Inc., Indianapolis, IN) of (in most cases) 11–18 days postnatal age; this age range optimizes the visualization of cells for the patch-clamp recordings and ensures functional properties similar to the adult rat (Ramoa and McCormick 1994). Animals were anesthetized within a few seconds by being placed in a glass chamber with a gauze soaked in isofluorane (AErrane, from Baxter Pharmaceutical, Inc., Deerfield, IL). Once the hind limb withdrawal reflex was absent, the animal was decapitated and the head submerged into an icy solution of artificial cerebrospinal fluid (ACSF, composed of, in mM: 126 NaCl, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 2 MgCl2, 2 CaCl2, and 10 glucose). The brain was removed in about 40s, blocked into a cube containing the thalamus, and glued (with instant Krazy Glue) onto the platform of a motorized vibratome (from WPI, Inc., Sarasota, FL); the platform was submerged into icy ACSF (continuously bubbled with a mixture of 95% O2 and 5% CO2), and 4–8, 400-μm-thick coronal slices with the nuclei of interest were obtained. The slices stayed in a beaker containing bubbled (95% O2 and 5% CO2) ACSF at 30 °C for about 30 min, then were kept at room temperature for the duration of the experiment. Individual slices were transferred to the recording chamber when needed.
Several experiments were performed in older animals (5–7 weeks). Given the technical difficulties associated with patch-clamp recordings in rats older than about 2 or 3 weeks (e.g., slow brain removal which can decrease cell quality, impaired cell visualization), some modifications were adopted in order to preserve cell quality and visibility in these experiments. Older animals (5–7 weeks) were deeply anaesthetized with a mixture of ketamine–xylazine (66 and 33 mg/kg, respectively). The animals were then transcardially perfused with 3 mL of a chilled (4 °C) sucrose–ACSF solution, consisting of (in mM): 206 sucrose, 2.8 KCl, 1 MgCl2, 1 CaCl2, 2 MgSO4, NaH2PO4, NaHCO3, 10 glucose, and 0.4 ascorbic acid. The brain was then removed (the perfusion plus removal of the brain were performed in 3–4 min) and sectioned at 300 μm while submerged in oxygenated sucrose–ACSF. The slices were obtained as described for the younger animals and placed in a chamber with normal ACSF. The goal of the sucrose–ACSF perfusion was 2-fold: perfusing the brain before slicing facilitated the visual localization of cells under the microscope (likely because of the clearance of the blood vessels). In addition, the substitution of NaCl for sucrose in the solution has been suggested to prevent swelling and lysis of brain cells during slice preparation (Aghajanian and Rasmussen 1989). The presences of ascorbic acid and of a low concentration of calcium are additional measures intended to improve cell viability by preventing oxidative damage and synaptic neurotransmitter release.
Intracellular Recordings
The data were gathered from current-clamp and continuous single electrode voltage-clamp recordings obtained in the whole-cell configuration from thalamic relay cells in 6 nuclei: 3 first order (the lateral geniculate nucleus, the ventral posterior nucleus, and the ventral portion of the medial geniculate body) and 3 higher order (the lateral posterior nucleus, the posterior medial nucleus, and the dorsal portion of the medial geniculate body). The liquid junction potential has not been subtracted from membrane potential values; it was calculated to be 12.6 mV with the solutions used in our experiments based on the junction potential calculator in Clampex (Molecular Devices).
Recordings were made in a standard visualized patch-clamp recording rig using a Zeiss microscope to help patch cells from the target nuclei (model Axioskop FS, Carl Zeiss, Inc., Thornwood, NY). A slice was kept in a chamber (located in the light path of the microscope) that had a volume of about 700 μL; the inflow rate of ACSF (warmed to 30 ± 2 °C before entering the chamber with a temperature controller from Warner Instruments, Hamden, CT) was kept at about 2 mL/min. The whole-cell configuration was achieved using glass micropipettes (pulled from borosilicate glass from Garner Glass, Claremont, CA) with tip resistances of 4–8 MΩ. The micropipette solution contained (in mM): 117 KGluconate, 13 KCl, 10 (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid ) (HEPES), 2 Na2ATP, 0.4 Na3GTP, 1 MgCl2, 0.07 CaCl2, and 0.1 ethylene glycol tetraacetic acid (EGTA). Only one cell was recorded in each slice.
After achieving the whole-cell configuration, each cell was injected with a set of negative and positive square current pulses of at least 400-ms duration and at different intensities. The recorded responses were used to verify the viability of the cell. Unstable cells (e.g., with shifting resting membrane potential) were discarded, as were cells with input resistance lower than 100 MΩ and cells with access resistance higher than 30 MΩ.
The recorded signals were amplified and filtered (30 kHz) with a Multiclamp 700B amplifier (Axon Instruments, Union City, CA). Data were acquired at 10 kHz, digitized using the AD converter Digidata 1440A from Axon Instruments, and recorded with software purchased from Axon Instruments (Clampex 8.2). Clampfit 8.2 (Axon Instruments) and Matlab (7.1 R14, The Mathworks, Inc., Natick, MA) were used for quantification and statistical analysis. Sample size, mean, and standard deviation were used to characterize each group of data. The underlying distribution of the parameters quantified was frequently difficult to determine, and nonparametric tests were chosen for sample comparisons: the χ2 test or the Fisher exact test (when the expected frequencies for χ2 were less than 5) were used to compare the frequencies of observed effects among nuclei; the 2-tailed Wilcoxon–Mann–Whitney test, to compare the distributions of 2 unpaired samples; the 2-tailed Wilcoxon signed rank test, for 2 sample paired comparisons; the Kruskal–Wallis test was used when more than 2 groups were compared; and the Brown-Forsythe, to test the homogeneity of group variances.
Pharmacological Agents
All drugs were bath applied. 5-Hydroxytryptamine hydrochloride (5-HT at 100 μM) or acetyl-β-methylcholine (MCh, at 250 μM) were bath applied for 20–40 s and the response recorded for 5–10 min (with a so-called gap-free protocol in Clampex). When used, antagonists were bath applied during at least 10 min previous to the application of the agonist to ensure effective antagonism. Drugs were purchased from Sigma-Aldrich (St Louis, MO): 5-hydroxytryptamine hydrochloride and MCh and from Tocris (Ellisville, MO): Methysergide maleate. All drugs were dissolved in deionized water to prepare stock solutions (stored at −20 °C) and then diluted to the final concentrations in the ACSF solution immediately before use.
Histology
A Nissl stain was used to visualize the nuclei in coronal sections (Fig. 1B). Briefly, sections were obtained with a freezing microtome, dried on subbed slides and hydrated through decreasing concentrations of ethanol to distilled water. The sections were then submerged in cresyl violet solution (0.5%) for 3 min, dehydrated and mounted. An Axiocam digital color camera (Carl Zeiss, Inc.) and AxioVision software (Rel. 4.5, Carl Zeiss, Inc.) were used to capture images of representative slices.
Results
A total of 160 thalamic relay cells with stable membrane potential and voltage-dependent properties were used for this study; because only one cell was recorded per slice (see Methods), this means that the data were obtained from 160 separate slice preparations. Three cells (1 in the lateral posterior nucleus, 1 in the posterior medial nucleus and 1 in the dorsal portion of the medial geniculate body) were also part of the data set reported in Varela and Sherman (2007); the remaining data were assembled for this study. Eighteen of the cells were recorded in older animals (5–7 weeks) and these data will be described separately. Of the 142 cells recorded in younger animals, there were 51 cells in first order nuclei, which included 16 cells from the ventral portion of the medial geniculate body, 20 from the (dorsal) lateral geniculate nucleus, and 15 from the ventral posterior nucleus. The 91 cells in higher order nuclei of young rats included 22 from the dorsal portion of the medial geniculate body, 35 from the lateral posterior nucleus, and 34 from the posterior medial nucleus. Figure 1B displays Nissl stains of coronal sections with the thalamic nuclei used in our recordings. Some experiments and analyses were only done in subsets of cells, and the appropriate sample sizes for these are included in Table 1.
