Abstract
The stop signal task (SST) is widely used to explore neural processes involved in cognitive control. By randomly intermixing stop and go trials and imposing on participants to respond quickly to the go but not the stop signal, the SST also introduces an indirect element of risk, which participants may avert by slowing down or ignore by responding “as usual,” during go trials. This “risk-taking” component of the SST has to our knowledge never been investigated. The current study took advantage of variability of go trial reaction time (RT) and compared the post-go go trials that showed a decrease in RT (risk-taking decision) and those post-go go trials that showed an increase in RT (“risk-aversive” decision) in 33 healthy individuals who underwent functional magnetic resonance imaging during the SST. This contrast revealed robust activation in bilateral visual cortices as well as left inferior parietal and posterior cingulate cortices, amygdala, and middle frontal gyrus (P < 0.05, family-wise error [FWE] corrected). Furthermore, we observed that the magnitude of amygdala activity is positively correlated with trait anxiety of the participants. These results thus delineated, for the first time, a neural analog of risk taking during stop signal performance, highlighting a novel aspect and broadening the utility of this behavioral paradigm.
Keywords: amygdala, frontolimbic, limbic, risk taking, ventromedial prefrontal
Introduction
The stop signal task (SST) is one of most widely used behavioral paradigms in studies of cognitive control, an “executive” function that allows individuals to restrain a habitual response, detect errors, and learn from errors by adapting our behavior. Many investigators have employed the SST to examine the neural processes of inhibitory control and to explore whether and how these processes are altered in psychiatric conditions in which deficits in cognitive control are implicated (Robbins 2007). For instance, the difficulty of a stop trial in the SST could be systematically manipulated by varying the time delay between the go and stop signals—the stop signal delay (SSD). By computing the probability of stop successes across a range of SSDs, one can estimate an inhibitory function or the stop signal reaction time (SSRT), to index individuals’ inhibitory ability (Logan and Cowan 1984; Logan et al. 1984; Logan 1994). Differences in the inhibitory function or SSRT have been used to characterize patients with attention deficit hyperactivity disorder (ADHD), obsessive compulsive disorder (OCD), and cocaine dependence among other clinical conditions (Crosbie and Schachar 2001; Johannes et al. 2001; Fillmore and Rush 2002; Chamberlain et al. 2006; Li, Milivojevic, et al. 2006; Alderson et al. 2007; Li, Huang, Yan, Bhagawar, et al. 2008; for a review, see also Li and Sinha 2008). Furthermore, by comparing individuals with short and long SSRT who otherwise do not differ in stop signal performance, one can potentially isolate the neural correlates of inhibitory control (Li, Huang, et al. 2006). In other studies, investigators contrasted stop errors with stop successes to examine medial cortical activity during error processing (Schmajuk et al. 2006; Chevrier et al. 2007; Li et al. forthcoming). In addition, we observed ventrolateral prefrontal cortical (VLPFC) activation during posterror slowing (PES) in a recent study where posterror go trials that increased in reaction time (RT) were compared with posterror go trials that did not increase in RT (Li, Huang, Yan, Paliwal, et al. 2008). This VLPFC activity appears to represent a specific neural signature of posterror behavioral adjustment. Such error-related responses have also been extensively investigated in children with ADHD (Schachar et al. 2004; Liotti et al. 2005) and patients with OCD (Ursu et al. 2003; Fitzgerald et al. 2005; Maltby et al. 2005; Hajcak et al. 2008; see also Nieuwenhuis et al. 2005). Overall, the SST has been of instrumental importance as a behavioral proxy in studies of the pathogenesis of neurological and psychiatric illnesses in which cortical deficits in cognitive control—or behavioral impulsivity—are involved (Bornovalova et al. 2005; Arnsten 2006; Winstanley et al. 2006; Chamberlain and Sahakian 2007).
Although cognitive control maintains homeostatic regulation, individuals venture out of their behavioral routines to seek new opportunities. This alternative aspect of impulsivity or the readiness to “take risk”—is a complex psychological process that contributes to the make up of human personality (Evenden 1999). Individuals show great variation in their propensity to take risk (Cohen et al. 2005; Galvan et al. 2007; Marsch et al. 2007). Although impulsive behaviors are mostly known to be associated with negative consequences, “risk taking” may have conferred distinct advantage in reproductive success in nonhuman primates (Berard et al. 1993; Mehlman et al. 1995). Impulsive male macaque monkeys migrate early from their natal group to seek sexual opportunities, whereas those that are excessively inhibited lose their edge in reproducing. Impulsivity is heritable. Studies of genetic linkage in humans, pedigree association in monkeys, and mouse inbreeding have all provided strong evidence for the heritability of impulsive behaviors (Swanson et al. 2001; Fairbanks et al. 2004; Isles et al. 2004).
Many studies have examined how brain responds when individuals take risks, typically in a paradigm involving reward contingencies (Paulus et al. 2003; Matthews et al. 2004; Ernst et al. 2005; Fukui et al. 2005; Kuhnen and Knutson 2005; Daw et al. 2006; Huettel et al. 2006; Paulus and Stein 2006; Yacubian et al. 2006; Coricelli et al. 2007; Eshel et al. 2007; Liu et al. 2007; Satterthwaite et al. 2007; Tobler et al. 2007; Tom et al. 2007). For instance, in individuals performing the Iowa gambling task, greater activation in the medial frontal gyrus is associated with risky choices, as compared with safe choices (Fukui et al. 2005). In a variant risk-related decision-making task, individuals showed greater activation in the right insula when selecting a risky as compared with a safe response (Paulus et al. 2003). Furthermore, such insula activation varied with individuals’ personality traits such as harm avoidance and neuroticism. Taken together, the frontolimbic circuits involving ventromedial prefrontal cortex (VMPFC), amygdala and insula, and structures involving reward and conflict processing, such as the ventral striatum and anterior cingulate cortex, are implicated in risk-taking behaviors.
The SST has to our knowledge never been used to investigate risk-taking behaviors. One characteristic of behavioral performance during the SST, in which the SSD tracked participants’ performance, is that participants demonstrate great variability in RT from one go trial to another. Although variation in RT is typical of any RT task, it may also result from an active decision process related to risk taking or “risk averting”. The rationale is that, with SSD and thus the difficulty of the stop trial varying constantly, participants are engaged in a process during which they need to meet the task requirements of being both accurate and fast. Thus, the RT of a go trial during which participants venture that no stop signal will follow is shorter than a go trial during which participants are aversive to such speculation. Therefore, the trial-to-trial variation in go RT involves a distinct albeit indirect component of risk taking.
The present study intended to examine this process in more details. The central question is that are distinct neural activations involved in go trials in which participants show a decrease in go trial RT (risk taking) as compared with those in which participants show an increase in go trial RT (risk averting) and vice versa? One important aspect of the stop signal performance needs to be considered in such investigation. That is, consistent with numerous previous studies, our recent work of the SST have shown that the RT of go trials that follow a stop trial (success or error) is longer compared with the RT of go trial that follow a go trial (Li, Huang, et al. 2006; Li, Milivojevic, et al. 2006; Li, Huang, Yan, Paliwal, et al. 2008; Rabbit 1966). Thus, to account for this contextual or “trial sequence” effect, we focused only on those go trials that immediately followed a go trial. We contrasted post-go go trials that decreased and increased, respectively, in RT, as compared with the mean RT of all post-go go trials that preceded the trial in question. We hypothesized that brain regions involved in risk taking, such as amygdala, insula, and medial frontal cortex would show greater activation during speeded as compared with delayed motor responses. An auxiliary goal of the study is to focus on individual variability and assess whether such risk-taking–related regional brain activation is associated with trait anxiety of the participants.
