Abstract
Objective
To evaluate the effectiveness of a xylitol pediatric topical oral syrup to reduce the incidence of dental caries of very young children.
Design
Randomized, double-blinded, controlled trial.
Setting
Communities in the Republic of the Marshall Islands.
Participants
108 children aged 9 to 15 months were screened and 100 were enrolled.
Intervention
Children were randomized and parents administered topical oral xylitol syrup two times (Xyl-2X, two xylitol 4.00 g/dose + one sorbitol dose) or three times (Xyl-3X, three xylitol 2.67 g/dose) per day (total 8 g) or control (one xylitol 2.67 g/dose + two sorbitol dose).
Outcome Measures
The outcome end-point of the study was the number of decayed primary teeth.
Results
Ninety-four of 100 children (mean±SD age, 15.0±2.7 months at randomization) with at least one follow-up exam were included in the intent-to-treat analysis. The mean±SD follow-up period was 10.5±2.2 months. Nearly 52% of children in the control condition had tooth decay compared to 40.6% among Xyl-3X and 24.2% among Xyl-2X conditions. The mean±SD number of decayed teeth was 1.9±2.4 for control, 1.0±1.4 for Xyl-3X, and 0.6±1.1 for Xyl-2X condition. Compared to controls, there was significantly fewer decayed teeth in the Xyl-2X (relative risk [RR], 0.30; 95% confidence interval [CI] 0.13, 0.66; P=.003) and Xyl-3X (RR, 0.50; 95% CI 0.26, 0.96; P=0.037) conditions. There was no statistical difference between the two xylitol treatment conditions (P=0.22).
Conclusion
Oral xylitol syrup administered topically two or three times each day at a total dose of 8 g was effective in preventing Early Childhood Caries.
Keywords: Xylitol, Early childhood caries, Tooth decay, Children, Infants, Preschool, Prevention, Randomized clinical trial
Introduction
Early Childhood Caries (ECC)—baby bottle tooth decay or nursing caries—describes severe tooth decay early in life. The Surgeon General’s Oral Health in America sounded the alarm that tooth decay is the most prevalent childhood chronic disease—five times more common than asthma.1 The Healthy People 2010 mid-term report noted the prevalence of ECC is rising.2 Poor children suffer rates twice that of their more affluent peers, and their disease is more likely to be untreated.1 Native American,3 Alaska,4 Hawaii Native and Pacific Island children5 suffer the highest rates of ECC and few have adequate access to dental care. Poor oral health impacts diet and nutrition and significantly diminishes quality of life.1 Yet, tooth decay is a disease that is, by and large, preventable.6
Addressing childhood oral health has become a primary initiative for the AAP and American Academy of Pediatric Dentistry (AAPD). Similarly, the U.S. D.H.H.S. National Call to Action to Promote Oral Health urges interdisciplinary training of health professional personnel in providing oral health education and preventive services to patients.7 Multi-modality and inter-disciplinary, e.g. medical, dental, and behavioral approaches to caries prevention in children are being aggressively sought. Xylitol, a naturally occurring polyol sweetener, is among these modalities that also include oral health education, dental risk assessment, topical fluorides and dental sealants.
Several bacterial species have been implicated in the cause of tooth decay. Foremost is mutans streptococci, a group of gram-positive microorganisms that represent a small proportion of the oral flora yet are highly damaging through their ability to colonize tooth surface and produce lactic acid demineralization leading to cavitation.8 Mutans streptococci implicated in human tooth decay include S. mutans and S. sobrinus.
Xylitol—approved by the U.S. Food and Drug Administration for use in food since 1963—has been shown to be an effective tooth decay preventive agent.9 Xylitol exerts selective antibacterial-like actions against mutans streptococci by disrupting glucose cell-wall transport and intracellular glycolysis thus inhibiting pathogen growth.10 It also reduces the adhesiveness of mutans streptococci to tooth biofilms.11 However, xylitol’s effectiveness is dependent on consumption of a minimum daily frequency and amount that is not found in foods or most xylitol containing products.12
Clinical studies of xylitol have almost exclusively involved chewing gum or lozenges and evaluated school age children and tooth decay in permanent teeth.12 An effective xylitol 2vehicle acceptable and safe for toddlers has been elusive. To address this gap, we conducted a randomized, double-blind, controlled trial using a xylitol topical syrup during primary tooth eruption in toddlers.
Methods
Enrollment Criteria
Children nine to 15 months old were eligible. Children were excluded if they: (1) were in the lower 10 percentile of U.S. standard weight and height, (2) had a history of esophageal or digestive disease, (3) had congenital craniofacial malformation, or (4) had a history of adenoidectomy, tympanostomy tubes, or tympanic membrane perforations. Exclusion criterion (4) was included because a second aim of the study was to evaluate the effect of xylitol in reducing acute otitis media, data, which is currently incompletely entered.
