Abstract
There is inconsistent evidence about the effect of reproductive history on women’s risk of pancreatic cancer. In the Million Women Study, a prospective cohort of middle-aged women in the UK, we examined associations between reproductive history and pancreatic cancer incidence and mortality, controlling for age, socioeconomic status, geographic region, body mass index, smoking and history of diabetes. During 7.1 million person-years of follow-up in 995,192 post-menopausal women there were 1,182 incident pancreatic cancers. Pancreatic cancer incidence and mortality did not vary significantly with age at menarche, number of children, age at first birth, breastfeeding, type of menopause, age at menopause or time since menopause. Any effect of reproductive history and pancreatic cancer risk in women is likely to be weak, if it exists at all.
Keywords: pancreatic cancer, reproductive history, parity, menopause, menarche
Introduction
Results from some studies (1-6), but not all (7-12), have suggested that the risk of pancreatic cancer in women may vary with reproductive factors such as age at menarche, parity, age at first birth, history of breastfeeding and age at menopause. Whether there is an association of pancreatic cancer risk with some reproductive factors remains an open question, in particular, parity, later age at menopause and surgical menopause (7, 11, 12).
The Million Women Study is a prospective cohort study of middle-aged women in the UK, recruited between 1996 and 2001. We have reported elsewhere on the increased risk of pancreatic cancer in women who have diabetes, who smoke, or have greater body mass index (13). In this report we present results on the relationship between female reproductive factors and pancreatic cancer incidence and mortality.
Materials and methods
The Million Women Study has been described in detail previously (14, 15). Between 1996 and 2001, 1.3 million middle-aged women invited for routine breast screening in the UK National Health Service completed a study questionnaire and gave informed consent for inclusion in the study, including follow-up for cancer incidence and mortality from any cause through the National Health Service Central Registers. The Oxford and Anglia Multi-Centre Research Ethics Committee approved the study.
The recruitment questionnaire (available at www.millionwomenstudy.org) included questions on age at menarche, the number of children, age at first birth (if any), current menopausal status, age at menopause, and medical history including history of bilateral oophorectomy. A question on length of breastfeeding in months for each birth was added to the baseline questionnaire after the first 9% were recruited.
We restricted this analysis to 995,192 post-menopausal women with no previous cancer (except non-melanoma skin cancer) aged between 50 and 64 at the time of recruitment. Women whose menopausal status at recruitment was masked by hysterectomy or by use of HRT before the natural menopause were considered post-menopausal only if they were aged 53 or greater at recruitment, as described previously (15). For analyses of type of menopause, age at menopause and time since menopause, we included only women whose menopausal type could clearly be defined as either natural menopause (592,306 women) or menopause attributed to bilateral oophorectomy (77,696 women), and with a known age at menopause or bilateral oophorectomy. Eligible women contributed person-years to analyses of incident cancer from the date of recruitment to the study until the date of diagnosis with pancreatic cancer, date of death or last date of follow-up, or date of loss to follow-up (e.g. through emigration), whichever was the earliest. Women registered with a cancer other than pancreatic cancer were censored at the date of diagnosis of that cancer. Cox proportional hazards models were applied using the Stata computing package, taking attained age as the underlying time variable, stratifying by geographical region (ten regions) and socioeconomic status (quintiles of deprivation index (16)) and adjusting for smoking (never, past, current), body mass index (in six categories with cut-points at 22.5, 25.0, 27.5, 30.0 and 32.5 kg/m2) and history of diabetes (yes or no), assigning missing values to a separate category where necessary. Time since menopause (in four categories with cut-points at 10, 15 and 20 years) was analysed as a time-dependent variable (17). Since for many variables (e.g. age at menopause) there is no natural reference category, variances are, where appropriate, estimated by treating the relative risks as floating absolute risks. Results for categorical variables are therefore presented in a plot of relative risks (RRs) and their corresponding floated standard errors (FSEs) and 95% floated confidence intervals (FCIs) (18). Results for dichotomous comparisons (all parous vs. nulliparous women, and bilateral oophorectomy vs. natural menopause) are presented in the text as RRs with conventional 95% confidence intervals. Proportional hazards assumptions were checked graphically.
