Abstract
Langerhans cells (LC) are antigen presenting cells (APC) that reside at the barrier surfaces. Mice expressing an ovalbumin peptide in the epidermis (K14-OVAp) were used to study CD8+ T cell responses to an epidermal self-antigen. Earlier results suggested that LC were the predominant APC, inducing a robust T cell response and autoimmunity. In this study we employed a whole protein model system, the K14-mOVA mouse, in which a transmembrane form of ovalbumin was expressed in keratinocytes. In contrast to K14-OVAp mice, T cells in K14-mOVA mice were activated, but did not expand and instead died by apoptosis. Furthermore, in double transgenic mice expressing both mOVA and OVAp, robust OT-I expansion occurred, indicating that tolerance to this antigen is not dominant and was due to lack of activating signals. We sought to identify the relevant APC in K14 mice using bone marrow chimeras, and found that radioresistant cells (presumably LC) were able to cross-present the OVA antigen from keratinocytes to naïve T cells in the lymph node. However, use of LC deficient mice indicated that LC were not required for the expansion of OT-I in K14-OVAp, or the deletion of OT-I in K14-mOVA mice. These data suggest that radioresistant non-Langerhans cells present self-antigen in K14-OVAp mice, and drive a robust CD8 T cell response.
Keywords: Dendritic cells, Langerhans cells, skin, peripheral tolerance
Introduction
Through central tolerance in the thymus, the developing T cell repertoire is actively purged of self-reactive T cells in an effort to avoid immune responses directed at self-tissues. This process, known as negative selection, is essential for avoiding autoimmunity, and mice with defects in negative selection develop autoimmune diseases throughout life (1, 2). Despite the ability of central tolerance to delete self reactive T cells in the thymus, some of these cells escape and are potential mediators of autoimmune disease (3–6). In the periphery, several mechanisms exist to enforce tolerance, including clonal ignorance, induction of anergy, clonal deletion, and control of T cell responses via CD4+ Foxp3+ Treg cells (7). In addition, the role of professional APC, specifically different dendritic cells (DC) 3 subsets, in peripheral tolerance is still not fully understood.
In order to study peripheral tolerance, many transgenic mouse models have been developed in which foreign antigens are expressed under tissue specific promoters. Peripheral tolerance can then be studied through the introduction of antigen-specific T cells to the periphery, avoiding the complicating factors of central tolerance. A widely studied model of peripheral tolerance is the RIP-mOVA transgenic mouse. In this system, a membrane-anchored model antigen consisting of ovalbumin fused to the transmembrane domain of transferrin receptor is expressed under the rat insulin promoter and produced in mouse pancreas and kidney (8). Introduction of OT-I T cells (CD8+ TCR transgenic specific for OVA-Kb (9)) via adoptive transfer resulted in their clonal deletion (10). Through these experiments it was determined that hematopoietic professional APC were responsible for presenting the self antigen in the lymph nodes draining tissues where the transgenic antigen was expressed (8).
The role of professional APC in this model of peripheral tolerance was highlighted in the initial experiments carried out in transgenic mice. Further experiments revealed the importance of a specific DC subset- the CD8α+ DC. It was shown in the RIP-mOVA system that clonal deletion of OT-I was dependent on the action of a CD8α+ DC subset (11). Other models of peripheral tolerance induction have also implicated CD8α+ DC as the relevant APC, as introduction of apoptotic cell associated antigens directly to the blood was shown to result in T cell tolerance (12). In similar systems, cell associated and soluble antigens were shown to be taken up and cross-presented to CD8+ T cells by CD8α+ DC (13, 14). In addition, targeting antigens for uptake by CD8α+ DC by fusing antigen to anti-DEC205 antibodies resulted in T cell deletion and tolerance (15). Through further studies, the CD8α+ DC subset is now understood to be very efficient at cross-presentation of both self and foreign antigens to CD8+ T cells (16).