Setting
The study was conducted in the Delap and Laura districts on Majuro atoll, Republic of the Marshall Islands (RMI). ECC is a serious health care problem in the Marshall Islands. The caries rate is two to three times that of the typical U.S. mainland community. The average child entering Head Start at age five has 6.8 untreated cavities and over 90% of children have at least one decayed tooth.13 Fifty-one percent of two year olds have at least one decayed tooth.14 Children drink water from rain catchments that do not contain appreciable fluoride and supplemental fluoride or topical fluoride treatments have not been available for toddlers. Diets are full of cheap high-energy foods. Breastfeeding is encouraged to age one but it is common practice to give infants and toddlers bottles containing sugar water or can fruit drinks.
Experimental Design
We compared two active treatment conditions each receiving 8 g of xylitol syrup orally per day divided into two (Xyl-2X, 4 g xylitol per dose) or three doses (Xyl-3X, 2.67 g xylitol per dose) to a control condition (a single 2.67 g xylitol dose). The Internal Review Committee (IRC) appointed by the Secretary of Health, RMI required that the control group receive a small amount of xylitol even though it was understood a placebo control would be more ideal and that evidence does not suggest a single dose of 2.67 g xylitol per day would have an effect.15,16
The study was controlled for frequency of daily syrup use where all groups received three syrup doses per day for 12 months. Groups taking xylitol syrup less than three times per day were given sorbitol syrup doses such that: Controls = one xylitol 2.67 g/dose + two sorbitol 2 g/dose; Xyl-2X = two xylitol 4 g/dose + one sorbitol 2 g/dose; and Xyl-3X = three xylitol 2.67 g/dose (Table 1). Sorbitol is another polyol sweetener that is noncariogenic but has not been demonstrated to have active protective effects against caries.17 All families received oral health education and free health care during the study period.
Table 1.
Topical oral syrups received by conditions
| Xyl-3X | Xyl-2X | Control | |
|---|---|---|---|
| Xylitol 4 g/dose | ---- | 2 doses | ---- |
| Xylitol 2.67 g/dose | 3 doses | ---- | 1 dose |
| Sorbitol 2 g/dose | ---- | 1 dose | 2 doses |
| Total xylitol dose/day | 8 g | 8 g | 2.67 g |
| Total polyol dose/day | 8 g | 10 g | 6.67 g |
We tested the hypothesis that xylitol syrup at 8 g per day given in two or three divided doses would reduce the incidence of cavitated carious lesions during primary tooth eruption compared to a control.
Intervention
Each syrup dose contained 8 mL of syrup. Table 2 shows the ingredients for each formulation. The syrups were matched for color, taste, and viscosity. Unicep (Sandpoint, ID) produced and packaged the syrups in a FDA compliant facility, and Sterigenics (Hayward, CA) gamma sterilized the syrups at a dose of 30 to 50 kGy. The syrups were produced in three batches during the study period to minimize storage. Each batch was periodically tested to monitor for common food product microbial contamination.
Table 2.
Ingredients of xylitol and sorbitol pediatric topical oral syrups.
| Syrup formulation in percent by weight (total volume = 8 mL/dose) |
||||
|---|---|---|---|---|
| Ingredient | Function | Xylitol 4.0 g/dose |
Xylitol 2.67 g/dose |
Sorbitol 2.0 g/dose |
| Water, USP Purified | 46.90 | 62.37 | 69.84 | |
| Xylitol | Active sweetener | 50.00 | 33.00 | 0.0 |
| Sorbitol | Non-active sweetener |
0.0 | 0.0 | 25.0 |
| Sodium Carboxymethylcellulose |
Thickening agent | 2.50 | 4.00 | 4.50 |
| Sucralose | Intense sweetener | 0.0 | 0.28 | 0.06 |
| Flavor, Strawberry | Flavor | 0.40 | 0.40 | 0.40 |
| Color, Carmine 50% | Color | 0.10 | 0.10 | 0.10 |
| Methyl Paraben | Preservative | 0.10 | 0.10 | 0.10 |
Human Subjects
The IRC of the Ministry of Health and the Secretary of Health approved the study. Approval was also obtained from the University of Washington Institutional Review Board. Informed consent of the parent was obtained.
Measures
The primary outcome end-point of the study being reported here was the number of decayed primary teeth, defined as cavitated carious lesions. A second outcome end-point was incidence of acute otitis media which will be reported in the near future. A single dental examiner was trained to the World Health Organization (1997) diagnostic protocol, and examined the teeth visually using a disposable dental mirror and artificial light. Compared to a gold standard examiner, the study examiner demonstrated excellent reliability for caries diagnosis (inter-rater correlation coefficient of 1.00 at the pre-syrup randomization dental exam and 0.96 at mid-study exam). The examiner was always blinded to group assignment.