Results
Table 1 shows the self-reported characteristics, at entry to the study, of the 995,192 post-menopausal women in this analysis. The cohort was followed for a mean 7.1 years for incident cancer (total 7.1 million person-years), during which time there were 1,182 incident cases of pancreatic cancer. The cohort was followed for a mean 8.9 years for mortality (total 8.8 million person-years), during which time there were 1,516 deaths from pancreatic cancer.
Table 1. Characteristics at recruitment of post-menopausal women included in this analysis.
| Population at risk | Incident cases of pancreatic cancer | Deaths from pancreatic cancer | |
|---|---|---|---|
| n | 995,192 | 1,182 | 1,516 |
| Mean years of follow-up | 7.1 for incidence; 8.9 for mortality. | ||
| Age in years (mean [SD]) | 57.3 (4.1) | 58.7 (3.9) | 58.7 (3.9) |
| Current smoker | 21% (194,617) | 33% (363) | 34% (482) |
| Body mass index in kg/m^2 (mean [SD]) | 26.3 (4.7) | 26.6 (4.9) | 26.4 (4.8) |
| History of diabetes | 3.0% (29,387) | 5.2% (61) | 4.6% (70) |
| Socioeconomic status: upper third | 33% (328,516) | 32% (379) | 31% (470) |
| Age at menarche (mean [SD]) | 13.0 (1.6) | 13.0 (1.6) | 13.1 (1.7) |
| Parous | 89% (885,015) | 87% (1,029) | 88% (1,339) |
| Number of children, among parous women (mean [SD]) | 2.4 (1.1) | 2.5 (1.2) | 2.5 (1.2) |
| Ever breast fed, among parous women | 69% (477,477) | 71% (533) | 70% (685) |
| Age at menopause (mean [SD]) * | 49.2 (3.7) | 49.3 (3.8) | 49.4 (3.8) |
In 670,002 women with natural menopause or bilateral oophorectomy.
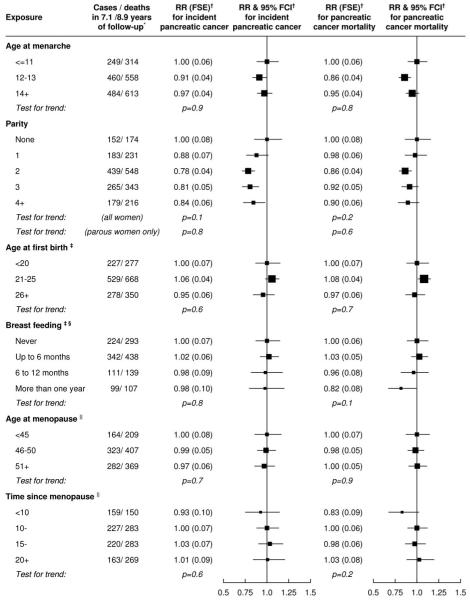
Figure 1 shows the incidence of pancreatic cancer by reproductive factors. Pancreatic cancer incidence did not vary significantly between categories of age at menarche, age at first birth, history of breastfeeding, age at menopause, or time since menopause. Compared to nulliparous women, all parous women combined were at lower risk of incident pancreatic cancer (p=0.01), but within parous women there was no trend with increasing number of children (p=0.8). Women with bilateral oophorectomy were at similar risk of pancreatic cancer to women with natural menopause (relative risk 0.96, 95% confidence interval 0.75-1.23, p=0.8). When we repeated analyses testing for associations with pancreatic cancer mortality instead of incident pancreatic cancer, results were similar, except that there was no significant difference in mortality between nulliparous women and all parous women combined (p=0.2)>.
Figure.
Relative risks of pancreatic cancer incidence by categories of age at menarche, parity, age at first birth, breastfeeding, type of menopause, age at menopause and time since menopause.
Footnotes to the figure:
* Totals may not always agree due to small numbers of missing values.