Materials and Methods
Subjects and Behavioral Task
Thirty-three healthy adults (20 men, 31 ± 6 years of age, 14 ± 2 years of education, all right-handed and using their right thumb to respond) were paid to participate in the study. All participants were free of major medical illness including neurological and psychiatric. None reported use of illicit substances. Our participants were assessed with the Maudsley Obsessive Compulsive Inventory (MOCI) as part of a preliminary study to investigate the neural correlates of obsessive compulsiveness (Hodgson and Rachman 1977). All subjects signed a written informed consent, in accordance to a protocol approved by the Yale Human Investigation Committee.
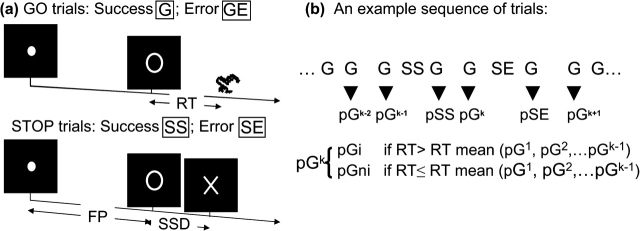
We employed a simple RT task in this stop signal paradigm (Li, Huang, et al. 2006; Fig. 1a). There were 2 trial types: “go” and “stop,” randomly intermixed in presentation. No other conditions constrained the presentation of the go and stop trials. A small dot appeared on the screen to engage attention and eye fixation at the beginning of a go trial. After a randomized time interval anywhere between 1 and 5 s (drawn from a uniform distribution), the dot turned into a circle, which served as an imperative stimulus, prompting subjects to quickly press a button. The circle vanished at button press or after 1 s had elapsed, whichever came first, and the trial terminated. A premature button press prior to the appearance of the circle also terminated the trial. Three quarters of all trials were go trials. The remaining 1 quarter were stop trials. In a stop trial, other than the fixation dot and go signal, an “X” or the “stop” signal appeared after and replaced the go signal. The subjects were told to withhold button press upon seeing the stop signal. Likewise, a trial terminated at button press or when 1 s had elapsed because the appearance of the stop signal. The SSD started at 200 ms and varied from one stop trial to the next according to a staircase procedure: If the subject succeeded in withholding the response, the SSD increased by 64 ms; conversely, if they failed, SSD decreased by 64 ms (Levitt 1970). There was an intertrial interval of 2 s. Subjects were instructed to respond to the go signal quickly while keeping in mind that a stop signal could come up in a small number of trials. Prior to the functional magnetic resonance imaging (fMRI) study, each subject had a practice session on the same behavioral task outside the scanner. In particular, both accuracy and response speed were emphasized to the participants (Li, Huang, Yan, Paliwal, et al. 2008). In the scanner each subject completed four 10-min runs of the task with the SSD updated automatically by the stimulus presentation program within each run and manually by the investigator across runs. The between-run duration was approximately 1 min, during which participants were instructed to close their eyes, rest, and stay still. Depending on the actual stimulus timing (trial varied in fore-period duration) and speed of response, the total number of trials varied slightly across subjects in an experiment (averaging ca. 315 go and 105 stop trials). The randomization procedure resulted in 74.9 (25.1) ± 1.2% go (stop) trials across subjects. With the staircase procedure, we anticipated that the subjects succeeded in withholding their response in approximately half of the stop trials. Furthermore, because of the variable fore period, go and stop trials were temporally well jittered.
Figure 1.
(a) Stop signal paradigm. In “go” trials (75%) observers responded to the go signal (a circle) and in stop trials (25%) they had to withhold the response when they saw the stop signal (an X). In both trials, the go signal appeared after a randomized time interval between 1 and 5 s (the fore-period or FP, uniform distribution) following the appearance of the fixation point. The stop signal followed the go signal by a time delay—the SSD. The SSD was updated according to a staircase procedure, whereby it increased and decreased by 64 ms following a stop success and stop error trial, respectively. There was an intertrial interval of 2 s. We distinguished go success (G) and go error (GE) and stop success (SS) and stop error (SE) trials during the task. (b) Go successes were further distinguished by their preceding trial; thus, pG trials were G trials preceded by a G trial, pSS trials were G trials preceded by a SS trial, and so on. Depending on whether they increased or did not increase in RT, compared with the mean RT of all preceding pG trials, pG trials were further grouped into pGi (post-go slowing) and pGni (post-go speeding; see Materials and Methods).
Following fMRI scan, participants were interviewed with the MOCI, which is a 30-item self-report questionnaire designed to measure obsessive compulsiveness, an anxiety personality trait (Hodgson and Rachman 1977). All items in the MOCI are answered true or false, yielding a maximum score of 30 with higher score indicating greater tendency toward obsessive compulsiveness and anxiety. Earlier studies have suggested that MOCI exhibit good reliability and predictive validity in nonclinical populations (Dent and Salkovskis 1986; Sternberger and Burns 1990).
Imaging Protocol
Conventional T1-weighted spin echo sagittal anatomical images were acquired for slice localization using a 3-T scanner (Siemens Trio, Erlangen, Germany). Anatomical images of the functional slice locations were next obtained with spin echo imaging in the axial plane parallel to the AC–PC line with time repetition (TR) = 300 ms, time echo (TE) = 2.5 ms, bandwidth = 300 Hz/pixel, flip angle = 60°, field of view = 220 × 220 mm, matrix = 256 × 256, and 32 slices with slice thickness = 4 mm and no gap. Functional, blood oxygen level–dependent (BOLD) signals were then acquired with a single-shot gradient echo planar imaging (EPI) sequence. Thirty-two axial slices parallel to the AC–PC line covering the whole brain were acquired with TR = 2000 ms, TE = 25 ms, bandwidth = 2004 Hz/pixel, flip angle = 85°, field of view = 220 × 220 mm, matrix = 64 × 64, and 32 slices with slice thickness = 4 mm and no gap. Three hundred images were acquired in each run for a total of 4 runs.
Data Analysis and Statistics
Data were analyzed with Statistical Parametric Mapping version 2 (SPM2, Wellcome Department of Imaging Neuroscience, University College London, London, UK). Images from the first 5 TRs at the beginning of each run were discarded to enable the signal to achieve steady state equilibrium between radiofrequency pulsing and relaxation. Images of each individual subject were first corrected for slice timing and realigned (motion corrected). A mean functional image volume was constructed for each subject for each run from the realigned image volumes. These mean images were normalized to an Montreal Neurological Institute (MNI) EPI template with affine registration followed by nonlinear transformation (Friston, Ashburner, et al. 1995; Ashburner and Friston 1999). The normalization parameters determined for the mean functional volume were then applied to the corresponding functional image volumes for each subject. Finally, images were smoothed with a Gaussian kernel of 10 mm at full width at half maximum. The data were high-pass filtered (1/128 Hz cutoff) to remove low-frequency signal drifts.