Procedures
The enrollment period extended from April and August 2006. After enrollment, children began a five-week non-treatment observation period during which time no syrup was given and children were visited by outreach workers at least twice per week to record loose stools or diarrhea symptoms (which are the most common side effects of consuming polyol sweeteners such as xylitol and sorbitol). This was followed by a three-week run-in period where the children were given one dose of xylitol (2.67 g) per day for the first week. The dose was increased by one dose each week to a maximum of three doses per day. This period was to allow the children’s digestive systems to adjust to the polyol and minimize unwanted side effects. A four-week washout period followed to ensure that the xylitol was cleared from the body before initiating the syrup randomization. The 12 months syrup randomization period began in August 2006 and the last participant completed follow-up in January 2008. Parents and outreach workers monitored children for side effects during all study periods. Loose stools and diarrhea episodes were recorded by the outreach workers during home visits.
Syrup doses were prepackaged for distribution in daily bags (three doses per bag) labeled with ID numbers and the day of the week, and placed in larger bags labeled with the week number (one to 52). Workers, who were not part of the study staff, packaged the syrups according to a schematic.
Locally hired outreach workers completed training and certification to conduct study protocols. Outreach workers provided oral health and polyol side effects education as well as trained and coached parents/caretakers in administering syrup topically to the teeth of children. A ratio of one outreach worker to 10 to 15 families was maintained throughout the study. The workers visited participating families at least twice per week from enrollment through the early part of the syrup randomization period then at least once per week there after to encourage adherence and develop strategies with families to resolve challenges. Parents kept calendar style logs to record administration of the syrup. Parents were also instructed to keep all used and un-used syrup doses in their pre-packed packages for collection, recording, and proper disposal by the workers.
Families received toys for their children and gift certificates good at local grocery stores as incentives throughout the study. The incentives were chosen with advice from a local advisory committee. Mothers and children were also given tee-shirts with the study logo and families periodically received toothpaste and toothbrushes for other members of the family. Twice during the study, parents and children were invited to community parties, first to receive a progress report on the study and then to hear the results.
Randomization
Subjects were assigned to ID numbers serially at enrollment where ID numbers had been randomly assigned to conditions by a statistician generated by a using block randomization and the sample function of the S-PLUS® statistical software (Insightful Corporation, Seattle, WA). Block sizes of 30 and 15 were used for the Laura district and 36 and 18 were used for the Delap district. All study team members were blinded until study completion except for the statistician.
Statistical Analysis
We estimated that the rate of decay, cavitated lesions for children at 24 months of age, to be 60% in the control group and 30% in the xylitol groups. Based on 0.8 power to detect a significant difference (P=0.05, two-sided) between xylitol and control groups, 32 children were required for each study group. To account for an expected 10% attrition, we planned to enroll 36 children per group.
Poisson regression analysis was used to compare the number of decayed teeth between the three study conditions, which included the natural logarithm of the follow-up time from the baseline dental exam following the syrup randomization protocol until the last follow-up (recorded) exam as an offset term. Generalized estimating equations with a robust variance estimator were used to fit the Poisson regression model in order to account for overdispersion due to multiple teeth per subject.18,19 Additional Poisson regression analyses adjusted for age at randomization, number of teeth at last follow-up, and study district were done. The prevented fraction and number needed to treat (NNT) were also calculated. NNT = 1/ARR (absolute risk reduction) and ARR = Control Event Rate – Experimental Event Rate. The data were analyzed using SAS (version 9.1.3) software package (SAS Institute, Inc., Carey, NC, USA, 2003).