Table 1.
Sample size and nucleus of origin for cells used on each experiment
| First order |
Higher order |
|||||
| VP | MGBv | LGN | POm | MGBd | LP | |
| 5-HT effect | 7,1M | 6 | 8 | 18, 1HP, 1M | 12, 2HP | 14, 4HP, 2M |
| Dose response | 0 | 0 | 1 | 0 | 0 | 2, 2HP |
| Input resistance | 4 | 6 | 4 | 10, 1 HP | 9, 2 HP | 7, 3 HP |
| Low Ca 2+ high Mg2+ | 3 | 2 | 6 | 3, 1 HP | 5, 2 HP | 3, 3 HP |
| 5-HT versus MCh | 6, 1NR | 5,5NR | 5,2NR | 15, 1 HP, 1M | 6, 2 HP | 10, 3 HP, 2M |
| Methysergide | 2,1M | 1 | 1 | 1HP | 1HP | 1HP |
Note: Summary of sample sizes used for quantification in each of the indicated experiments (rows; see text for details), origin of the cells in columns. HP = indicates a cell that was hyperpolarized; M = serotonergic effect was mixed (hyperpolarization followed by depolarization); NR = cell did not respond to 5-HT; if nothing is indicated the effect was depolarization. Abbreviations: VP = ventral posterior nucleus; MGBv = ventral portion of the medial geniculate body; LGN = lateral geniculate nucleus; POm = posterior medial nucleus; MGBd = dorsal portion of the medial geniculate body; LP = lateral posterior nucleus.
Overview of Effects
In 122 of the 142 cells from young animals (first and higher order, independent of their response to 5-HT), direct current (DC) injection was used to keep the cells at around −60 to −65 mV in order to minimize voltage-dependent effects in our measures. A cell was considered to have a response to the bath application of 5-HT (at 100 μM unless otherwise indicated) whenever a change in membrane potential larger than 1 mV occurred in the 2 min following the end of the agonist application; the membrane potential of the cells showed an average standard deviation of 0.512 ± 0.305 mV (measured in the 1 min before the start of the 5-HT application in 30 cells, 5 from each nucleus), therefore, a change of 1 mV represents about twice the standard deviation of the control membrane potential of the cells. This occurred in 132 (93%) of the cells. Figure 2A–C displays representative examples of the responses. Figure 2A shows a current-clamp recording of a small depolarization that we commonly observed in response to the application of 5-HT to first order relay cells; in this case the example was from the lateral geniculate nucleus. The downward deflections through the recording were current pulses used to estimate input resistance (see below). The insets below the main traces in Figure 2A–D show the response of the cell to a hyperpolarizing and a depolarizing current pulse; the rebound activation of a substantial IT is typical of relay cells and distinguishes them from interneurons (McCormick and Pape 1988).
Figure 2.
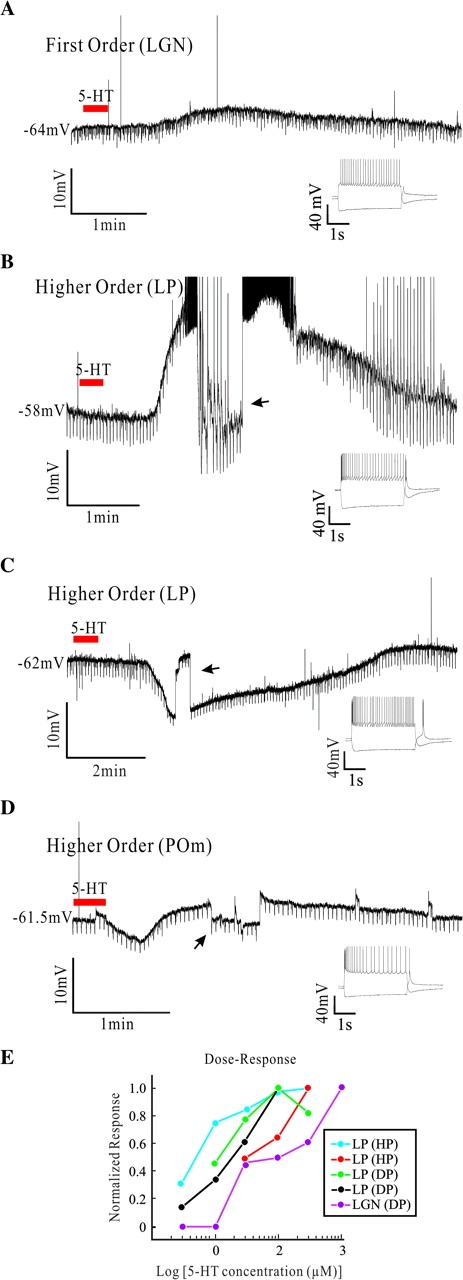
Effects of 5-HT on first and higher order relay cells. (A) Representative example of a first order relay cell's response to 5-HT (100 μM, ≈30 s). Downward deflections correspond to negative current pulses (−10 pA, 400 ms, 3–4 s between pulses) used to measure input resistance. Below the right part of the voltage trace is an inset showing the cell responses to steps of current injection, and similar insets are shown in (B)-(D). (B) Example representative of depolarizing responses observed in higher order relay cells. The upward spikes are truncated action potentials. The arrow here and in (C) indicates a period of adjustment of DC current injection. (C) Example representative of hyperpolarizing responses observed in higher order relay cells. (D) Example of a higher order cell showing a mixed response. (E) Dose–response curves for 5 cells, and they are labeled regarding nucleus of origin and response to 5-HT (DP, depolarizing; HP, hyperpolarizing). Abbreviations for nuclei as in Figure 1.
To quantify the change in membrane potential caused by 5-HT, we selected those cells in which the peak of the effect could easily be identified during current-clamp recordings, thus leaving out cells recorded only in voltage-clamp (for the dose–response experiments below) and cells in which DC injection was modified during the response in order to apply current protocols (e.g., for input resistance measures, see below); we also selected only those cells in which 5-HT was applied during the first 30 min of recording. Cells meeting these criteria in first order nuclei included 21 of the 41 cells that were depolarized by 5-HT, and in higher order nuclei, included 44 cells of the 74 depolarized and 7 of the 13 hyperpolarized by 5-HT. For these 21 first order cells, the average depolarization at the peak of the response to 20–40 s application of 5-HT was 4.84 ± 3.82 mV (mean ± SD here and below), and the amplitude was not different among the 3 first order nuclei (P > 0.1, Kruskal–Wallis).
Figure 2B,C show examples of responses from higher order cells. Figure 2B illustrates a typical depolarizing response from a lateral posterior nucleus relay cell, and the average depolarization for the 44 cells meeting the abovementioned criteria was 11.52 ± 7.13 mV. The size of the depolarizing responses was significantly larger in higher than in first order nuclei (P < 0.0001, Mann–Whitney). With the higher order sample, the depolarizing responses were much smaller in the 14 cells from the posterior medial nucleus (7.88 ± 5.97 mV) compared with the 18 cells of the lateral posterior nucleus (12.97 ± 5.94 mV) and the 12 in the dorsal portion of the medial geniculate body (15.3 ± 7.89 mV; P < < 0.001, Kruskal–Wallis). The depolarization was sometimes large enough to evoke action potentials as shown in the example of Figure 2B (the spikes have been truncated for presentation purposes); in that same figure, the DC level was briefly adjusted (indicated with an arrow here and below) to bring the membrane potential to the control level in order to perform comparable measurements of input resistance (see below); the upward deflections in the last minutes of recording are due to partial activation of IT, which was occasionally partially de-inactivated by the negative pulses used for testing input resistance, although without evoking sodium spikes.