† RR, FSE and FCI denote relative risk, floating standard error and floating confidence interval controlling for age, region, socioeconomic status, smoking, body mass index and diabetes as described in the text.
‡ In parous women only, adjusting additionally for level of parity.
§ The question about breastfeeding was added to the questionnaire after the first 9% of women were recruited.
|| In women with natural menopause or bilateral oophorectomy
Discussion
Reproductive factors including age at menarche, parity, age at first birth, breastfeeding, type of menopause, age at menopause and time since menopause were not associated with risk of pancreatic cancer in this cohort.
The relationship between parity and pancreatic cancer risk has been inconsistent in previous studies (7-12). When all parous women were grouped together, there was a slightly lower risk than in nulliparous women, for pancreatic cancer incidence and not mortality, and the finding for disease incidence may be due to chance. There was no trend in risk with increasing number of children in all women or in parous women, either for incidence or mortality.
In this study we did not see any difference in risk between women with natural menopause and women whose menopause was attributed to bilateral oophorectomy. Of two previous prospective studies that have examined pancreatic cancer risk in relation to type of menopause, one found an elevated risk of incident pancreatic cancer in women with hysterectomy or bilateral oophorectomy compared to women with natural menopause (11), but another found no such association with pancreatic cancer mortality (9).
Most previous studies have found no association between pancreatic cancer risk and age at menopause (7, 9, 10). One recent study reported that women with a later menopause were at lower risk of incident pancreatic cancer, especially in women with a surgical menopause (11). By contrast, another study reported that women with a later menopause were at higher risk (12). Our study adds to the evidence that there is little or no association between age at menopause and risk of pancreatic cancer. Breastfeeding history has been considered by just two previous prospective studies of pancreatic cancer (7, 12). Neither found significant results for breastfeeding, although one found a possible interaction between parity and breastfeeding (12). With more cases of pancreatic cancer than both these studies combined, our results provide further evidence against an association between breastfeeding history and pancreatic cancer risk.
Early studies reported increased risk of pancreatic cancer in women with early menarche (1, 2). Our results are consistent with those from more recent studies in finding no association between pancreatic cancer risk and age at menarche (7-12). Previous studies have reported both positive (2) and inverse (19) associations between risk of pancreatic cancer and earlier age at first birth, but most studies find no such association, as do we (7-12).
To our knowledge, the results presented here include more women with incident pancreatic cancer (as opposed to pancreatic cancer mortality) than any previous prospective study. As in most large epidemiological studies, reproductive history was self-reported. However, the prospective design of the study means that information on reproductive factors was recorded before follow-up began and should not be influenced by a subsequent diagnosis of cancer.
In conclusion, in this large prospective study we found no strong evidence of any associations between reproductive history and pancreatic cancer incidence or mortality.
Acknowledgments
We are grateful to the women participating in the study and to the staff of the participating breast screening centres and co-ordinating centre. The Million Women Study is supported by Cancer Research UK, the Medical Research Council, and the UK National Health Service breast screening programme
References