Results
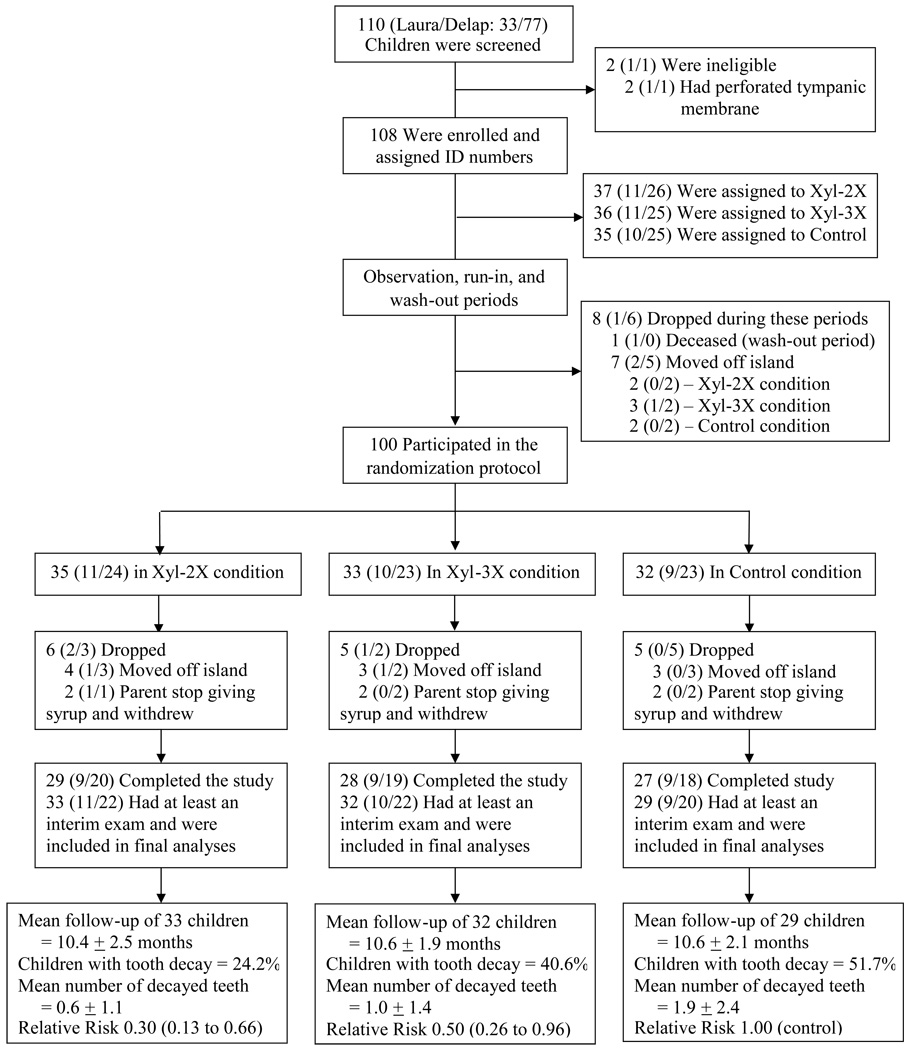
Of the 108 children enrolled (Laura, N=32 and Delap, N=76), eight children dropped out during the observation, run-in, or wash-out period leaving 100 children who participated in the syrup randomization protocol. Of these, 94 had at least one follow-up caries assessment (interim or final exam) and were included in the final analysis; 84 completed the study (Figure 1). Table 3 shows the characteristics of the 94 children included in the final analysis by study conditions. Subjects in the Xyl-3X condition were about two months younger than subjects in the Xyl-2X and the Control conditions at initiation of the randomization protocol (ANOVA, P=0.002). Mean syrup exposure time was similar between the conditions (ANOVA, p=0.94). The syrup compliance rate during the study was high, over 90% for each condition, based on actual counts of daily consumed and un-consumed syrup doses. Results of periodic syrup microbial testing showed no changes compared to immediately after production testing.
Figure 1.
Enrollment, Pre- and Post-Randomization Protocol, Follow-up, and Outcomes in the Xylitol pediatric oral topical syrup trial in the Marshall Islands.
Table 3.
Characteristics of the 94 children included in the final analysis* for each condition in the clinical trial.
| Xyl-2X | Xyl-3X | Control | Total | |
|---|---|---|---|---|
| N (Laura/Delap) | 33 (11/22) | 32 (10/22) | 29 (9/20) | 94 (30/64) |
| Female sex – no. (%) | 19 (57.8%) | 18 (56.2%) | 14 (48.3%) | 51 (54.3%) |
| Age at randomization – months ± SD† | 15.9 ± 2.6 | 13.7 ± 2.4 | 15.6 ± 2.7 | 15.0 ± 2.7 |
| Follow-up time‡ – months ± SD | 10.4 ± 2.5 | 10.6 ± 1.9 | 10.6 ± 2.1 | 10.5 ± 2.2 |
94 children had at least one exam follow-up exam during the study period and were included in this intent-to-treat analysis. Of these, 84 completed all follow-up exams and 10 who dropped out after having an interim exam.
SD= Standard Deviation
Syrup randomization follow-up period was 12 months. However, some of the 94 children included in the analysis dropped out after their interim exam and thus reduced the mean follow-up time.
Caries Hypothesis Test
Table 4 gives the caries outcomes after exposure to the intervention for a mean of 10.5 months: 51.7% of the controls vs. 41.6% of group Xyl-3X and 24.2% of group Xyl-2X had tooth decay. No teeth were missing or lost to tooth decay. Compared to controls, the number of decayed teeth was significantly less in both the Xyl-2X condition (relative risk [RR], 0.30; 95% confidence interval [CI] 0.13 to 0.66; P=0.003) and the Xyl-3X condition (RR, 0.50; 95% CI 0.26 to 0.96; P=0.037). There was no significant difference between the two xylitol conditions (P=0.22). The results after controlling for study site, age at entry, and number of teeth at follow-up were similar.