Four main types of trial outcome were first distinguished: go success (G), go error (GE), stop success (SS), and stop error (SE) trial (Fig. 1b). G trials were divided into those that followed a G (pG), GE (pGE), SS (pSS), and SE (pSE) trial and pG, pSS, and pSE trials were further divided into those that increased in RT (pGi, pSSi, and pSEi, respectively) and those that did not increase in RT (pGni, pSSni, and pSEni), to allow the isolation of neural processes involved in post-G, post-SS, and post-SE behavioral adjustment. To determine whether a pG/pSS/pSE trial increased or did not increase in RT, it was compared with the pG trials that preceded it in time during each session. The pG trials that followed the pG/pSS/pSE trial were not included for comparison because these subsequent pG trials could not have a causal effect on the pG/pSS/pSE trial in question, in terms of how participants adjust their response speed. A single statistical analytical design was constructed for each individual subject, using the general linear model (GLM) with the onsets of go signal in each of these trial types convolved with a canonical hemodynamic response function (HRF) and with the temporal derivative of the canonical HRF and entered as regressors in the model (Friston, Holmes, et al. 1995). Realignment parameters in all 6 dimensions were also entered in the model. Serial autocorrelation of the time series was corrected by a first-degree autoregressive or AR(1) model (Friston et al. 2000; Della-Maggiore et al. 2002). The GLM estimated the component of variance that could be explained by each of the regressors.
In the first-level analysis, we contrasted pGni versus PGi for individual subjects to identify the neural correlates of risk taking. We also contrasted pSSi versus pSSni and pSEi versus pSEni, to compare the current results with previous findings of postconflict and posterror adjustment in RT, respectively (Li, Huang, Yan, Paliwal, et al. 2008). The contrast images for individual subjects were employed for random effect analysis (Penny and Holmes 2004). Brain regions were identified using an atlas (Duvernoy 2003; Mai et al. 2008). In region of interest (ROI) analysis, we used MarsBaR (Brett et al. 2002; http://marsbar.sourceforge.net/) to derive for each individual subject the effect size of activity change for the ROIs. Functional ROIs were defined based on activated clusters from whole brain analysis. All voxel activations are presented in MNI coordinates. Cross correlation with Pearson regression was performed for the effect size of activity change between the ROIs.
Results
General Stop Signal Performance
Across all 33 subjects, the mean and median go trial RT were 539 ± 106 ms (mean ± standard deviation [SD]) and 529 ± 111 ms, respectively, consistent with the right-skewed distribution of RT in an RT task. Our subjects scored 97.9 ± 1.7% of go trials and 50.4 ± 2.0% of stop trials. Across subjects, the RT of stop error trials were significantly shorter than the RT of go trials (P < 0.001, paired t-test). Furthermore, the RT and SSD of stop error trials were positively correlated (all P values < 0.01, 0.370 < R values < 0.927, Pearson regression). The latter 3 findings suggested that their overall performance was adequately tracked by the staircase procedure. Across subjects, there were 109 ± 16 (mean ± SD) of pGi and 104 ± 17 of pGni trials across subjects. The pGi increase in RT was 63 ± 10 ms, whereas the pGni decrease in RT was 47 ± 8 ms. The magnitude of RT increase or decrease, each during delayed (risk-averting) and speeded (risk-taking) response, was thus approximately half a SD of the mean go trial RT.
Neural Substrates of Speeded as Compared with Delayed Motor Response during the SST
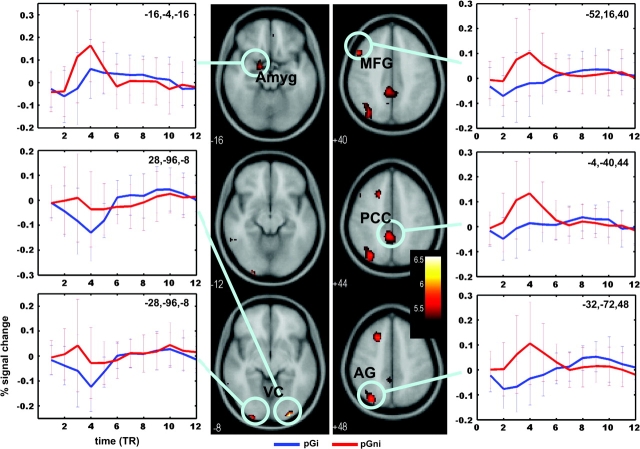
Compared with post-go go trial that increased in RT (pGi), post-go go trials that decreased in RT (pGni) engaged greater activity in bilateral visual cortices (x =28, y = −96, z = −8, 640 mm3, voxel Z = 5.89; x = −28, y = −100, z = −8, 448 mm3, voxel Z = 5.24), left amygdala (x = −16, y = −4, z = −16, 704 mm3, voxel Z = 4.93), middle and superior frontal gyri (x = −52, y = 16, z = 40, 1216 mm3, voxel Z = 5.22; x = −20, y = 20, z = 48, 1920 mm3, voxel Z = 5.20), inferior parietal cortex (x = −32, y = −72, z = 48, 4096 mm3, voxel Z = 5.20), and posterior cingulate cortex (PCC) and precuneus (x = −4, y = −40, z = 44, 3456 mm3, voxel Z = 5.13; x = −20, y = −68, z = 20, 3136 mm3, voxel Z = 4.91), at P < 0.05, corrected for FWE of multiple comparisons and 5 voxels in extent of activation (Fig. 2). Thus, speeded as compared with delayed responses involved a distinct pattern of regional brain activation during the SST. At a lower statistical threshold, a number of other brain regions also showed greater activity for this contrast (P < 0.001, uncorrected and 10 voxels in the extent of activation, Fig. 3). These brain regions as identified from SPM are summarized in Table 1. Notably, a cluster of 5504 mm3 in the VMPFC was identified with a peak voxel Z value of 4.49. We made this cluster a ROI because many previous studies have implicated the VMPFC and the functional connection between the VMPFC and amygdala in risk-taking, affective processes and autonomic responses during affective processing (see Discussion; Fig. 4a,b). Also worth noting was a small cluster in the right posterior insula (Table 1). Conversely, no brain regions showed greater activation during delayed (risk-aversive) compared with speeded (risk-taking) motor responses (P < 0.01, uncorrected).
Figure 2.
At a threshold of P < 0.05, corrected for family-wise error of multiple comparisons, post-go speeding as compared with post-go slowing elicited greater activation in bilateral visual cortices; left amygdala, middle, and superior frontal gyri; inferior parietal cortex; and PCC. Blood oxygen level–dependent contrasts are superimposed on a T1-structural image in axial sections. Color bar represents voxel T value. For each functional cluster of activation, we also show the time course of percentage of signal change. The peristimulus time histogram is estimated using MarsBaR based on the finite impulse response model.
Figure 3.