Figure 2C shows an example (taken from the lateral posterior nucleus) of the second most frequent effect in higher order cells: a hyperpolarizing response to 5-HT. The hyperpolarization measured in the 7 higher order relay cells meeting the abovementioned criteria was −11.46 ± 3.55 mV and was followed by a small depolarization (2.45 ± 0.77 mV). Strictly speaking this qualifies as a “mixed” response, however we will use the term “hyperpolarizing” to refer to cells showing an effect like that on Figure 2C, because the hyperpolarization was clearly longer and larger than in the cells with “mixed” response described below.
The fourth type of response (“mixed”) was observed in 4 cells, one first order from the ventral posterior nucleus and the rest higher order from the posterior medial nucleus (1) and lateral posterior nucleus (2), and an example is illustrated in Figure 2D. Here, the response to 5-HT consisted of a small hyperpolarization followed by a depolarization. In these cells, the hyperpolarization was −4.74 ± 4.32 mV, and the depolarization, 4.21 ± 1.39 mV. This type of mixed response could be clearly distinguished from the primarily hyperpolarizing effect (followed by a small depolarization) described above: first, the duration of the hyperpolarization was substantially longer in “hyperpolarized” cells than in “mixed-response” cells (258.84 ± 0.7 s vs. 52.61 ± 2.44 s; P < 0.007); and, second, the ratio of the amplitude of the second part of the response (depolarization) to the amplitude of the initial hyperpolarization was also different (1.28 ± 0.65 in mixed responses and 0.24 ± 0.13 in hyperpolarized cells; P < 0.007).
Dose–Response Curves
For the examples shown in all the figures in this paper, and in all the cells for which data have been further quantified, 5-HT was used at a concentration of 100 μM. This was selected after assessing the dose–response relationship to 5-HT application in a subset of 5 cells (including first and higher order cells and cells that either depolarized or hyperpolarized to 5-HT application; see Table 1 for details). The cells were recorded in continuous single electrode voltage-clamp (held at −60 mV), and were exposed to decreasing concentrations of 5-HT, from 1 mM to 3 μM, bath applied to the cell. Not all the concentrations were used in all the cells. The maximum current evoked by each concentration was recorded and the absolute maximum current for each cell was used to normalize its responses. A semilogarithmic plot of these normalized data is shown in Figure 2E. A concentration of 100 μM was chosen routinely, because this was expected to evoke a substantial but not saturating response in most cells.
Population Responses
Figure 3 summarizes the frequency of each of the responses for the whole population in first and higher order nuclei (Fig. 3A), the difference in the size of the depolarization in first and higher order nuclei (Fig. 3B), and the distribution of responses in each individual nucleus (Fig. 3C). Of the 42 first order relay cells that responded to 5-HT, 41 (97.6%) were purely depolarized by the 5-HT application (Fig. 3A), the other cell showed a mixed response. The relative frequency of responses was different in higher order nuclei. Of the 91 higher order relay cells that responded to 5-HT, 74 (81.3%) showed a pure depolarization, 13 showed a hyperpolarization (14.4%), and 3 showed a mixed response (3.3%). The frequency of the hyperpolarizing response was significantly different between first and higher order thalamic nuclei (P < 0.012, χ2 test), and the number of nonresponding cells was significantly greater among the first order relay cells (P < 0.001, χ2 test).
Figure 3.
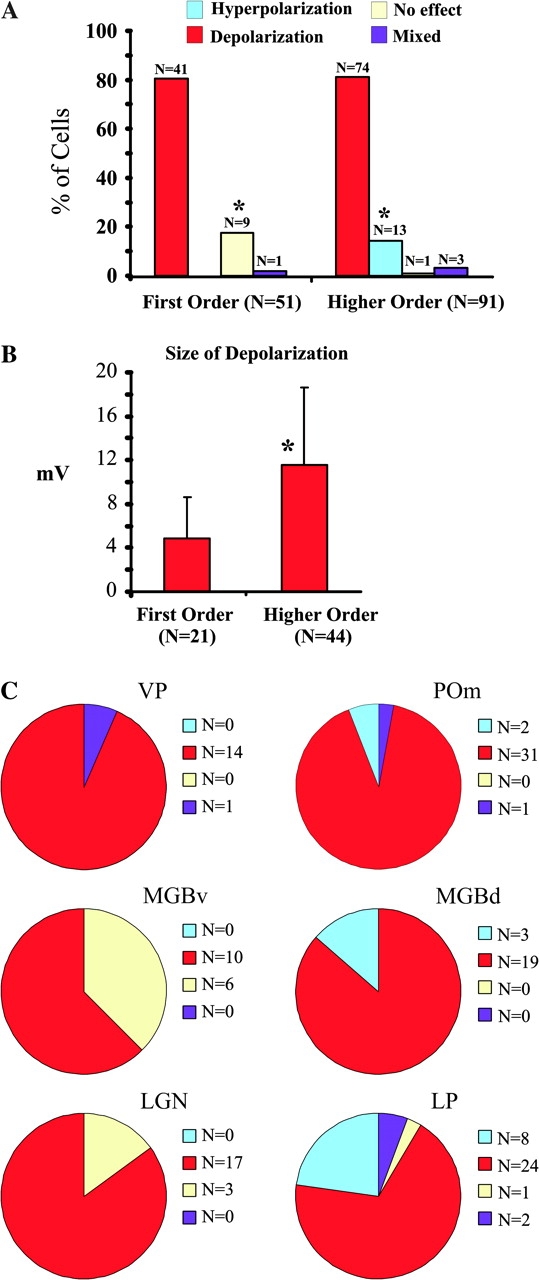
Population responses to 5-HT. (A) Frequency of the different effects of 5-HT on first and higher order relay cells. (B) Size of the depolarizing effect in first order and higher order nuclei. (C) Distribution of the effects in individual nuclei (first order in left column and higher order in right column). Abbreviations for nuclei as in Figure 1. Asterisks indicate significant difference.
As noted above, another difference between first and higher order nuclei was the size of the depolarizing response, which is summarized in Figure 3B. Evoked depolarizations were smaller for 21 first order relay cells than for 44 higher order cells (4.84 ± 3.82 mV vs. 11.52 ± 7.13 mV; P < 0.001 on a Mann–Whitney U-test).
Regarding the types of responses in individual nuclei, the most consistent effects in first order nuclei (Fig. 3C, left column), were found in the ventral posterior nucleus, where only one cell showed a mixed response and the remaining 14 were depolarized to 5-HT. The sample from the ventral portion of the medial geniculate body included 10 cells (62.5%) showing depolarization and 6 (37.5%) that did not respond. Finally, the lateral geniculate nucleus sample included 17 cells (85%) showing depolarization and 3 (15%) not responding. Statistically, the ventral portion of the medial geniculate body stands out as having significantly more nonresponsive cells than the other first order nuclei (P = 0.02, Fisher exact test).