- 1.Bueno De Mesquita HB, Maisonneuve P, Moerman CJ, Walker AM. Anthropometric and reproductive variables and exocrine carcinoma of the pancreas: A population-based case-control study in The Netherlands. International Journal of Cancer. 1992;52:24–29. doi: 10.1002/ijc.2910520106. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez E, La Vecchia C, D’Avanzo B, Negri E. Menstrual and reproductive factors and pancreatic cancer risk in women. International Journal of Cancer. 1995;62:11–14. doi: 10.1002/ijc.2910620104. [DOI] [PubMed] [Google Scholar]
- 3.Ji BT, Hatch MC, Chow W-H, et al. Anthropometric and reproductive factors and the risk of pancreatic cancer: A case-control study in Shanghai, China. International Journal of Cancer. 1996;66:432–437. doi: 10.1002/(SICI)1097-0215(19960516)66:4<432::AID-IJC4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 4.Karlson BM, Wuu J, Hsieh CC, Lambe M, Ekbom A. Parity and the risk of pancreatic cancer: A nested case-control study. International Journal of Cancer. 1998;77:224–227. doi: 10.1002/(sici)1097-0215(19980717)77:2<224::aid-ijc10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 5.Duell EJ, Holly EA. Reproductive and menstrual risk factors for pancreatic cancer: A population-based study of San Francisco Bay Area women. American Journal of Epidemiology. 2005;161:741–747. doi: 10.1093/aje/kwi104. [DOI] [PubMed] [Google Scholar]
- 6.Lo AC, Soliman AS, El-Ghawalby N, et al. Lifestyle, occupational, and reproductive factors in relation to pancreatic cancer risk. Pancreas. 2007;35:120–129. doi: 10.1097/mpa.0b013e318053e7d3. [DOI] [PubMed] [Google Scholar]
- 7.Skinner HG, Michaud DS, Colditz GA, et al. Parity, reproductive factors, and the risk of pancreatic cancer in women. Cancer Epidemiology Biomarkers and Prevention. 2003;12:433–438. [PubMed] [Google Scholar]
- 8.Navarro Silvera SA, Miller AB, Rohan TE. Hormonal and reproductive factors and pancreatic cancer risk: A prospective cohort study. Pancreas. 2005;30:369–374. doi: 10.1097/01.mpa.0000160301.59319.ba. [DOI] [PubMed] [Google Scholar]
- 9.Teras LR, Patel AV, Rodriguez C, Thun MJ, Calle EE. Parity, other reproductive factors, and risk of pancreatic cancer mortality in a large cohort of U.S. Women (United States) Cancer Causes and Control. 2005;16:1035–1040. doi: 10.1007/s10552-005-0332-4. [DOI] [PubMed] [Google Scholar]
- 10.Lin Y, Kikuchi S, Tamakoshi A, et al. Association of menstrual and reproductive factors with pancreatic cancer risk in women: Findings of the Japan collaborative cohort study for evaluation of cancer risk. Journal of Gastroenterology. 2006;41:878–883. doi: 10.1007/s00535-006-1869-z. [DOI] [PubMed] [Google Scholar]
- 11.Prizment AE, Anderson KE, Hong CP, Folsom AR. Pancreatic cancer incidence in relation to female reproductive factors: Iowa women’s health study. Journal of the Pancreas. 2007;8:16–27. [PubMed] [Google Scholar]
- 12.Heuch I, Jacobsen BK, Albrektsen G, Kvaele G. Reproductive factors and pancreatic cancer risk: A Norwegian cohort study. British Journal of Cancer. 2008;98:189–193. doi: 10.1038/sj.bjc.6604095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens RJ, Roddam AW, Spencer EA, et al. Factors associated with incident and fatal pancreatic cancer in a cohort of middle-aged women. International Journal of Cancer. 2009 doi: 10.1002/ijc.24196. (in press). DOI 10.1002/ijc.24196. [DOI] [PubMed] [Google Scholar]
- 14.Million Women Study Collaborative Group The Million Women Study: Design and characteristics of the study population. Breast Cancer Research. 1999;1:73–80. doi: 10.1186/bcr16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Million Women Study Collaborative Group Breast cancer and hormone-replacement therapy in the Million Women Study. The Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 16.Townsend P, Phillimore P, Beattie A. Health and deprivation: inequality and the North. Croom Helm; London: 1988. [Google Scholar]
- 17.Collett D. Modelling survival data in medical research. Chapman & Hall / CRC Press; USA: 2003. (Texts in Statistical Science). [Google Scholar]
- 18.Easton DF, Peto J, Babiker AGAG. Floating absolute risk: An alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Statistics in Medicine. 1991;10:1025–1035. doi: 10.1002/sim.4780100703. [DOI] [PubMed] [Google Scholar]
- 19.Kreiger N, Lacroix J, Sloan M. Hormonal factors and pancreatic cancer in women. Annals of Epidemiology. 2001;11:563–567. doi: 10.1016/s1047-2797(01)00219-8. [DOI] [PubMed] [Google Scholar]