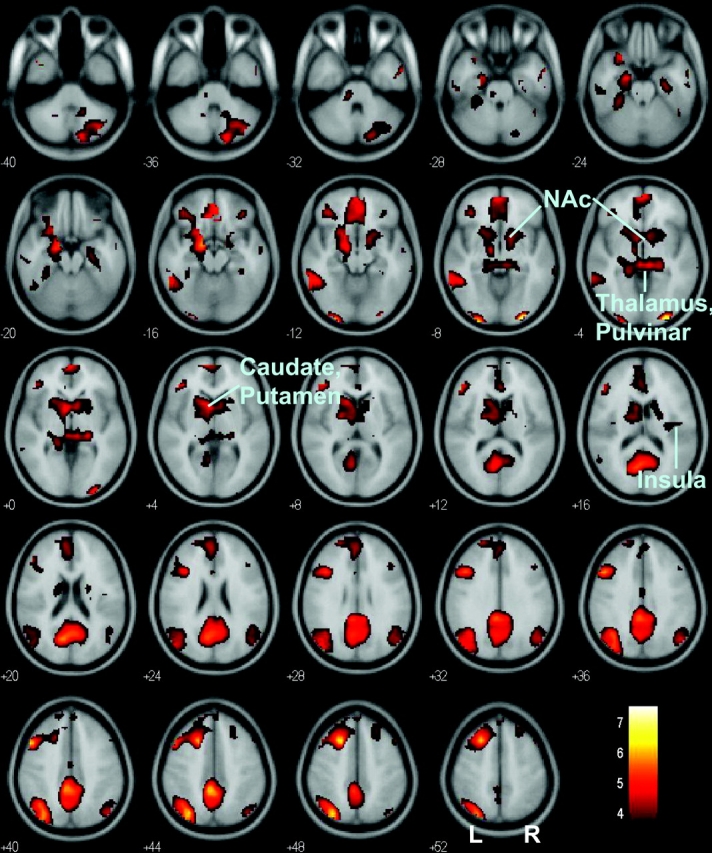
At a more relaxed threshold (P < 0.001, uncorrected and 10 voxels in extent of activation), many other regions also showed greater activation during post-go speeding, compared with post-go slowing. These regions included the VMPFC, ventral and dorsal striatum, thalamus, and right posterior insula. Absence of activity was noted in the anterior insula. Blood oxygen level–dependent signals were overlaid on a T1-structural image in axial slices from z = −40 to z = +52, with adjacent sections 4 mm apart. Color scale represents voxel T value. Orientation is neurological: R, right; NAc, Nucleus Accumbens.
Table 1.
Regional brain activity during risk taking
| Cluster size (voxels) | Voxel Z value | MNI coordinate (mm) |
Identified brain region and approximate BA | ||
| x | y | z | |||
| 34 | 5.89 | 28 | −96 | −8 | Visual cortex, BA 18 |
| 35 | 5.24 | −28 | −100 | −8 | Visual cortex, BA 18 |
| 2588 | 5.22 | −52 | 16 | 40 | Middle frontal G, BA 8 |
| 5.20 | −20 | 20 | 48 | Superior frontal S, BA 8 | |
| 5.13 | −4 | −44 | 44 | Precuneus/posterior cingulate gyrus, BA 31 | |
| 362 | 5.20 | −32 | −72 | 48 | Angular G, BA 39 |
| 4.75 | −40 | −60 | 40 | Angular G, BA 39 | |
| 110 | 4.51 | −60 | −48 | −12 | Middle temporal G, BA 37 |
| 30 | 4.34 | 56 | 0 | −32 | Inferior temporal G, BA 20 |
| 3.38 | 56 | −8 | −16 | Inferior temporal G, BA 20 | |
| 175 | 4.31 | 16 | −84 | −36 | Cerebellum |
| 4.07 | 44 | −64 | −44 | Cerebellum | |
| 152 | 4.07 | 48 | −72 | 40 | Angular G, BA 39 |
| 15 | 3.90 | −12 | −32 | −32 | Cerebellum |
| 16 | 3.88 | 36 | 36 | −16 | Orbitofrontal G, BA 47 |
| 21 | 3.66 | −56 | −24 | −28 | Cerebellum |
| 26 | 3.60 | 48 | 20 | 48 | Middle frontal G, BA 8 |
| 41 | 3.57 | 24 | 32 | 48 | Middle frontal G, BA 8 |
| 15 | 3.49 | 32 | −16 | 16 | Insula, posterior part, BA 13 |
| 11 | 3.39 | 0 | −8 | 36 | Cingulate G |
Note: P < 0.001, uncorrected, and 10 voxels in extent of activation. G, gyrus; S, sulcus; BA, Brodmann area
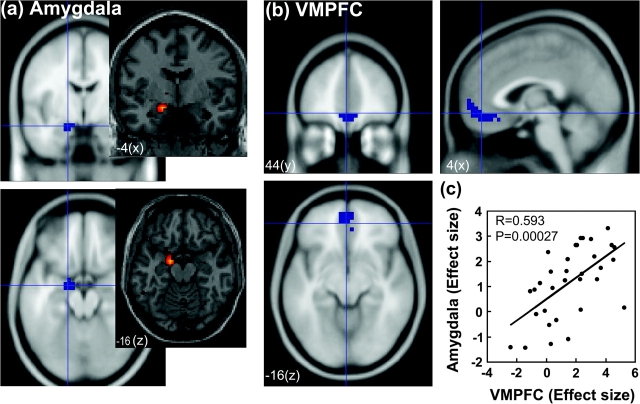
Figure 4.
Functional ROI was identified of the amygdala (a) and the VMPFC (b). (c) Effect sizes of activity during post-go speeding > post-go slowing were linearly correlated in the 2 structures.
ROI Analyses
We made ROIs based on the results obtained in whole-brain analyses. We derived the effect size of the contrast “speeded response > delayed response” for bilateral visual cortices, left amygdala, PCC/precuneus, middle and superior frontal gyri, and VMPFC and cross-correlated the effect sizes with pairwise linear regressions. Because of multiple comparisons, we guarded the type-I error at an α = 0.05/10 = 0.005 because of a total of 5 functional groups of ROIs: visual cortices, PCC/precuneus, middle/superior frontal gyri, VMPFC, and amygdala. The results showed that bilateral visual cortical activations were highly correlated (P < 0.0001, R = 0.610, Pearson regression) but not with any other ROIs (all P values > 0.1). In contrast, PCC/precuneus, middle and superior frontal gyri, and VMPFC were also correlated in activity (all P values < 0.0002, 0.606 < R values < 0.807). Amygdala showed a moderate correlation in activity with the VMPFC (P < 0.0003, R = 0.593, Fig. 4c) and PCC (P < 0.004, R = 0.487) but not with any other ROIs (all P values > 0.02).
The total MOCI score ranged from 1 to 15 with an average of 7.0 ± 4.1 (mean ± SD), below the mean of 18.9 and 9.3 reported, respectively, for obsessional and nonobsessional neurotic individuals (Hodgson and Rachman 1977). We observed a significant linear correlation between amygdala activity and total MOCI score (P < 0.001, R = 0.576). Activations in the PCC (P > 0.3) or VMPFC (P > 0.3) did not correlate with this anxiety trait.
Post-go and Posterror Speeding Involves Distinct Neural Circuits
Does “post-go speeding” indeed engage neural processes distinct from RT adjustment under other trial contexts? We showed in our previous work that the ventrolateral prefrontal cortex is involved in PES, but no brain regions were specifically activated for the reverse contrast: “posterror speeding” > PES (Li, Huang, Yan, Paliwal, et al. 2008). We thus examined whether the functional ROIs identified from the current study also activated for the contrast posterror speeding > PES with small volume correction (SVC). Note that our subjects showed an RT increase of 122 ± 38 ms during PES and an RT decrease of 75 ± 39 ms during posterror speeding, comparable to the post-go measures (see results on General Stop Signal Performance). Despite the RT differences in posterror adjustment, the results showed a small cluster of 640 mm3 in the ventromedial prefrontal region (x = −12, y = 48, z = −4, voxel Z = 2.00) and 2 other clusters each of 384 mm3 (x = −6, y = −56, z = 24, voxel Z = 1.93) and of 192 mm3 (x = −4, y = −36, z = 44, voxel Z = 1.84) in the precuneus/PCC region, at P < 0.05, uncorrected, with SVC. None of these clusters survived an uncorrected P < 0.01. No activations could be identified in the amygdala.