Among the higher order nuclei (Fig. 3C, right), 31 of 34 relay cells in the posterior medial nucleus were depolarized (91.2%), 2 were hyperpolarized (5.9%), and 1 showed a mixed response (2.9%). In the dorsal division of the medial geniculate body 19 of 22 cells (86.4%) were depolarized and the remaining 3 (11.8%) showed hyperpolarizing responses. The lateral posterior sample of 35 cells was the most heterogeneous among the higher order nuclei, including 24 (68.6%) depolarizing cells, 8 hyperpolarizing (22.9%), 2 with mixed responses (5.7%), and 1 unresponsive (2.9%). Statistical analysis shows that the number of hyperpolarizing cells is not significantly different across higher order nuclei (P > 0.1, Fisher exact test).
In a previous report (Varela and Sherman 2007), we failed to find morphological differences between cells in first and higher order nuclei and anatomical features were not further explored here. Comparing the resting membrane potential (measured within a minute after achieving the whole-cell configuration) across nuclei revealed some singularities. Overall, we found no difference between the membrane potential values in the first and higher order groups (P = 0.13, Brown–Forsythe; P = 0.90, Mann–Whitney). The 42 first order relay cells in which we measured this parameter had an average of −66.97 ± 6.18 mV compared with the −67.00 ± 4.93 mV of 61 higher order cells. Within first order nuclei, the 14 lateral geniculate cells were significantly more hyperpolarized (−71.51 ± 5.57 mV; P = 0.004, Kruskal–Wallis) than the sample of 14 cells from the ventral posterior nucleus (−63.97 ± 5.97 mV) or the 14 cells in the ventral portion of the medial geniculate body (−65.43 ± 4.40 mV). Among the nonhyperpolarizing higher order cells, the 17 relay cells that we measured from the dorsal portion of the medial geniculate body had an average resting membrane potential of −68.98 ± 3.45 mV, significantly different (P = 0.01, Mann–Whitney) than cells in 22 cells from the posterior medial nucleus (−65.04 ± 5.12 mV); the 22 cells measured in the lateral posterior were at −67.44 ± 5.18 mV on average. Cells hyperpolarized by 5-HT did not differ in resting membrane potential from the rest of higher order cells nor from the first order cells (P = 0.43, Kruskal–Wallis).
In order to rule out the possibility that the hyperpolarizing responses occur exclusively in young animals and disappear at older developmental stages, or that, alternatively, they become widespread occurring in both first and higher order nuclei, we recorded from 18 first and higher order cells in older animals (5–7 weeks). Of these 18 cells, 11 were in higher order nuclei (6 in the posterior medial, 4 in the dorsal portion of the medial geniculate body and one in the lateral posterior nucleus) and 7 were in first order nuclei (5 in the lateral geniculate, 1 in the ventral portion of the medial geniculate body and 1 in the ventral posterior nucleus). The recordings were done under identical conditions to those described for the younger animals (e.g., DC was adjusted in 16 of the 18 cells to hold them around −60 to −65 mV, 5-HT was bath applied for 30–40 s and the effect was recorded in current-clamp).
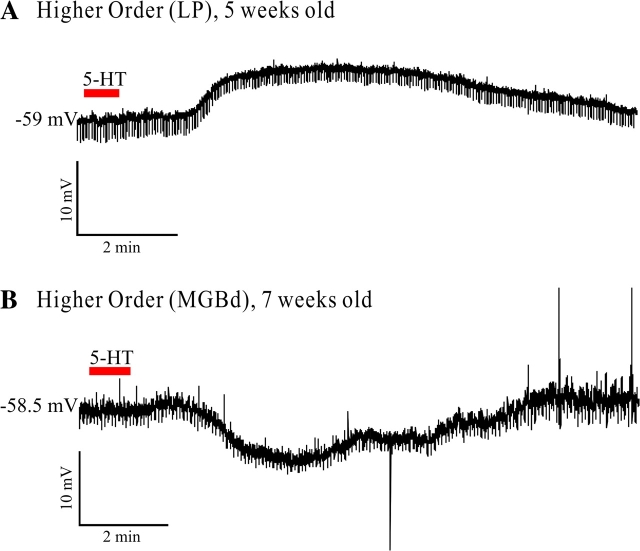
Two examples of typical responses from higher order relay cells of older animals are shown in Figure 4A,B. Figure 4A shows the depolarizing response of a cell from the lateral posterior nucleus of a 5-week-old rat. Figure 4B shows a hyperpolarizing response caused by 5-HT in a cell from the dorsal portion of the medial geniculate body of a 7-week-old rat. In the higher order sample from older animals, we found 2 cells (i.e., 18.18% of the higher order cells) that were hyperpolarized by 5-HT (both were in the dorsal portion of the medial geniculate body), 7 of the cells showed small depolarizations, 1 cell in the posterior medial nucleus presented a mixed response and another cell in the dorsal medial geniculate body had no response to 5-HT application. No hyperpolarizations were found in first order relay cells. Four of the 7 first order cells recorded in older rats responded with small depolarizations to the application of 5-HT, and the other 3 cells (2 in the lateral geniculate nucleus and one in the ventral portion of the medial geniculate body) showed no response. Therefore, the variety of 5-HT-mediated responses observed in higher order relay cells of young animals exist also in older animals in higher order nuclei and the hyperpolarizations are not observed in first order nuclei.
Figure 4.
Effect of 5-HT in older animals. (A) Current-clamp recording showing the effect of bath-applied 5-HT on a cell from the dorsal medial geniculate body of a 5-week-old rat. (B) Current-clamp recording of the effect of bath-applied 5-HT on a cell from the lateral posterior nucleus of a 7-week-old rat.
Whole-cell patch-clamp recordings from rat slices older than about 3 weeks pose a number of technical difficulties, among them, achieving a proper seal between cell and micropipette becomes more troublesome (because of the lack of visibility through the slice) and there is an increased probability of cell deterioration (given the longer time employed in brain removal). Because of these technical problems, the controls described below were only performed in young animals.
Further Analysis of Responses of Relay Cells to 5-HT
Determining the specific conductances involved in each of the responses to serotonergic activation was beyond the scope of this paper. However, by applying small negative current pulses through the duration of the recording we were able to test for general changes in input resistance. Similarly, by repeating the application of the agonist during the blockade of synaptic transmission, we were able to determine the existence of a direct component of the responses. Table 1 reports the number of cells from each nucleus used for these experiments.
Changes in Input Resistance
In order to estimate input resistance, a negative current pulse of −10 pA and 400 ms was applied every 3 or 4 s during the current-clamp recordings. Sometimes these pulses were sufficiently long and hyperpolarizing to evoke rebound activation of IT; an example can be seen during the last minutes of the recording in Figure 2B. Because the changes in membrane potential evoked by the agonist could also affect membrane resistance (e.g., by modifying voltage-dependent conductances), in the subset of cells used to quantify changes in input resistance, appropriate DC injection was used during the peak of the effect to bring the membrane voltage back to the initial baseline level (e.g., Fig. 5A,B). The subset of cells for which we made these measurements is provided in Table 1.
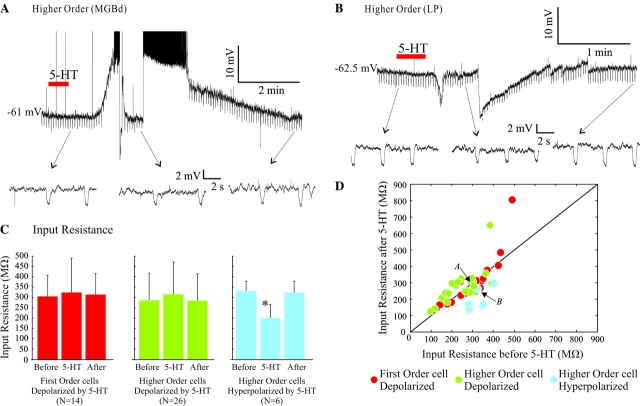
Figure 5.