Table 4.
Percent of children with tooth decay and number of decayed teeth for the 94 children included in the final analysis.
| Condition | Percent with Decayed teeth |
No. teeth at last exam, Mean ± SD* |
No. decayed teeth Mean ± SD [Max]† |
Relative Risk‡ (95% CI) |
|---|---|---|---|---|
| Control (N=29) | 51.7 | 17.2 ± 2.5 | 1.9 ± 2.4 [8] | 1.00 |
| Xyl-2X (N=33) | 24.2 | 17.2 ± 2.9 | 0.6 ± 1.1 [4] | 0.30 (0.13, 0.66) |
| Xyl-3X (N=32) | 40.6 | 16.6 ± 3.2 | 1.0 ± 1.4 [6] | 0.50 (0.26, 0.96) |
94 children had at least one exam during the study period and were included in this intent-to-treat analysis. Of these, 84 completed all follow-up exams and 10 who dropped out after having an interim exam.
SD = Standard Deviation.
Max = maximum range.
Non-adjusted analysis. Generalized estimating equations with a robust variance estimator were used to fit the Poisson regression in order to account for overdispersion due to multiple teeth per subject.
Wald test for comparison with control condition. Score test for overall condition effect (X2=7.26; P-value = 0.027).
Prevented Fraction and Number Needed to Treat
The incidence rates (unadjusted model) for decayed primary teeth were:
Control condition: 2.20 decayed primary teeth per year
Xyl-2X condition: 0.66 decayed primary teeth per year
Xyl-3X condition: 1.10 decayed primary teeth per year
The Prevented Fraction ranged from 50% (Xyl-3X vs. Control: 100 × (2.2 − 1.1)/2.2) to 70% (Xyl-2X vs. Control: 100 × (2.2 − 0.66)/2.2) and the Number Needed to Treat ranged from was 10 with Xyl-3X to 4 with Xyl-2X (Xyl-3X vs. Control: 1/(51.7% - 41.6%); Xyl-2X vs. Control: 1/(51.7% - 24.2%)).
Side Effects
The proportions of children that experienced loose stools or diarrhea during the run-in (10.0%) and wash-out (8.7%) periods were less than that during the observation period (18.5%) immediately after enrollment. Expected side effects such as loose stools or diarrhea during the syrup randomization period (11.3% total) occurred at rates similar across the three groups (Xyl-3X, 10.6%; Xyl-2X, 11.7%, Control, 11.4%) and similar to the pre-syrup randomization periods. The children experienced no serious adverse events during the syrup randomization protocol.
Discussion
Children with Early Childhood Caries are three times more likely to develop tooth decay in their permanent teeth.20 Childhood tooth decay has a negative impact on oral health-related quality of life21 which then improves after dental treatment.22,23 Minority and poor children in the U.S. and U.S. associated states and territories in the Pacific have very high rates of ECC, while access to care is limited and has not improved with enhanced Medicaid coverage under Early and Periodic Screening, Diagnostic, and Treatment (EPDST).24 In this study, more than half (51.7%) of the control group children experienced tooth decay before their third birthday. This rate is extraordinary but consistent with previous rate reported by Tut et al.14 and rates for minority and American Indian and Alaska Native children.
The results suggest that exposure to 8 g of xylitol in a twice-daily topical oral syrup during primary tooth eruption could prevent up to 70% of the decayed teeth. Dividing the 8 g into three doses did not increase the effectiveness of the treatment. These results provided evidence for the first time that xylitol is effective for the prevention of decay in primary teeth of toddlers.