Discussion
Speeded as Compared with Delayed Motor Responses in the SST Engages Distinct Brain Regions
Although consistent with the right-skewed distribution typical of a RT task, the go trial RT of our participants was above 500 ms, suggesting that participants were “weighing” their options during go responses. The current study addressed the question whether trial-by-trial variation in go RT could reflect a decision process indirectly related to risk taking. To this end, we accounted for limited contextual effects and examined go trials that were immediately preceded by another go trial. By contrasting post-go go trials that decreased and increased in RT, we have identified distinct brain circuits that appear to reflect this risk-taking decision during the SST.
Activation of bilateral visual cortices, angular gyrus, and middle/superior frontal gyri could result from greater attention and response readiness directed to the impending go signal (Brefczynski and DeYoe 1999; Smith et al. 2000; Mort et al. 2003; Slotnick et al. 2003; Luks and Simpson 2004; Thiel et al. 2005; Tamm et al. 2006; Macaluso et al. 2007; Hanakawa et al. forthcoming; Teitti et al. 2008). Activity in the amygdala and ventral and dorsal striatum likely reflected greater saliency associated with risk-taking as compared with risk-averting decisions during the SST (Berns et al. 2005, 2006; Johnstone et al. 2006; van Reekum et al. 2007; Williams et al. 2007; Zink et al. 2004, 2006). Affective responses as engaged during risk-related behaviors may lead to greater activation of the amygdala, VMPFC, and the PCC, consistent with a role of these brain regions in mediating emotion and autonomic arousal (Mangina and Beuzeron-Mangina 1996; Fredrikson et al. 1998; Nagai et al. 2004; Ohira et al. 2006; Stark et al. 2006; Cheng et al. 2007; Eippert et al. 2007). The finding of greater amygdala activity during post-go speeding is consistent with the vast literature linking this subcortical structure to risk-related behavior in imaging studies (Bechara et al. 1999, 2003; Kahn et al. 2002; Cohen and Ranganath 2005; Hsu et al. 2005; Vorhold et al. 2007) and in animal and human lesion studies (Klüver and Bucy 1938; Zola-Morgan et al. 1991; Brand et al. 2007; for a review, see Davis 1992; LeDoux 1998; Davidson et al. 2000). In particular, the correlation of amygdala activity with an anxiety trait suggests that the contrast between post-go speeding and slowing captured an important dimension of individual differences in risk taking, sensation seeking, and harm avoidance. This unique aspect of stop signal performance has heretofore never been reported in the literature. This to our knowledge also appears to be the first report of a trait measure of affective processes involved in a cognitive motor task.
Notably, greater activation is observed in the left than the right hemisphere during risk taking in the SST (Fig. 2). This result is consistent with previous studies demonstrating an association between individual risk-taking tendency and left-hemispheric activity (Drake 1985; Drake and Ulrich 1992; for further assessment of this issue, see, however, McElroy and Seta 2004; Gallagher and Dagenbach 2007). Also broadly relevant is the recent observation that inactivation by transcranial magnetic stimulation of the right but not left prefrontal cortex induced risk-taking behaviors in a gambling task (Knoch et al. 2006). Conversely, activation by direct current stimulation of the right prefrontal cortex diminished risk-taking behavior (Fecteau et al. 2007). Note that, however, right-hemispheric activity is clearly evident at a more liberal threshold. More studies are needed to pursue interhemispheric mechanisms of risk-taking behavior.
Overall, speeded (risk-taking) as compared with delayed (risk-averting) go responses in the SST appeared to activate many similar brain regions as engaged in behavioral tasks involving an explicit reward. One notable difference is the anterior insula, which is extensively implicated in mediating risk-taking decisions (Paulus et al. 2003; Kuhnen and Knutson 2005; Huettel 2006; Lee et al. forthcoming; Preuschoff et al. 2008). Previous studies have suggested an important role of the anterior insula in processing error (Taylor et al. 2007), processing unpleasant effect during mental satiation (Mojzisch and Schulz-Hardt 2007), mediating the difference between expected and experienced bodily states (Paulus and Stein 2006), and subserving neural representations of feeling and self bodily states and social emotions related to others (Singer 2007). The lack of anterior insula activation in the current study presumably could reflect the absence of these cognitive and affective processes during post-go speeding in the SST. For instance, perhaps because no monetary reward was at stake, little negative affect was evoked during post-go speeding, despite the motivational saliency of this decision. Risk taking in the SST may thus partake in some but not all of the risk-related cognitive and affective processes that have been observed in earlier work in which material loss was involved.
Post-go Speeding versus Posterror Speeding
Compared with a risk-averting decision as shown in post-go slowing in go trial RT, a risk-taking decision involving post-go speeding activates the left angular gyrus; bilateral visual cortices; left amygdala, middle, and superior frontal gyri; PCC and precuneus; and VMPFC. None of these brain areas showed comparable activity during posterror speeding as in post-go speeding. Although posterror speeding represents a decision process in response to an external and relatively infrequent event (stop error, comprising approximately 12.5% of all trials), post-go speeding seems to reflect an endogenous, moment-to-moment process that governs participants’ readiness to take risk during the SST. These results thus appear to provide a functional dissociation of the 2 aspects of behavioral impulsivity during the SST, with posterror responses mediated by cognitive control and post-go responses mediated by a tendency or the lack thereof to take risk and/or seek sensation. Further studies are warranted to investigate whether a similar risk-taking process would also manifest in other cognitive control tasks, such as the Stroop, Simon, and flankers paradigm, in which a trade-off between speed and accuracy could potentially elicit the same conundrum—to slow down or not to slow down—in the participants.
Ventromedial Prefrontal and Amygdala Activity during Risk-Taking Responses
Studies have implicated the VMPFC in the representation of sympathetic arousal (Damasio 1994). For instance, Nagai et al. (2004) showed that activity in this brain region covaried negatively with basal skin conductance level; higher VMPFC activity was associated with lower physiological arousal. Consistent with its role in emotional and autonomic regulation, medial prefrontal cortex was more activated when subjects suppressed negative affect evoked by arousing and aversive pictures, along with attenuated activity in subcortical limbic regions including the nucleus accumbens and amygdala (Phan et al. 2005).
Other studies have demonstrated a critical role of the VMPFC–amygdala connection in affect regulation (for a review, see Li and Sinha 2008). In particular, patients with anxiety such as posttraumatic stress disorder are known to have decreased VMPFC activity along with increased amygdala reactivity to affect-laden stimuli (Bremner et al. 1999, 2003; Shin et al. 1999; Rauch et al. 2000; Lanius et al. 2001, 2004; Liberzon et al. 2003; Bishop et al. 2004; Lindauer et al. 2004; Shin et al. 2004; Britton et al. 2005; for a review, see also Liberzon and Phan 2003). It has been hypothesized that the failure of prefrontal regulation of amygdala activity underlies the pathogenesis of anxiety states. Furthermore, many imaging studies have documented prefrontal dysregulation of subcortical activity in individuals who are genetically vulnerable to developing anxiety disorders (Hariri et al. 2002, 2003, 2005; Bertolino et al. 2005; Pezawas et al. 2005; Meyer-Lindenberg et al. 2006).
The current result of a positive correlation between VMPFC and amygdala activity during risk taking in the SST thus needs to be reconciled with these previous findings. One explanation is that the VMPFC and amygdala were engaged in the SST as a result of an endogenous process—a decision for speeded response despite the risk of encountering the stop signal, whereas participants responded to exogenous affective stimuli in these earlier studies. These paradigms might differ with respect to top-down versus bottom-up processing. Another possible explanation is that our participants are healthy individuals. Thus, in contrast to the patients examined in the bulk of previous studies, healthy individuals may demonstrate correlated VMPFC response to amygdala activity, reflecting cognitive appraisal of the risk-taking decisions (Ochsner and Gross 2005). Another possibility is that a risk-taking decision as in post-go speeding is not an affectively negative process, as in the studies in which participants experienced traumatic, fear-evoking, or other categorically unpleasant stimuli. In fact, one is tempted to speculate that, for individuals who are prone to sensation seeking, post-go speeding could even be a psychologically positive event. More generally, amygdala activity in the SST may be more related to the saliency rather than to negative affect associated with risk-taking decisions. These clearly are conjectures that need to be tested in the future.