Effect of 5-HT on input resistance. (A, B) DC current injection during the peak of the effect was adjusted to bring the potential back to the baseline membrane potential to compare input resistance at the same voltage level. Expanded traces below each main trace show the negative current pulses (−10 pA, 400 ms; 3–4 s between pulses) used to measure input resistance. (C) Average input resistance (±SD) before, during and after the effect of 5-HT for first order depolarizing (left), higher order depolarizing (center), and higher order hyperpolarizing (right) cells. Asterisk indicates significant difference. (D) Input resistance before and during the effect of 5-HT effect for all the cells measured. Arrows locate examples shown in (A) and (B).
In these experiments, we observed no significant change in input resistance in cells that were depolarized by 5-HT, neither in first nor in higher order relay cells (Fig. 5C). The average change in input resistance was an increase of 3.95 ± 21.88% in 14 first order and 13.33 ± 25.07% in 26 higher order cells (Fig. 5D) and the difference with the control input resistance was not significant for either group (P > 0.9 for first order and P > 0.13 for higher order, Wilcoxon signed rank). This suggests that the depolarizations might be mediated by both the opening and closing of ion channels.
During hyperpolarizing responses in 6 higher order relay cells, the input resistance was decreased an average of 39.6 ± 14.84% (Fig. 5C,D), and the difference with control was significant (P = 0.03, Wilcoxon signed rank). The reduction in input resistance is in agreement with previously reported results showing that the hyperpolarizing effect results from the opening of K+ channels, thereby increasing membrane conductance (Monckton and McCormick 2002).
A comparison of the initial (control) input resistance between higher order relay cells hyperpolarized and depolarized by 5-HT showed no significant difference (P = 0.23, Mann–Whitney). On average, the 14 first order relay cells that depolarized to 5-HT had input resistances of 305.08 ± 101.84 MΩ, 26 higher order relay cells that were depolarized had input resistances of 285.51 ± 132.3 MΩ, and 6 higher order relay cells that were hyperpolarized had input resistances of 330.68 ± 47.13 MΩ.
Evidence that Serotonergic Effects are Direct
To demonstrate that at least some of the 5-HT effects were postsynaptic, resulting from direct activation of 5-HT receptors on the recorded cells, we repeated the 5-HT application while blocking synaptic transmission. Because neurotransmitter release from nerve terminals requires extracellular Ca2+ (see Oheim et al. 2006 for a review), and it is antagonized by extracellular Mg2+ (Douglas 1968) a bathing solution (ACSF) containing low Ca2+ (0.5 mM) and high Mg2+ (8 mM) concentrations is often used to block synaptic release. A potential concern when using a low Ca2+ bathing solution is the change in membrane excitability. Divalent cations can influence gating and permeation in most voltage-dependent channels by neutralizing the membrane negative surface charge (Piccolino and Pignatelli 1996). However, all divalent ions, including Mg2+, have these effects (Piccolino and Pignatelli, 1996) and by using both a decreased concentration of Ca2+ and an increased concentration of Mg2+ synaptic release can be blocked without modifying excitability.
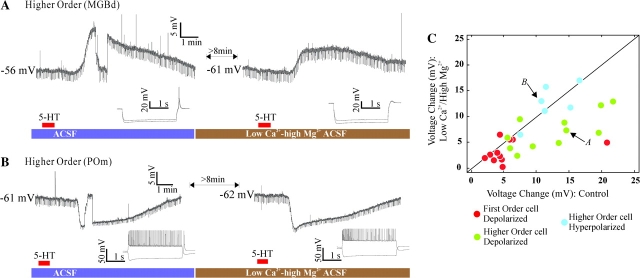
Figure 6A illustrates the effect of 5-HT under the blockade of synaptic release for a higher order relay cell in the dorsal medial geniculate body; 5-HT application caused a depolarization that persisted in low Ca2+ (0.5 mM) and high Mg2+ ACSF. Figure 6B shows the analogous result for a higher order relay cell from the posterior medial nucleus for which the hyperpolarizing response to 5-HT also persisted after blockade of synaptic transmission. The insets below the traces in Figure 6A,B show a confirmation of the effect of the low Ca2+ and high Mg2+ solution, namely, they show that the low threshold Ca2+ spikes are gone in the presence of low Ca2+ and high Mg2+ as the bathing solution.
Figure 6.
Direct postsynaptic effects of 5-HT. (A) Higher order relay cell depolarized by 5-HT in normal ACSF (left) is also depolarized by 5-HT in presence of low Ca2+ (0.5 mM)-high Mg2+ (8 mM) ACSF (right), which blocks synaptic transmission. (B) Effect on the response of a higher order relay cell hyperpolarized by 5-HT. (C) Population results for the low Ca2+-high Mg2+ experiments, showing the peak effect evoked by 5-HT before and in the presence of low Ca2+-high Mg2+ ACSF. Arrows locate examples shown in (A) and (B).
Blocking synaptic transmission with low Ca2+ and high Mg2+ as the bathing solution yielded similar results in the 28 relay cells tested. The sample included 11 first order and 11 higher order relay cells with depolarizing responses to 5-HT, and 6 higher order with hyperpolarizing responses (see Table 1 for specific nuclei of origin). Given that cells with mixed response were rare (2.3% of first order and 3.3% of higher order responsive cells) the control was not performed on this group. The response amplitude was reduced in the presence of low Ca2+ and high Mg2+ relative to control both in the first order group (P = 0.02, Wilcoxon signed rank) and in the higher order cells that depolarized in response to 5-HT (P = 0.007, Wilcoxon signed rank). This was in contrast to the hyperpolarizing effect in higher order cells, in which the response remained virtually the same even during synaptic blockade (P = 0.84, Wilcoxon signed rank).
Figure 6C plots the maximum change in membrane potential evoked by 5-HT in control ACSF versus in the low Ca2+-high Mg2+ condition for all cells tested. The average response change in the low Ca2+ and high Mg2+ control was a decrease of 31.11 ± 41.79% for the 11 first order relay cells and 36.53 ± 29.72% for the 11 higher order cells depolarized by 5-HT. The overall effect in the 6 cells hyperpolarized by 5-HT was a decrease of 4.55 ± 22.3%. The percentage of change in the response was not significantly different across the 3 groups (first order, higher order depolarizing and higher order hyperpolarizing; P = 0.05, Kruskal–Wallis). The decrease in the amplitude of the depolarizing responses to 5-HT when applied in the presence of the low Ca2+ and high Mg2+ solution could be due to several factors. For example, 5-HT might be activating presynaptic receptors on excitatory terminals in the control situation; or Ca2+ could be contributing to the control response. The specific synaptic and ionic mechanisms of the serotonergic responses requires further investigation. Nonetheless, the persistence of most of the serotonergic effect in the low Ca2+ and high Mg2+ solution lets us conclude that most or all of the responses seen in our population of relay cells are caused by the postsynaptic activation of serotonergic receptors on the recorded relay cells.