There is some consensus among researchers that a habitual xylitol exposure of five to 10 g divided into at least three daily periods of consumption is needed for a therapeutic effect. However, we previously evaluated the response of mutans streptococci in plaque--a surrogate marker for tooth decay--to varying frequencies of xylitol chewing gum consumption for five weeks at a standard daily dose (10.3 g/day) among adults. The study found a linear reduction in mutans streptococci with increasing frequency (zero to five) of xylitol chewing gum consumption but the reduction with twice daily chewing did not reach statistical significance.32 That study had inherent weaknesses, a short follow-up and a surrogate marker, which may not withstand comparison to a more rigorous long RCT with tooth decay as the endpoint. Moreover, the act of chewing and suckling are potent stimulators of salivary flow which enhances the clearance of food debris, oral bacteria, and acid buffering capacity benefiting the remineralization of enamel and protecting from tooth decay.17 Chewing gum and lozenges studies where controls also used gum or lozenges may under estimate the reduction of mutans streptococci and tooth decay by xylitol. Given the absence of potential effects attributable to chewing or suckling which are inherent in xylitol chewing gum and lozenges studies, the results found in this study more accurately reflect the effects of xylitol. Finally, xylitol given during tooth eruption and colonization is suggested to have maximal protective effects.19
The greater reduction seen with Xyl-2X (8 g xylitol + 2 g sorbitol/day) compared to Xyl-3X (8 g xylitol/day) begs the question of xylitol and sorbitol synergistic effects. However, it is important to appreciate that the difference observed between the experimental groups was not statistically significant. Furthermore, a 40 months chewing gum study in Belize did not find support for xylitol/sorbitol synergism. The study found that at similar total daily polyol doses, the xylitol only groups were most effective in caries reduction followed by the xylitol+sorbitol groups then sorbitol alone compared to no-gum control group.25
The study had a low drop-out rate (16%) and parents showed high compliance to the study protocol. Parent accepted the syrups. Children tolerated the polyols dose of six to 10 g per day and experienced relatively few and minor side effects of laxation. Side effects rates were comparable between experimental and control groups and between the run-in and randomized periods. This is in agreement with a recent study which concluded that xylitol solution at dosages of five to 7.5 grams per day were well-tolerated by toddlers age 6 to 36 months.26
Some have expressed concern about using sweet substances for the prevention of ECC. Literature on infant and toddler foods and taste preference is sparse. The literature does suggest that infants have an innate predilection for sweet taste.27 However, a wide variety of experiential factors influence flavor preferences during childhood.28–30 Higher sweet products consumption has been associated with urbanization and lower socio-economic status.30,31 Nevertheless, there is no published evidence that long-term consumption of specific sweet food during early childhood increases predilection for sweet food in general as a juvenile or adult. Similarly, there is no published literature on long-term xylitol exposure and future sweet food preference. On the other hand, xylitol based products have been widely available and consumed in Finland and Northern Europe for several decades without reports of undesirable effects in later years.32
Featherstone and colleagues argued that a multi-modality approach including antimicrobial therapy, professionally administered topical fluorides and fluoridated water should be used where possible for the prevention of tooth decay.33 The prevented fraction, the proportion of disease occurrence in a population averted due to a protective risk factor or public health intervention, in this study ranged from 50 to 70% for the xylitol treatment groups. The number needed to treat ranged from 10 to 4 to prevent a child from developing tooth decay. These findings provide support for the use of xylitol syrup along with fluorides, particularly for high risk population. The results also support the position of the AAPD34 and the NIH Consensus Statement: Diagnosis and Management of Dental Caries Throughout Life35 that xylitol is an important tool for the prevention of dental caries. More work is needed to develop vehicles and strategies for the public health application of xylitol. In populations with high rates of tooth decay, xylitol interventions are likely to be cost-effective.
Conclusion
This study is the first to demonstrate that xylitol topical syrup at 8 g per day divided into two or three doses given during primary tooth eruption in children 15 to 25 months of age reduces tooth decay and could prevent up to 70 percent of decayed teeth.
Supplementary Material
Acknowledgments
This was a special study setting and the circumstances deserve to be recorded here. The Republic of the Marshall Islands was previously a protectorate of the United States following World War II. Previous to independence, the U.S. conducted nuclear testing in the islands causing adverse effects on the population36 and resulting in distrust of any research done by mainlanders.37 In this environment, this study was the first controlled trial with children that has been permitted. These conditions placed special conditions on the Ministry of Health and the investigators to conduct themselves ethically. Extensive debate went on prior to approval of the study and community involvement and openness was stressed throughout. The authors of this paper include both University of Washington investigators and local personnel. The results have been reported back to the communities and the Ministry of Health, and considerable technical assistance has been provided to the Republic of the Marshall Islands Dental Department in order to assist in meeting the excessive burdens of tooth decay, periodontal or gum disease and oral cancer. As a result, serious efforts are underway to reduce the levels of oral disease in this population including cooperative studies addressing tooth decay and periodontal disease in uncontrolled diabetes, which is also quite common. We hope this study might be an example of what might go on in other racially and ethnically diverse communities in the U.S. where distrust regarding studies done by outsiders continues to be high.
The authors especially recognize Ms. Justina Langidrik, RMI Secretary of Health who has strongly supported efforts to find a solution to Early Childhood Caries in RMI. The authors express appreciation to Dr. Kyaw Tut, Chief of Dental Services, RMI and Maryann Mea, Fermina Briand, Melaia Joran, Lia Langibelik, Nelly Bikajela, Lisa Joran and Theresa Kedi for assistance in carrying out the study. Appreciation is also extended to Dr. Stella Yu of the Maternal and Child Health Bureau, HRSA for her oversight and patience with the many delays in accomplishing this work. The authors acknowledge the technical assistance of Ross Craig of Danisco USA. Danisco donated the raw materials used to make the syrups in this study.