The mechanism by which professional APC can induce both T cell peripheral tolerance and activation is still under investigation. However, a significant amount of data suggests that in the steady state, DC express low levels of molecules that promote T cell activation, including MHC, costimulatory molecules, and inflammatory cytokines. Antigen presentation by these DC results in T cell tolerance via the mechanisms discussed previously. It is only following maturation of the DC in response to inflammatory signals that upregulation of MHC, costimulatory molecules and cytokine production provide the signals necessary for T cell activation (17). This dichotomy was demonstrated in the model system where anti-DEC205 coupled antigen could induce T cell activation if targeted to mature DC with antiCD40 in the skin (18). In addition, it was shown in a model of inducible expression of LCMV antigens that immature or resting DC induce tolerance to these antigens, but activated / mature DC induced T cell activation (19). These studies support the idea that the maturation state of the presenting DC is crucial in determining the T cell response to self antigens.
In contrast to this paradigm, some models in which transgenic self-antigens are expressed under skin specific promoters do not result in peripheral tolerance. We developed a transgenic mouse model in which the OVA peptide recognized by OT-I T cells, was expressed under the control of the human keratin-14 promoter, providing for expression in stratified epithelia including the epidermis of the skin (6). Adoptive transfer of low numbers of naïve OT-I T cells specific for the OVAp antigen resulted in a robust expansion of OT-I T cells, and induction of autoimmunity occurred with adoptive transfer of high numbers of OT-I T cells. Surprisingly, the expansion of OT-I T cells was driven by radioresistant APC. Furthermore, antigen presented by E-cadherin+ APC in the lymph node could stimulate T cells (20). These data implicated Langerhans cells (LC), the APC that reside in the epidermis of the skin and other barrier surfaces. These cells encounter naïve T cells in the lymph nodes, as they have been shown to migrate to skin draining lymph nodes, transporting skin antigens in the steady state (21). These cells were the likely APC, as LC are radioresistant (22) and express E-Cadherin (23). Other models of skin self antigens have been developed using the same human K14 promoter. K14-mOVA mice were developed by Stephen Katz and coworkers, in which a membrane bound form of ovalbumin protein was expressed. Introduction of OT-I T cells via adoptive transfer also resulted in an autoimmune skin disease, and LC from K14-mOVA skin explants activated OT-I T cells (24). In addition, a model using the Keratin 5 promoter to drive a membrane bound transgenic ovalbumin protein was developed by Azukizawa et al. K5-mOVA mice also develop an autoimmune skin disease upon introduction of OT-I T cells (25).
These transgenic models of skin self antigens suggest that LC antigen presentation in the steady state might induce constitutive T cell activation, as opposed to the tolerance induced by the CD8α+ DC in other model systems. The differential outcomes observed in antigen presentation by different DC subsets could reflect a difference in activation status and the inability of LC to induce tolerance in the steady state. This is possible, as LC that have migrated to draining lymph nodes in the steady state have higher expression of the molecules needed for T cell activation such as MHC I and II, costimulatory molecules (B7-1/2) and inflammatory cytokines (IL-12) (26). In addition, conditioning of these cells in K14-OVAp mice, which results in down regulation of costimulatory molecules, correlated with a reduced ability to stimulate T cell responses (27).
We sought to determine the role of LC in presentation of skin self-antigens using the K14-OVAp model system and a new K14-mOVA transgenic model. This transgene uses the human K14 promoter to drive expression of the same membrane-anchored ovalbumin/transferrin receptor fusion protein used in the RIP-mOVA studies discussed above. In contrast to previously described K14 transgenic model systems, OT-I T cells are activated in these K14-mOVA mice, but do not expand or induce autoimmunity, and the abortive response results in OT-I apoptosis. The deletion of OT-I in the model is not dependent upon CD4 T cells, and the expansion observed in K14-OVAp mice is dominant in double transgenic mice. Finally, we observed that although LC can present antigen in both transgenic models, they are not required for the expansion observed in K14-OVAp or deletion observed in K14-mOVA. In both K14 transgenic model systems used in this study antigen presentation by a radioresistant cell other than LC can drive expansion of OT-I T cells. These results clarify and advance our understanding of peripheral tolerance mechanisms that control self-reactive T cells.