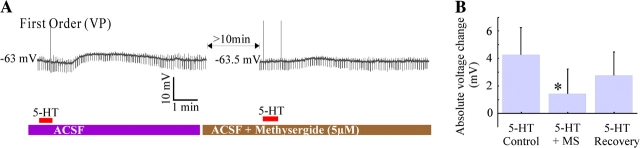
A large number of serotonergic receptor types exist (reviewed in Bockaert et al. 2006) and completely determining the specific contributors for the observed responses is beyond the scope of this study. Nevertheless, the partial serotonergic antagonist methysergide (5 μM) was used in 8 cells (3 hyperpolarized by 5-HT; see Table 1 for cell origins). Methysergide, which appears to block 5-HT1, 5-HT2, and 5-HT7 receptors, was continuously bath applied for at least 10 min before 5-HT was tested. Six of the cells showed a decrease in the response in the presence of the blocker (by 80 ± 23.22%; P = 0.03, Wilcoxon signed rank); however, one cell from the ventral posterior nucleus and a hyperpolarizing cell from the dorsal medial geniculate body did not change their response in the presence of methysergide. An example of the effect of methysergide in a cell recorded in the ventral posterior nucleus is shown in Figure 7A, and the results for all the cells tested are summarized in Figure 7B. Of the receptors affected by methysergide, 5-HT1A has been implicated in the hyperpolarization of thalamic cells (Monckton and McCormick 2002); also, 5-HT7 was reportedly responsible for the depolarization of cells in the thalamic anterior dorsal nucleus by Chapin and Andrade (2001a). Our results are in agreement with the results of Monckton and McCormick (2002) and Chapin and Andrade (2001a) and suggest that receptors other than 5-HT1, 5-HT2, and 5-HT7 might be present in some cells.
Figure 7.
Effect of Methysergide (an antagonist for 5-HT1, 5-HT2, and 5-HT7 receptors) on 5-HT response. (A) Effect for a first order relay cell depolarized by 5-HT. (B) Voltage change caused by 5-HT before (control), during (+MS), and after (recovery) the application of methysergide (MS).
Comparison of Serotonergic and Muscarinic Activation
We have recently reported similar effects to cholinergic activation to those shown here: namely, that although all first order and most higher order relay cells are depolarized by muscarinic agonists, a subset of higher order relay cells are hyperpolarized (Varela and Sherman 2007). To see if there are correlations between the effects of the 2 modulatory systems, we tested the activation of both muscarinic and serotonergic receptors in a group of 24 first order and 40 higher order relay cells. The results are displayed in Table 2.
Table 2.
MCh and 5-HT effect
| N = 64 | First order | 5-HT effect |
Higher order | 5-HT effect |
||||||
| HP | DP | Mixed | NR | HP | DP | Mixed | NR | |||
| MCh effect | HP | 0 | 0 | 0 | 0 | HP | 1 | 6 | 1 | 0 |
| DP | 0 | 15 | 0 | 5 | DP | 5 | 18 | 2 | 0 | |
| Mixed | 0 | 0 | 0 | 1 | Mixed | 0 | 7 | 0 | 0 | |
| NR | 0 | 2 | 0 | 1 | NR | 0 | 0 | 0 | 0 | |
Note: Comparison of MCh and 5-HT effects in individual first and higher order relay cells. Rows display effect of MCh, columns effect of 5-HT. HP = hyperpolarization; DP = depolarization; NR = no response.
We used the same methods as before (Varela and Sherman 2007), that is, a general muscarinic agonist, MCh (used at 250 μM) was bath applied and the response recorded in current-clamp mode. Of the 24 first order cells, 17 were depolarized by 5-HT, and 15 of them were also depolarized by muscarinic activation; the other 2 did not respond to MCh. Of the 7 cells that did not respond to 5-HT, 5 were depolarized by MCh; one showed a mixed response to MCh, and one did not respond.
Of the 40 higher order relay cells depolarized by 5-HT, 18 were depolarized by MCh, 6 were hyperpolarized, and 6 showed a mixed response. For the 3 cells with mixed response to 5-HT, MCh depolarized 2 of them and evoked a hyperpolarizing response in the other. Interestingly, the 6 cells that were hyperpolarized by 5-HT or the 8 hyperpolarized by MCh were commonly depolarized by the other agonist, and only in one cell did the response have the same hyperpolarizing sign in both applications. However, there was no statistical difference between the frequency of cells that presented the same response (hyperpolarization or depolarization) to both neurotransmitters and the frequency of those with opposite responses (P > 0.56; Fisher exact test). In other words, no significant correlation was found between the effects of the 2 neurotransmitters in our sample.
Discussion
Modulatory systems, such as those originating in cholinergic and serotonergic brainstem centers, innervate both thalamic nuclei that receive cortical drivers (higher order nuclei) and those that do not (first order nuclei). We report that the effect of 5-HT on membrane potential is different at the population level between first and higher order relay cells. We found that depolarization was effectively the sole direct effect in first order relay cells. Most higher order relay cells were also depolarized by 5-HT, but the depolarizations were much larger. In addition, the higher order nuclei included a significant subset of cells (14.3%) that were hyperpolarized by 5-HT. Furthermore, as expected by chance, this subset appeared to be largely nonoverlapping with previously described higher order relay cells that are hyperpolarized by activation of muscarinic receptors (Varela and Sherman 2007).
Comparison with Previous Studies
Only a few studies (summarized in Table 3) have tested the effect of 5-HT in several thalamic nuclei under the same conditions. Our finding of hyperpolarizing cells in higher order thalamic nuclei is in agreement with the extensive study by Monckton and McCormick (2002) in ferret brain slices. This included primarily nuclei that have been classified as higher order (lateral posterior, lateral dorsal, pulvinar, mediodorsal) and, in those, the common response to 5-HT was a hyperpolarization. Our results extend the investigation of serotonergic effects to a different species and demonstrate the existence of different responses to 5-HT in first and higher order nuclei.
Table 3.
Previous in vitro reports of serotonergic effect in the thalamus
| Reference | Species | Age | Nuclei (no. of cells) |
5-HT effect | |
| Monckton and McCormick (2002) | Ferret | 6–24 weeks | Pulvinar (97) | Lateral dorsal (9) | Most common effect: hyperpolarization; some depolarizations reported in the lateral geniculate (% of cells not specified) |
| Lateral posterior (9) | Anterior ventral (7) | ||||
| Mediodorsal (6) | Central lateral (13) | ||||
| Lateral geniculate (7) | Center median (10) | ||||
| Medial geniculate (5) | |||||
| Ventral posterior (4) | |||||
| Pape and McCormick (1989) | Guinea pig | 200–300 g | Lateral and medial geniculate (125 cells in guinea pig; 6 cells in cat) | Depolarization (only effect reported) | |
| Cat (2 animals) | 2–12 months | ||||
| McCormick and Pape (1990) | Guinea pig | 200–300 g | Lateral and medial geniculate (21) | Depolarization (only effect reported) | |
| Cat (4 animals) | 2–12 months | ||||
| Chapin and Andrade (2001a) | Rat | P28–42 | Anterior dorsal (123) | 98.4% depolarized in anterior dorsal. Depolarization, hyperpolarizations and no response reported for the anterior ventral (% not specified) | |
| Anterior ventral (10) | |||||
Note: Previous in vitro studies. Rows indicate previous in vitro reports that have tested the effect of 5-HT in several thalamic nuclei. Columns briefly summarize information that is relevant to compare previous studies with the results presented here.
The sample of cells recorded by Monckton and McCormick (2002) in first order nuclei (dorsal lateral geniculate nucleus, ventral posterior and possibly the ventral portion of the medial geniculate body) was much smaller; nevertheless, in these nuclei, depolarizing responses to 5-HT were reported as exceptional and, on average, the effect consisted of a small (<1 mV) hyperpolarization.