Conclusions
To summarize, we have identified a distinct array of brain regions during speeded as compared with delayed go responses in the SST. These regional activities may represent an indirect neural analog of risk-taking behavior in the SST. Activation in a wide array of cortical and subcortical regions was observed, indicating the psychological complexity of such risk-taking behavior. These results thus could potentially broaden the utility of the SST as a tool to study behavioral impulsivity in healthy individuals as well as in patients with impulse control and substance use disorders.
Funding
Clinician Scientist in Substance Abuse Research K12 (PI: B. Rounsaville) and I/START and R01 grants from National Institute on Drug Abuse; Alcoholic Beverage Medical Research Foundation; Clinical Translational Science Award from the Yale Clinical Center of Investigation (PI: R. Sherwin) to C.-s.Li; State of Connecticut, Department of Mental Health and Addictions Services; a pilot project grant from the Psychotherapy Development Center of Yale University.
Acknowledgments
Conflict of Interest: None declared.
References
- Alderson RM, Rapport MD, Kofler MJ. Attention-deficit/hyperactivity disorder and behavioral inhibition: a meta-analytic review of the stop-signal paradigm. J Abnorm Child Psychol. 2007;35:745–758. doi: 10.1007/s10802-007-9131-6. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry. 2006;67(Suppl 8):7–12. [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Role of the amygdala in decision-making. Ann N Y Acad Sci. 2003;985:356–369. doi: 10.1111/j.1749-6632.2003.tb07094.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berard JD, Nuernberg P, Epplen JT, Schmidtke J. Male rank, reproductive behaviors, and reproductive success in free-ranging rhesus macaques. Primates. 1993;34:481–489. [Google Scholar]
- Berns GS, Chappelow J, Cekic M, Zink CF, Pagnoni G, Martin-Skurski ME. Neurobiological substrates of dread. Science. 2006;312:754–758. doi: 10.1126/science.1123721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, Chappelow J, Zink CF, Pagnoni G, Martin-Skurski ME, Richards J. Neurobiological correlates of social conformity and independence during mental rotation. Biol Psychiatry. 2005;58:245–253. doi: 10.1016/j.biopsych.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Arciero G, Rubino V, Latorre V, De Candia M, Mazzola V, Blasi G, Caforio G, Hariri AR, Kolachana B, et al. Variation of human amygdala response during threatening stimuli as a function of 5′HTTLPR genotype and personality style. Biol Psychiatry. 2005;57:1517–1525. doi: 10.1016/j.biopsych.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan JS, Brett M, Lawrence A. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Lejuez CW, Daughters SB, Zachary Rosenthal M, Lynch TR. Impulsivity as a common process across borderline personality and substance use disorders. Clin Psychol Rev. 2005;25:790–812. doi: 10.1016/j.cpr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Brand M, Grabenhorst F, Starcke K, Vandekerckhove MM, Markowitsch HJ. Role of the amygdala in decisions under ambiguity and decisions under risk: evidence from patients with Urbach-Wiethe disease. Neuropsychologia. 2007;45:1305–1317. doi: 10.1016/j.neuropsychologia.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Brefczynski JA, DeYoe EA. A physiological correlate of the “spotlight” of visual attention. Nat Neurosci. 1999;2:370–374. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- Bremner J, Narayan M, Staib L, Southwick S, McGlashan T, Charney D. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J, Vythilingam M, Vermetten E, Southwick S, McGlashan T, Staib L, Soufer R, Charney D. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biol Psychiatry. 2003;53:879–889. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-P. Region of interest analysis using an SPM toolbox. Abstract presented at the 8th International Conference on Functional Mapping of the Human Brain. 2002 June 2–6; Sendai, Japan. Available le on CD-ROM in NeuroImage, 16(2), abstract 497. [Google Scholar]
- Britton J, Phan K, Taylor S, Fig L, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Blackwell AD, Robbins TW, Sahakian BJ. Motor inhibition and cognitive flexibility in obsessive-compulsive disorder and trichotillomania. Am J Psychiatry. 2006;163:1282–2124. doi: 10.1176/ajp.2006.163.7.1282. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Sahakian BJ. The neuropsychiatry of impulsivity. Curr Opin Psychiatry. 2007;20:255–261. doi: 10.1097/YCO.0b013e3280ba4989. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Richards J, Helmstetter FJ. Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learn Mem. 2007;14:485–490. doi: 10.1101/lm.632007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier AD, Noseworthy MD, Schachar R. Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Hum Brain Mapp. 2007;28:1347–1358. doi: 10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Ranganath C. Behavioral and neural predictors of upcoming decisions. Cogn Affect Behav Neurosci. 2005;5:117–126. doi: 10.3758/cabn.5.2.117. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Young J, Baek JM, Kessler C, Ranganath C. Individual differences in extraversion and dopamine genetics predict neural reward responses. Brain Res Cogn Brain Res. 2005;25:851–861. doi: 10.1016/j.cogbrainres.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Coricelli G, Dolan RJ, Sirigu A. Brain, emotion and decision making: the paradigmatic example of regret. Trends Cogn Sci. 2007;11:258–265. doi: 10.1016/j.tics.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Crosbie J, Schachar R. Deficient inhibition as a marker for familial ADHD. Am J Psychiatry. 2001;158:1884–1890. doi: 10.1176/appi.ajp.158.11.1884. [DOI] [PubMed] [Google Scholar]
- Damasio AR. New York: Crosset/Putnam; 1994. Descartes’ error: emotion, reason and the human brain. [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation–a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Daw ND, O'Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441:876–879. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Maggiore V, Chau W, Peres-Neto PR, McIntosh AR. An empirical comparison of SPM preprocessing parameters to the analysis of fMRI data. Neuroimage. 2002;17:19–28. doi: 10.1006/nimg.2002.1113. [DOI] [PubMed] [Google Scholar]
- Dent HR, Salkovskis PM. Clinical measures of depression, anxiety and obsessionality in non-clinical sample. Behav Res Ther. 1986;24:689–691. doi: 10.1016/0005-7967(86)90066-5. [DOI] [PubMed] [Google Scholar]
- Drake RA. Lateral asymmetry of risky recommendations. Pers Soc Psychol Bull. 1985;11:409–415. [Google Scholar]
- Drake RA, Ulrich G. Line bisecting as a predictor of personal optimism and desirability of risky behaviors. Acta Psychol. 1992;79:219–226. doi: 10.1016/0001-6918(92)90058-l. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The human brain, second edition. Wien (Austria): Springer-Verlag; 2003. [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Hum Brain Mapp. 2007;28:409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, London ED. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacol. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Newman TK, Bailey JN, Jorgensen MJ, Breidenthal SE, Ophoff RA, Comuzzie AG, Martin LJ, Rogers J. Genetic contributions to social impulsivity and aggressiveness in vervet monkeys. Biol Psychiatry. 2004;55:642–647. doi: 10.1016/j.biopsych.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, Pascual-Leone A. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: a direct current stimulation study. J Neurosci. 2007;27:12500–12505. doi: 10.1523/JNEUROSCI.3283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SF. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biol Psychiatry. 2005;57:287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Fredrikson M, Furmark T, Olsson MT, Fischer H, Andersson J, Långström B. Functional neuroanatomical correlates of electrodermal activity: a positron emission tomographic study. Psychophysiology. 1998;35:179–185. [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Polone J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995;2:165–189. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Friston KJ, Josephs O, Zarahn E, Holmes AP, Rouquette S, Poline J. To smooth or not to smooth? Bias and efficiency in fMRI time-series analysis. Neuroimage. 2000;12:196–208. doi: 10.1006/nimg.2000.0609. [DOI] [PubMed] [Google Scholar]