Materials and Methods
Mice
C57BL6 (B6) and B6.PL-Thy1a (Thy1.1 congenic) mice were purchased from The Jackson Laboratory and the National Cancer Institute. OT-I TCR transgenic mice were produced as described (9), and crossed to B6.PL-Thy1a mice to provide Thy1.1 congenic OT-I.PL. IFNα/βR−/− IL-12R−/− OT-I and Perforin−/− OT-I were obtained from Matthew Mescher. K14-OVAp transgenic mice were generated as described (28, McGargill, 2002 #135). K14-mOVA mice were generated by and obtained from Ben Rich at Harvard University. K14-mOVA mice were generated by inserting a fusion protein containing amino acids 1–118 of the human transferring transmembrane receptor fused to amino acids 139–386 of chicken ovalbumin (29), into the human keratin 14 promoter containing plasmid K14-HGX as described (30). K14-OVAp mice crossed to B6.PL-Thy1a (Thy1.1/Thy1.2) were used as recipients for IFNα/βR−/− IL-12R−/− OT-I and Perforin−/− OT-I. H-2Kbm1 mice were purchased from The Jackson Laboratory and backcrossed to both K14-OVAp and K14-mOVA mice to H-2Kbm1 homozygosity. Langerin-DTREGFP knock-in mice were obtained from Bernard Malissen (31), and were crossed to K14-OVAp and K14-mOVA mice. Mice that were heterozygous for the designated K14 transgene and the Langerin-DTREGFP knockin were used for all experiments. All mice were treated in accordance with federal guidelines approved by the University of Minnesota Institutional Animal Care and Use Committee.
Antibodies
Fluorochrome conjugated antibodies to CD8α, Thy1.1, Thy1.2, CD44, CD69, CD27, CD54, CD49d, CD11c, I-Ab and CD45.2, and biotinylated antibodies to CD137w (4-1BB), and CD25 were purchased from eBioscience, BD PharMingen, or Biolegend. Y3 and 5F1 antibodies were conjugated to FITC and biotin, respectively. The anti-EpCAM antibody used to determine LC chimerism was purified from G8.8 hybridoma supernatant (obtained from Andrew Farr, Seattle, WA), and biotinylated. Streptavidin-APC or Streptavidin-FITC from eBioscience or Streptavidin-PerCP from BD Pharmingen was used as a secondary with biotinylated antibodies. Intracellular staining for cleaved Caspase 3 was performed following fixation in 0.5% formaldehyde and permeabilization in 90% methanol. Purified anti-cleaved Caspase 3 antibody was purchased from Cell Signaling Technologies, and Alexa-647 conjugated goat-anti-rabbit IgG (Invitrogen) was used as the secondary antibody. All flow cytometry was acquired on either a BD FACScalibur or LSR II and analyzed with Flow Jo software (Treestar).
T cell Adoptive Transfer
OT-I T cells were isolated from lymph nodes of OT-I.PL, OT-I, IFNα/βR−/− IL-12R−/− OT-I or Perforin−/− OT-I mice and purified by negative selection using magnetic cell sorting MACS (Miltenyi Biotech). Naïve (CD44low) OT-I T cells were purified by labeling the cell suspension with FITC conjugated antibodies to B220, I-Ab, CD4 and CD44 (BD pharmingen). Following staining, the cells were incubated with anti-FITC MACS Microbeads and bead labeled cells were depleted using MACS LS columns. Cell purity (>95%) was determined by flow cytometry prior to adoptive transfer. Cells were transferred into host mice intravenously. A total of 5 × 104 OT-I were transferred into K14-OVAp and K14-mOVA mice to determine expansion at day 6. In adoptive transfers for OT-I phenotyping in K14-mOVA and K14-OVAp, 1 × 106 OT-I T cells were transferred. For some experiments, OT-I were labeled with CFSE (Molecular Probes) to determine cell division as previously described (32). Transferred cells were detected in recipient mice by making single cell suspensions of lymph nodes and spleen at varying times after transfer. Flow cytometry analysis using CD8α and the appropriate congenic Thy1 marker. Fold expansion of OT-I was calculated by determining the total number of OT-I recovered in the lymphoid tissue and dividing this by the number of cells that were adoptively transferred.
CD4 Depletion
In vivo depletion of CD4+ cells was done by intravenous injection of anti-CD4 antibody ascites (GK1.5). 100µg of GK1.5 was injected i.v. every two days starting at day 8 prior to adoptive transfer, and continuing until harvest. CD4 depletion was analyzed in each animal at time of harvest using flow cytometry to identify CD4 T cells.