It is not clear why the depolarizing responses commonly found in first and higher order in our study were not found in the ferret. Age differences appear to not be responsible for the differences because we have not observed an increase in the relative number of cells that are hyperpolarized by 5-HT in older animals (similar in age to those recorded by Monckton and McCormick 2002). Previous reports from the same group (Pape and McCormick 1989; McCormick and Pape 1990) described small depolarizing responses as the predominant effect on guinea pig and cat lateral geniculate nucleus and medial geniculate body. In rats, Chapin and Andrade (2001a) reported depolarizing responses to 5-HT in the first order anterior dorsal nucleus that were similar in amplitude to the ones we report here in higher order nuclei. Altogether, the evidence suggests that there may be species differences in terms of the first order responses and the frequency of the hyperpolarizing cells in higher order nuclei; it also raises the possibility that the existence of substantial hyperpolarizing responses only on higher order nuclei has been preserved even across mammalian orders.
The larger depolarizations observed in higher order compared with first order relay cells may arise from a number of reasons (e.g., the existence of different types of serotonergic receptors, the overall number of receptors present, the sensitivity of the receptors, the level of activation of second messenger cascades). Clearly, further experiments are needed to find out the explanation for the larger depolarizations in higher order relay cells.
Direct Effect and Mechanisms
Bath application of 5-HT can in principle affect a recorded relay cell either directly (i.e., acting on postsynaptic receptors on the recorded cell) or indirectly, by affecting its afferents. Presynaptic effects on extra-thalamic neurons that innervate thalamic relay cells, including cortical and brainstem, can be ignored, because these cells are not included in our slices; similarly, there are essentially no interneurons to innervate our sample of relay cells outside of the lateral geniculate nucleus (Arcelli et al. 1997). Nonetheless, 5-HT can affect presynaptic terminals even when the soma of origin is not present.
The results of the experiments with low Ca2+ and high Mg2+ ACSF indicate that the effects of serotonergic activation are mostly evoked by activation of receptors on the relay cells. The fact that the responses (particularly the depolarization) are decreased (although not completely eliminated) in the presence of low Ca2+ and high Mg2+ ACSF suggests the participation of presynaptic receptors. It is also possible that Ca2+ contributes to the current activated by serotonergic receptors (e.g., through Ih channels; Pape and McCormick 1989; McCormick and Pape 1990; Chapin and Andrade 2001b), which would explain why the responses are smaller when the extracellular Ca2+ concentration is decreased. Further experiments are needed to clarify the role of Ca2+ and presynaptic activation on the responses to 5-HT.
The array of serotonergic receptors that could mediate the responses is particularly broad; fifteen genes encoding functional 5-HT receptors have been cloned in mammalian brain (Bockaert et al. 2006). Of these, a few are reportedly expressed in the thalamus (5-HT1A, Pazos et al. 1987; Aznar et al. 2003; 5-HT2C, Sharma et al. 1997; 5-HT6, Roberts et al. 2002; 5-HT7, Varnäs et al. 2004), but most remain to be explored. The partial blockade of the responses by the antagonist methysergide indicates that multiple receptors are involved. 5-HT1A receptors have been implicated in the hyperpolarizing response in the thalamus by opening K+ channels (Monckton and McCormick 2002). This is consistent with our finding of a decrease in input resistance during the hyperporlarizing response. The receptor mediating the depolarization could not be identified in guinea pig and cat lateral geniculate nucleus and medial geniculate body (McCormick and Pape 1990), whereas 5-HT7 was reported to mediate the depolarization in the rat anterior dorsal nucleus (Chapin and Andrade 2001a). The receptor 5-HT7 is also present in the human thalamic anterior nuclei, pulvinar and mediodorsal nucleus (Varnäs et al. 2004) and could thus mediate responses in other thalamic regions. Our results are consistent with the involvement of 5-HT7 receptors as reported by Chapin and Andrade (2001a); on the other hand, methysergide can also block the effect of 5-HT1 and 5-HT2 receptors and, in addition, the responses were not always completely blocked by methysergide, suggesting that yet other receptors could participate. More data are needed regarding the specific receptors involved in the various responses.
Functional Implications
First and Higher Order Classification
The results presented here add to the growing evidence of differences in the modulatory systems that innervate first and higher order nuclei, reinforcing this classification. The higher order nuclei, but not the first order, are heterogeneous in terms of their responses to both cholinergic (Varela and Sherman 2007) and serotonergic afferents. In particular, both modulatory systems contribute to hyperpolarize a substantial amount of relay cells in higher order nuclei. More data are needed regarding the role of other modulators to determine if all modulatory systems have the ability to produce differential effects in first and higher order nuclei. Further study of the cells hyperpolarized by 5-HT and/or MCh would be of particular interest, as only more evidence can determine the extent to which these cells represent a unique class of relay cell present only in higher order nuclei.
In addition, the thalamic reticular nucleus (TRN) provides input to both first and higher order nuclei. Our results raise the possibility that the effect of 5-HT may differ in the first and higher order portions of TRN.
Relationship to Firing Mode
The fact that 2 modulatory systems can hyperpolarize a group of higher order relay cells is interesting in the context of certain intrinsic firing properties of thalamic relay cells. These cells can respond to incoming input in one of 2 modes depending on the activation state of an inward voltage-dependent Ca2+ current (IT). When relatively depolarized beyond roughly –60 mV, IT is inactivated and the cell responds in tonic mode: the response is a train of action potentials, the number of which increases fairly linearly with increasing input amplitude. If hyperpolarized beyond roughly −60 mV, IT is deinactivated, and the cell will respond to a sufficiently large depolarization in burst mode: the response is a burst of action potentials with a highly nonlinear input/output relationship (Smith et al. 2000; Sherman 2001) but with an improved signal to noise ratio thought to underlie better detectability (Guido et al. 1995; Reinagel et al. 1999; Sherman 2001). The burst mode of response is also particularly effective at activating postsynaptic cortical cells (Swadlow and Gusev 2001).
By modifying the resting potential of relay cells, modulatory inputs, such as cholinergic and serotonergic inputs, can strongly influence response mode. Activity in cholinergic and serotonergic brainstem centers that innervate thalamus increases during awake states (Kayama et al. 1992; Steriade and McCarley 2005). Our data predict that higher order thalamic nuclei may have a subset of neurons that predominantly use burst mode of response during awake behavior, a subset missing from first order nuclei. Thus, both cholinergic and serotonergic afferents could be responsible for the higher proportion of bursting found in recordings of spontaneous activity of cells in several higher order thalamic nuclei in the awake monkey (Ramcharan et al. 2005).
Funding
Funded by USPHS grants EY03038 and DC008794.
Acknowledgments
Conflict of Interest: None declared.