- Fukui H, Murai T, Fukuyama H, Hayashi T, Hanakawa T. Functional activity related to risk anticipation during performance of the Iowa gambling task. Neuroimage. 2005;24:253–259. doi: 10.1016/j.neuroimage.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Gallagher P, Dagenbach D. Manipulating noise frequencies alters hemispheric contributions to decision making. Brain Cogn. 2007;64:42–49. doi: 10.1016/j.bandc.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: who is at risk? Dev Sci. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Franklin ME, Foa EB, Simons RF. Increased error-related brain activity in pediatric obsessive-compulsive disorder before and after treatment. Am J Psychiatry. 2008;165:116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Dimyan MA, Hallett M. Motor planning, imagery, and execution in the distributed motor network: a time-course study with functional MRI. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn036. doi:10.1093/cercor/bhn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Drabant E, Munoz K, Kolachana B, Mattay VS, Egan MF, Weinberger D. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger D. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitoro A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hodgson RJ, Rachman S. Obsessional-compulsive complaints. Behav Res Ther. 1977;15:389–395. doi: 10.1016/0005-7967(77)90042-0. [DOI] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Huettel SA. Behavioral, but not reward, risk modulates activation of prefrontal, parietal, and insular cortices. Cogn Affect Behav Neurosci. 2006;6:141–151. doi: 10.3758/cabn.6.2.141. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006;49:765–775. doi: 10.1016/j.neuron.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Isles AR, Humby T, Walters E, Wilkinson LS. Common genetic effects on variation in impulsivity and activity in mice. J Neurosci. 2004;24:6733–6740. doi: 10.1523/JNEUROSCI.1650-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Mantey M, Nager W, Rada D, Müller-Vahl KR, Emrich HM, Dengler R, Münte TF, Dietrich D. Altered inhibition of motor responses in Tourette syndrome and obsessive-compulsive disorder. Acta Neurol Scand. 2001;104:36–43. doi: 10.1034/j.1600-0404.2001.00308.x. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Oakes TR, Davidson RJ. The voice of emotion: an FMRI study of neural responses to angry and happy vocal expressions. Soc Cogn Affect Neurosci. 2006;1:242–249. doi: 10.1093/scan/nsl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Yeshurun Y, Rotshtein P, Fried I, Ben-Bashat D, Hendler T. The role of the amygdala in signaling prospective outcome of choice. Neuron. 2002;33:983–994. doi: 10.1016/s0896-6273(02)00626-8. [DOI] [PubMed] [Google Scholar]
- Klüver H, Bucy PC. An analysis of certain effects of bilateral temporal lobectomy in the rhesus monkey, with special reference to “psychic blindness”. J Psychol. 1938;5:33–54. [Google Scholar]
- Knoch D, Gianotti LR, Pascual-Leone A, Treyer V, Regard M, Hohmann M, Brugger P. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J Neurosci. 2006;26:6469–6472. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Lanius R, Williamson P, Densmore M, Boksman K, Neufeld RW, Gati J, Menon RS. The nature of traumatic memories: a 4-T fMRI functional connectivity analysis. Am J Psychiatry. 2004;161:36–44. doi: 10.1176/appi.ajp.161.1.36. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Fear and the brain: where have we been, and where are we going? Biol Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- Lee TM, Chan CC, Han SH, Leung AW, Fox PT, Gao JH. An event-related fMRI study on risk taking by healthy individuals of high or low impulsiveness. Neurosci Lett. 2008;438:138–141. doi: 10.1016/j.neulet.2008.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1970;49:467–477. [PubMed] [Google Scholar]
- Li C-SR, Huang C, Constable T, Sinha R. Imaging response inhibition in a stop signal task—neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Huang C, Yan P, Bhagawar Z, Milivojevic V, Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine dependent men. Neuropsychopharmacol. 2008;33:1798–1807. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Huang C, Yan P, Paliwal P, Constable RT, Sinha R. Neural correlates of post-error slowing during a stop signal task—a functional magnetic resonance imaging study. J Cogn Neurosci. 2008;20:1021–1029. doi: 10.1162/jocn.2008.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Milivojevic V, Kemp K, Hong K, Sinha R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend. 2006;85:205–212. doi: 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Li C-SR, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Yan P, Chao HH-A, Sinha R, Paliwal P, Constable RT, Lee TW, Zhang S Forthcoming. Error-specific medial cortical and subcortical activity during the stop signal task—a functional magnetic resonance imaging study. Neuroscience. doi: 10.1016/j.neuroscience.2008.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Britton J, Phan K. Neural correlates of traumatic recall in posttraumatic stress disorder. Stress. 2003;6:151–156. doi: 10.1080/1025389031000136242. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Phan K. Brain-imaging studies of posttraumatic stress disorder. CNS Spectr. 2003;8:641–650. doi: 10.1017/s109285290000883x. [DOI] [PubMed] [Google Scholar]
- Lindauer RJ, Booij J, Habraken JB, Uylings HB, Olff M, Carlier IV, den Heeten GJ, van Eck-Smit BL, Gersons BP. Cerebral blood flow changes during script-driven imagery in police officers with posttraumatic stress disorder. Biol Psychiatry. 2004;56:853–861. doi: 10.1016/j.biopsych.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Liotti M, Pliszka SR, Perez R, Kothmann D, Woldorff MG. Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex. 2005;41:377–388. doi: 10.1016/s0010-9452(08)70274-0. [DOI] [PubMed] [Google Scholar]
- Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci. 2007;27:4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: a user's guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory and language. San Diego (CA): Academic Press; 1994. pp. 189–239. [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: a theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Luks TL, Simpson GV. Preparatory deployment of attention to motion activates higher-order motion-processing brain regions. Neuroimage. 2004;22:1515–1522. doi: 10.1016/j.neuroimage.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Frith CD, Driver J. Delay activity and sensory-motor translation during planned eye or hand movements to visual or tactile targets. J Neurophysiol. 2007;98:3081–3094. doi: 10.1152/jn.00192.2007. [DOI] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T. Atlas of the human brain, third edition. New York: Academic Press; 2008. [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, O'Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage. 2005;24:495–503. doi: 10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Mangina CA, Beuzeron-Mangina JH. Direct electrical stimulation of specific human brain structures and bilateral electrodermal activity. Int J Psychophysiol. 1996;22:1–8. doi: 10.1016/0167-8760(96)00022-0. [DOI] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Badger GJ, Quesnel KJ. The anatomy of risk: a quantitative investigation into injection drug users’ taxonomy of risk attitudes and perceptions. Exp Clin Psychopharmacol. 2007;15(2):195–203. doi: 10.1037/1064-1297.15.2.195. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Simmons AN, Lane SD, Paulus MP. Selective activation of the nucleus accumbens during risk-taking decision making. Neuroreport. 2004;15:2123–2127. doi: 10.1097/00001756-200409150-00025. [DOI] [PubMed] [Google Scholar]