Funded by HRSA Maternal and Child Health Bureau R40MC03622 and National Institute of Dental and Craniofacial Research U54 DE14254.
Abbreviations
- ECC
early childhood caries
- AAP
American Academy of Pediatrics
- AAPD
American Academy of Pediatric Dentistry
- DHHS
Department of Health and Human Services
Footnotes
Financial disclosure and conflict of interest: The authors have no financial interest in companies that produce xylitol or xylitol containing products or have conducted research funded by these companies.
Trial Registration International Standard Randomized Controlled Trial Number ISRCTN84269958
References
- 1.USPHS. Oral health in America: a report of the Surgeon General. J Calif Dent Assoc. 2000 Sep;28(9):685–695. [PubMed] [Google Scholar]
- 2.Oral health in America: a report of the Surgeon General. J Calif Dent Assoc. 2000 Sep;28(9):685–695. [PubMed] [Google Scholar]
- 3.Broderick E, Mabry J, Robertson D, Thompson J. Baby bottle tooth decay in Native American children in Head Start centers. Public Health Rep. 1989 Jan–Feb;104(1):50–54. [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly M, Bruerd B. The prevalence of baby bottle tooth decay among two native American populations. J Public Health Dent. 1987 Spring;47(2):94–97. doi: 10.1111/j.1752-7325.1987.tb01983.x. [DOI] [PubMed] [Google Scholar]
- 5.Greer MH, Tengan SL. Dental caries in early childhood among Native Hawaiians. Pacific Health Dialog. 1998;5(2):332–336. [Google Scholar]
- 6.Hale KJ. Oral health risk assessment timing and establishment of the dental home. Pediatrics. 2003 May;111(5 Pt 1):1113–1116. doi: 10.1542/peds.111.5.1113. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Dental and Craniofacial Research; National Call to Action to Promote Oral Health. 2003 Spring; Spring; NIH Publication No.(03-5303) [PubMed]
- 8.Fejerskov O, Kidd EAM. Dental caries : the disease and its clinical management. 2nd ed. Oxford, UK: Ames; Iowa, USA: Blackwell Munksgaard; 2008. [Google Scholar]
- 9.Milgrom P, Ly KA, Rothen M. Xylitol-how does it work and is there a dose response? Adv Dent Res. 2008:in press. [Google Scholar]
- 10.Miyasawa-Hori H, Aizawa S, Takahashi N. Difference in the xylitol sensitivity of acid production among Streptococcus mutans strains and the biochemical mechanism. Oral Microbiol Immunol. 2006 Aug;21(4):201–205. doi: 10.1111/j.1399-302X.2006.00273.x. [DOI] [PubMed] [Google Scholar]
- 11.Soderling E. Xylitol, mineralisation, mutans streptococci, and dental plaque. Adv Dent Res. 2008 doi: 10.1177/0895937409335642. In Press. [DOI] [PubMed] [Google Scholar]
- 12.Ly KA, Milgrom P, Rothen M. Xylitol, sweeteners, and dental caries. Pediatr Dent. 2006 Mar–Apr;28(2):154–163. discussion 192-158. [PubMed] [Google Scholar]
- 13.Tut OK, Greer MH, Milgrom P. Republic of the Marshall Islands: planning and implementation of a dental caries prevention program for an island nation. Pac Health Dialog. 2005 Mar;12(1):118–123. [PubMed] [Google Scholar]
- 14.Tut O, Milgrom P. Prevalence of Early Childhood Caries in Majuro, RMI; Proceedings of the National Oral Health Conference; April 29 to May 1; Boston, MA. 2002. [Google Scholar]
- 15.Ly KA, Milgrom P, Roberts MC, Yamaguchi DK, Rothen M, Mueller G. Linear response of mutans streptococci to increasing frequency of xylitol chewing gum use: a randomized controlled trial [ISRCTN43479664] BMC Oral Health. 2006;6:6. doi: 10.1186/1472-6831-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milgrom P, Ly KA, Roberts MC, Rothen M, Mueller G, Yamaguchi DK. Mutans streptococci dose response to xylitol chewing gum. J Dent Res. 2006 Feb;85(2):177–181. doi: 10.1177/154405910608500212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imfeld T. Chewing gum--facts and fiction: a review of gum-chewing and oral health. Crit Rev Oral Biol Med. 1999;10(3):405–419. doi: 10.1177/10454411990100030901. [DOI] [PubMed] [Google Scholar]
- 18.Hardin JW, Hilbe J. Generalized estimating equations. Boca Raton, Fla: Chapman & Hall/CRC; 2003. [Google Scholar]