References
- Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomic and functional topography of the dorsal raphe nucleus. Ann N Y Acad Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. doi: 10.1002/syn.890030406. [DOI] [PubMed] [Google Scholar]
- Arcelli P, Frassoni C, Regondi MC, De Biasi S, Spreafico R. GABAergic neurons in mammalian thalamus: a marker of thalamic complexity? Brain Res Bull. 1997;42:27–37. doi: 10.1016/s0361-9230(96)00107-4. [DOI] [PubMed] [Google Scholar]
- Aznar S, Qian Z, Shah R, Rahbek B, Knudsen GM. The 5-HT1A serotonin receptor is located on calbindin- and parvalbumin-containing neurons in the rat brain. Brain Res. 2003;959:58–67. doi: 10.1016/s0006-8993(02)03727-7. [DOI] [PubMed] [Google Scholar]
- Barthó P, Freund TF, Acsady L. Selective GABAergic innervation of thalamic nuclei from zona incerta. Eur J Neurosci. 2002;16:999–1014. doi: 10.1046/j.1460-9568.2002.02157.x. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Bécamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006;326:553–572. doi: 10.1007/s00441-006-0286-1. Epub 2006 Aug 1. [DOI] [PubMed] [Google Scholar]
- Bokor H, Frere SG, Eyre MD, Slezia A, Ulbert I, Luthi A, Acsady L. Selective GABAergic control of higher-order thalamic relays. Neuron. 2005;45:929–940. doi: 10.1016/j.neuron.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Chapin EM, Andrade R. A 5-HT(7) receptor-mediated depolarization in the anterodorsal thalamus. I. Pharmacological characterization. J Pharmacol Exp Ther. 2001a;297:395–402. [PubMed] [Google Scholar]
- Chapin EM, Andrade R. A 5-HT(7) receptor-mediated depolarization in the anterodorsal thalamus. II. Involvement of the hyperpolarization-activated current I(h) J Pharmacol Exp Ther. 2001b;297:403–409. [PubMed] [Google Scholar]
- Douglas WW. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968;34:451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lima AD, Singer W. The serotoninergic fibers in the dorsal lateral geniculate nucleus of the cat: distribution and synaptic connections demonstrated with immunocytochemistry. J Comp Neurol. 1987;258:339–351. doi: 10.1002/cne.902580303. [DOI] [PubMed] [Google Scholar]
- Gonzalo-Ruiz A, Lieberman AR, Sanz-Anquela JM. Organization of serotoninergic projections from the raphe nuclei to the anterior thalamic nuclei in the rat: a combined retrograde tracing and 5-HT immunohistochemical study. J Chem Neuroanat. 1995;8:103–115. doi: 10.1016/0891-0618(94)00039-v. [DOI] [PubMed] [Google Scholar]
- Guido W, Lu SM, Vaughan JW, Godwin DW, Sherman SM. Receiver operating characteristic (ROC) analysis of neurons in the cat's lateral geniculate nucleus during tonic and burst response mode. Vis Neurosci. 1995;12:723–741. doi: 10.1017/s0952523800008993. [DOI] [PubMed] [Google Scholar]
- Guillery RW. Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. J Anat. 1995;187(Pt 3):583–592. [PMC free article] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003;26:331–343. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Kayama Y, Ohta M, Jodo E. Firing of ‘possibly’ cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res. 1992;569:210–220. doi: 10.1016/0006-8993(92)90632-j. [DOI] [PubMed] [Google Scholar]
- Kirifides ML, Simpson KL, Lin RC, Waterhouse BD. Topographic organization and neurochemical identity of dorsal raphe neurons that project to the trigeminal somatosensory pathway in the rat. J Comp Neurol. 2001;435:325–340. doi: 10.1002/cne.1033. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Acetycholine inhibits identified interneurons in the cat lateral geniculate nucleus. Nature. 1988;334:246–248. doi: 10.1038/334246a0. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol. 1990;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monckton JE, McCormick DA. Neuromodulatory role of serotonin in the ferret thalamus. J Neurophysiol. 2002;87:2124–2136. doi: 10.1152/jn.00650.2001. [DOI] [PubMed] [Google Scholar]
- Mooney DM, Zhang L, Basile C, Senatorov VV, Ngsee J, Omar A, Hu B. Distinct forms of cholinergic modulation in parallel thalamic sensory pathways. Proc Natl Acad Sci USA. 2004;101:320–324. doi: 10.1073/pnas.0304445101. Epub 2003 Dec 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oheim M, Kirchhoff F, Stuhmer W. Calcium microdomains in regulated exocytosis. Cell Calcium. 2006;40:423–439. doi: 10.1016/j.ceca.2006.08.007. Epub 2006 Oct 25. [DOI] [PubMed] [Google Scholar]
- Pape HC, McCormick DA. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989;340:715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain—III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience. 1987;21:97–122. doi: 10.1016/0306-4522(87)90326-5. [DOI] [PubMed] [Google Scholar]
- Piccolino M, Pignatelli A. Calcium-independent synaptic transmission: artifact or fact? Trends Neurosci. 1996;19:120–125. doi: 10.1016/s0166-2236(96)80017-8. [DOI] [PubMed] [Google Scholar]
- Ramcharan EJ, Gnadt JW, Sherman SM. Higher-order thalamic relays burst more than first-order relays. Proc Natl Acad Sci USA. 2005;102:12236–12241. doi: 10.1073/pnas.0502843102. Epub Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoa AS, McCormick DA. Developmental changes in electrophysiological properties of LGNd neurons during reorganization of retinogeniculate connections. J Neurosci. 1994;14:2089–2097. doi: 10.1523/JNEUROSCI.14-04-02089.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinagel P, Godwin D, Sherman SM, Koch C. Encoding of visual information by LGN bursts. J Neurophysiol. 1999;81:2558–2569. doi: 10.1152/jn.1999.81.5.2558. [DOI] [PubMed] [Google Scholar]
- Roberts JC, Reavill C, East SZ, Harrison PJ, Patel S, Routledge C, Leslie RA. The distribution of 5-HT(6) receptors in rat brain: an autoradiographic binding study using the radiolabelled 5-HT(6) receptor antagonist [(125)I]SB-258585. Brain Res. 2002;934:49–57. doi: 10.1016/s0006-8993(02)02360-0. [DOI] [PubMed] [Google Scholar]
- Sánchez-González MA, García-Cabezas MA, Rico B, Cavada C. The primate thalamus is a key target for brain dopamine. J Neurosci. 2005;25:6076–6083. doi: 10.1523/JNEUROSCI.0968-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Punhani T, Fone KC. Distribution of the 5-hydroxytryptamine2C receptor protein in adult rat brain and spinal cord determined using a receptor-directed antibody: effect of 5,7-dihydroxytryptamine. Synapse. 1997;27:45–56. doi: 10.1002/(SICI)1098-2396(199709)27:1<45::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci. 2001;24:122–126. doi: 10.1016/s0166-2236(00)01714-8. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators. Proc Natl Acad Sci USA. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the thalamus and its roles in cortical function. Cambridge (MA): The MIT Press; 2005. [Google Scholar]
- Smith GD, Cox CL, Sherman SM, Rinzel J. Fourier analysis of sinusoidally driven thalamocortical relay neurons and a minimal integrate-and-fire-or-burst model. J Neurophysiol. 2000;83:588–610. doi: 10.1152/jn.2000.83.1.588. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCarley RW. Brainstem control of wakefulness and sleep. New York: Plenum; 2005. [Google Scholar]
- Swadlow HA, Gusev AG. The impact of ‘bursting’ thalamic impulses at a neocortical synapse. Nat Neurosci. 2001;4:402–408. doi: 10.1038/86054. [DOI] [PubMed] [Google Scholar]
- Van Horn SC, Sherman SM. Fewer driver synapses in higher order than in first order thalamic relays. Neuroscience. 2007;146:463–470. doi: 10.1016/j.neuroscience.2007.01.026. Epub 2007 Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela C, Sherman SM. Differences in response to muscarinic activation between first and higher order thalamic relays. J Neurophysiol. 2007;98:3538–3547. doi: 10.1152/jn.00578.2007. Epub 2007 Oct 17. [DOI] [PubMed] [Google Scholar]
- Varnäs K, Thomas DR, Tupala E, Tiihonen J, Hall H. Distribution of 5-HT7 receptors in the human brain: a preliminary autoradiographic study using [3H]SB-269970. Neurosci Lett. 2004;367:313–316. doi: 10.1016/j.neulet.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- Wang S, Eisenback MA, Bickford ME. Relative distribution of synapses in the pulvinar nucleus of the cat: implications regarding the “driver/modulator” theory of thalamic function. J Comp Neurol. 2002;454:482–494. doi: 10.1002/cne.10453. [DOI] [PubMed] [Google Scholar]