- McElroy T, Seta J. On the other hand am I rational? Hemispheric activation and the framing effect. Brain Cogn. 2004;55:572–580. doi: 10.1016/j.bandc.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, Suomi SJ, Linnoila M. Correlation of CSF 5-HIAA concentration with sociality and the timing of emigration in free-ranging primates. Am J Psychiatry. 1995;152:907–913. doi: 10.1176/ajp.152.6.907. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci USA. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojzisch A, Schulz-Hardt S. Being fed up: a social cognitive neuroscience approach to mental satiation.ch A, Schulz-Hardt S. Ann N Y Acad Sci. 2007;1118:186–205. doi: 10.1196/annals.1412.006. [DOI] [PubMed] [Google Scholar]
- Mort DJ, Perry RJ, Mannan SK, Hodgson TL, Anderson E, Quest R, McRobbie D, McBride A, Husain M, Kennard C. Differential cortical activation during voluntary and reflexive saccades in man. Neuroimage. 2003;18:231–246. doi: 10.1016/s1053-8119(02)00028-9. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Critchley H, Featherstone E, Trimble M, Dolan R. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a “default mode” of brain function. Neuroimage. 2004;22:243–251. doi: 10.1016/j.neuroimage.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Nielen MM, Mol N, Hajcak G, Veltman DJ. Performance monitoring in obsessive-compulsive disorder. Psychiatry Res. 2005;134:111–122. doi: 10.1016/j.psychres.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ohira H, Nomura M, Ichikawa N, Isowa T, Iidaka T, Sato A, Fukuyama S, Nakajima T, Yamada J. Association of neural and physiological responses during voluntary emotion suppression. Neuroimage. 2006;29:721–733. doi: 10.1016/j.neuroimage.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Penny W, Holmes AP. Random-effects analysis. In: Frackowiak RSJ, Ashburner JT, Penny WD, Zeki S, editors. Human brain function. San Diego (CA): Elsevier; 2004. pp. 843–850. [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant E, Verchinski B, Munoz K, Kolachana B, Egan M, Mataay V, Hariri A, Weinberger D. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan P, Moore G, Uhde T, Tancer M. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbit PMA. Errors and error correction in choice-response tasks. J Exp Psychol. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin M, Lasko N, Orr S, Pitman R. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Green L, Myerson J, Parker J, Ramaratnam M, Buckner RL. Dissociable but inter-related systems of cognitive control and reward during decision making: evidence from pupillometry and event-related fMRI. Neuroimage. 2007;37:1017–1031. doi: 10.1016/j.neuroimage.2007.04.066. [DOI] [PubMed] [Google Scholar]
- Schachar RJ, Chen S, Logan GD, Ornstein TJ, Crosbie J, Ickowicz A, Pakulak A. Evidence for an error monitoring deficit in attention deficit hyperactivity disorder. J Abnorm Child Psychol. 2004;32:285–293. doi: 10.1023/b:jacp.0000026142.11217.f2. [DOI] [PubMed] [Google Scholar]
- Schmajuk M, Liotti M, Busse L, Woldorff MG. Electrophysiological activity underlying inhibitory control processes in normal adults. Neuropsychologia. 2006;44:384–395. doi: 10.1016/j.neuropsychologia.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Shin L, Porr S, Carson M, Rauch S, Macklin M, Lasko N, Peters P, Metzger L, Dougherty D, Cannisatraro P, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lsko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am J Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Singer T. The neuronal basis of empathy and fairness. Novartis Found Symp. 2007;278:20–30. [PubMed] [Google Scholar]
- Slotnick SD, Schwarzbach J, Yantis S. Attentional inhibition of visual processing in human striate and extrastriate cortex. Neuroimage. 2003;19:1602–1611. doi: 10.1016/s1053-8119(03)00187-3. [DOI] [PubMed] [Google Scholar]
- Smith AT, Singh KD, Greenlee MW. Attentional suppression of activity in the human visual cortex. Neuroreport. 2000;11:271–277. doi: 10.1097/00001756-200002070-00010. [DOI] [PubMed] [Google Scholar]
- Stark R, Wolf OT, Tabbert K, Kagerer S, Zimmermann M, Kirsch P, Schienle A, Vaitl D. Influence of the stress hormone cortisol on fear conditioning in humans: evidence for sex differences in the response of the prefrontal cortex. Neuroimage. 2006;32:1290–1298. doi: 10.1016/j.neuroimage.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Sternberger LG, Burns GL. Maudsley obsessional-compulsive inventory: obsessions and compulsions in a nonclinical sample. Behav Res Therapy. 1990;28:337–340. doi: 10.1016/0005-7967(90)90086-x. [DOI] [PubMed] [Google Scholar]
- Swanson J, Posner M, Fusella J, Wasdell M, Sommer T, Fan J. Genes and attention deficit hyperactivity disorder. Curr Psychiatry Rep. 2001;3:92–100. doi: 10.1007/s11920-001-0005-2. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Parietal attentional system aberrations during target detection in adolescents with attention deficit hyperactivity disorder: event-related fMRI evidence. Am J Psychiatry. 2006;163:1033–1043. doi: 10.1176/ajp.2006.163.6.1033. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13:160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- Teitti S, Määttä S, Säisänen L, Könönen M, Vanninen R, Hannula H, Mervaala E, Karhu J. Non-primary motor areas in the human frontal lobe are connected directly to hand muscles. Neuroimage. 2008;40:1243–1250. doi: 10.1016/j.neuroimage.2007.12.065. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Nicotine modulates reorienting of visuospatial attention and neural activity in human parietal cortex. Neuropsychopharmacol. 2005;30:810–820. doi: 10.1038/sj.npp.1300633. [DOI] [PubMed] [Google Scholar]
- Tobler PN, O'Doherty JP, Dolan RJ, Schultz W. Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. J Neurophysiol. 2007;97:1621–1632. doi: 10.1152/jn.00745.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychol Sci. 2003;14:347–353. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- van Reekum CM, Urry HL, Johnstone T, Thurow ME, Frye CJ, Jackson CA, Schaefer HS, Alexander AL, Davidson RJ. Individual differences in amygdala and ventromedial prefrontal cortex activity are associated with evaluation speed and psychological well-being. J Cogn Neurosci. 2007;19:237–248. doi: 10.1162/jocn.2007.19.2.237. [DOI] [PubMed] [Google Scholar]
- Vorhold V, Giessing C, Wiedemann PM, Schütz H, Gauggel S, Fink GR. The neural basis of risk ratings: evidence from a functional magnetic resonance imaging (fMRI) study. Neuropsychologia. 2007;45:3242–3250. doi: 10.1016/j.neuropsychologia.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Williams LM, Felmingham K, Kemp AH, Rennie C, Brown KJ, Bryant RA, Gordon E. Mapping frontal-limbic correlates of orienting to change detection. Neuroreport. 2007;18:197–202. doi: 10.1097/WNR.0b013e328010ff80. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacubian J, Gläscher J, Schroeder K, Sommer T, Braus DF, Büchel C. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J Neurosci. 2006;26:9530–9537. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Chappelow J, Martin-Skurski M, Berns GS. Human striatal activation reflects degree of stimulus saliency. Neuroimage. 2006;29:977–983. doi: 10.1016/j.neuroimage.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Alvarez-Royo P, Clower RP. Independence of memory functions and emotional behavior: separate contributions of the hippocampal formation and the amygdala. Hippocampus. 1991;1:207–220. doi: 10.1002/hipo.450010208. [DOI] [PubMed] [Google Scholar]