- 19.Hujoel PP, Makinen KK, Bennett CA, et al. The optimum time to initiate habitual xylitol gum-chewing for obtaining long-term caries prevention. J Dent Res. 1999 Mar;78(3):797–803. doi: 10.1177/00220345990780031301. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Wang W. Predicting caries in permanent teeth from caries in primary teeth: an eight-year cohort study. J Dent Res. 2002 Aug;81(8):561–566. doi: 10.1177/154405910208100812. [DOI] [PubMed] [Google Scholar]
- 21.Do LG, Spencer A. Oral health-related quality of life of children by dental caries and fluorosis experience. J Public Health Dent. 2007;67(3):132–139. doi: 10.1111/j.1752-7325.2007.00036.x. Summer. [DOI] [PubMed] [Google Scholar]
- 22.Sheiham A. Dental caries affects body weight, growth and quality of life in pre-school children. Br Dent J. 2006 Nov 25;201(10):625–626. doi: 10.1038/sj.bdj.4814259. [DOI] [PubMed] [Google Scholar]
- 23.Filstrup SL, Briskie D, da Fonseca M, Lawrence L, Wandera A, Inglehart MR. Early childhood caries and quality of life: child and parent perspectives. Pediatr Dent. 2003 Sep–Oct;25(5):431–440. [PubMed] [Google Scholar]
- 24.Fisher MA, Mascarenhas AK. Does Medicaid improve utilization of medical and dental services and health outcomes for Medicaid-eligible children in the United States? Community Dent Oral Epidemiol. 2007 Aug;35(4):263–271. doi: 10.1111/j.1600-0528.2007.00341.x. [DOI] [PubMed] [Google Scholar]
- 25.Makinen KK, Bennett CA, Hujoel PP, et al. Xylitol chewing gums and caries rates: a 40-month cohort study. J Dent Res. 1995 Dec;74(12):1904–1913. doi: 10.1177/00220345950740121501. [DOI] [PubMed] [Google Scholar]
- 26.Vernacchio L, Vezina RM, Mitchell AA. Tolerability of oral xylitol solution in young children: implications for otitis media prophylaxis. Int J Pediatr Otorhinolaryngol. 2007 Jan;71(1):89–94. doi: 10.1016/j.ijporl.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawless H. Sensory development in children: research in taste and olfaction. J Am Diet Assoc. 1985 May;85(5):577–582. 585. [PubMed] [Google Scholar]
- 28.Liem DG, Mennella JA. Sweet and sour preferences during childhood: role of early experiences. Dev Psychobiol. 2002 Dec;41(4):388–395. doi: 10.1002/dev.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borzekowski DL, Robinson TN. The 30-second effect: an experiment revealing the impact of television commercials on food preferences of preschoolers. J Am Diet Assoc. 2001 Jan;101(1):42–46. doi: 10.1016/S0002-8223(01)00012-8. [DOI] [PubMed] [Google Scholar]
- 30.Maciel SM, Marcenes W, Watt RG, Sheiham A. The relationship between sweetness preference and dental caries in mother/child pairs from Maringa-Pr, Brazil. Int Dent J. 2001 Apr;51(2):83–88. doi: 10.1002/j.1875-595x.2001.tb00827.x. [DOI] [PubMed] [Google Scholar]
- 31.Jamel HA, Sheiham A, Cowell CR, Watt RG. Taste preference for sweetness in urban and rural populations in Iraq. J Dent Res. 1996 Nov;75(11):1879–1884. doi: 10.1177/00220345960750111001. [DOI] [PubMed] [Google Scholar]
- 32.Maguire A, Rugg-Gunn AJ. Xylitol and caries prevention--is it a magic bullet? Br Dent J. 2003 Apr 26;194(8):429–436. doi: 10.1038/sj.bdj.4810022. [DOI] [PubMed] [Google Scholar]
- 33.Young DA, Featherstone JD, Roth JR, et al. Caries management by risk assessment: implementation guidelines. J Calif Dent Assoc. 2007 Nov;35(11):799–805. [PubMed] [Google Scholar]
- 34.Policy on the Use of Xylitol in Caries Protection. AAPD Reference Manual. 2006;29(7):36–37. Online accessed [2008, 03, 15] ( http://www.aapd.org/media/policies_guidelines/p_xylitol.pdf), Online accessed [2008, 2003, 2015] http://www.aapd.org/media/policies_guidelines/p_xylitol.pdf.
- 35.Diagnosis and Management of Dental Caries Throughout Life. NIH Consensus Statement. 2001 March 26–28;18(1):1–24. [PubMed] [Google Scholar]
- 36.Pollock NJ. Health transitions, fast and nasty: the case of Marshallese exposure to nuclear radiation. Pac Health Dialog. 2002 Sep;9(2):275–282. [PubMed] [Google Scholar]
- 37.Williams DP, Hamptom A. Barriers to Health Services Perceived by Marshallese Immigrants. Journal of Immigrant Health. 2005 October;7(4) doi: 10.1007/s10903-005-5129-8. 2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.