Radiation Bone Marrow Chimeras
Single cell suspensions of bone marrow cells from indicated donor mice were prepared and depleted of mature T cells by complement mediated cytotoxicity with 30H12 (anti-Thy1.2; ATCC, Manassas, VA) as described (33). Bone marrow cells were then injected i.v. (5×106 cells/recipient) into lethally irradiated recipient mice (1000 rads). Hematopoietic chimerism in skin and lymphoid organs was determined by staining with antibodies against H-2Kb: clone Y3 (recognizes Kb and Kbm1) and clone 5F1 (does not recognize Kbm1) for flow cytometry analysis. Dendritic cells from skin draining lymph nodes and spleen were prepared by digestion with collagenase D (Sigma, St. Louis, MO) and EDTA as previously described (34). Epidermal cells were prepared by limited trypsinization and dissociation of epidermal sheets by pipetting in DNase as previously described (35).
LC Depletion with Diphtheria Toxin
LC were systemically depleted in mice by I.P. injection of 1µg Diphtheria Toxin (List Biological Laboratories), as previously described (31).
Results
OT-I T cells exhibit different outcomes in K14-OVAp and K14-mOVA mice
Previous studies in our lab focused on understanding the autoimmune disease induced by a CD8+ T cell response to a transgenic peptide from ovalbumin (OVA) expressed under the human K14 promoter. In this system TCR transgenic OT-I T cells, specific for the OVA peptide SIINFEKL, were activated and induced autoimmunity in K14-OVAp transgenic mice, which express the OVA peptide in stratified epithelia. This study further suggested that Langerhans cells (LC) were the antigen presenting cell (APC) that initiated the OT-I T cell response and induced autoimmunity (20). In order to determine if similar responses occurred to protein antigens, we employed the K14-mOVA transgenic mouse model in which a portion of the ovalbumin protein is fused to the transmembrane domain of the transferrin receptor to provide membrane localization. The T cell response to mOVA was assessed via an adoptive transfer system in which naïve (CD44low) OT-I CD8+ T cells were transferred into K14-mOVA hosts and identified using the congenic marker Thy1.1.
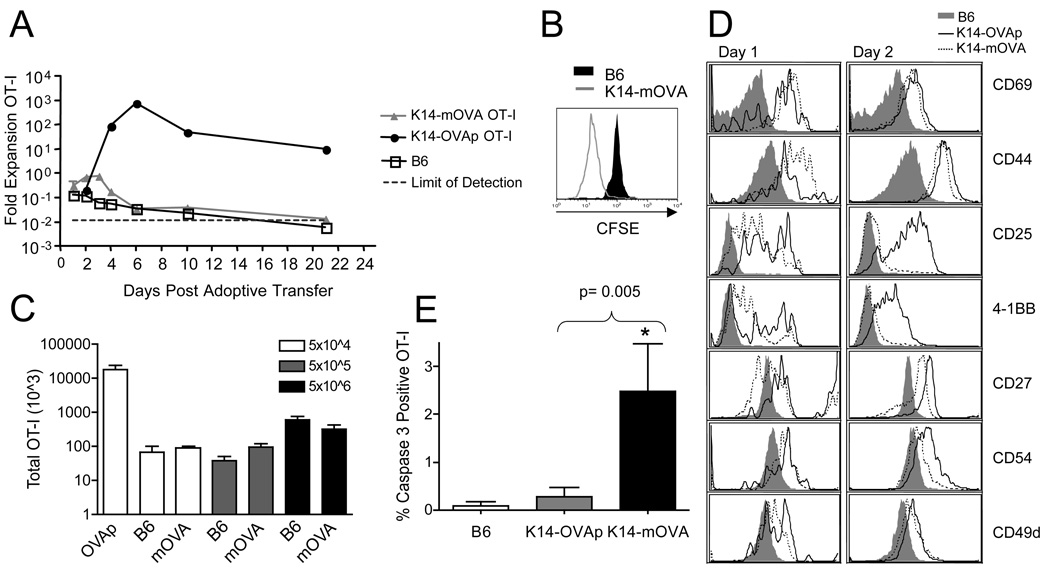
We examined OT-I T cell responses in K14-OVAp and K14-mOVA mice over a three-week time course. OT-I T cells in K14-mOVA mice underwent activation and division (Figure 1A–B), but only underwent a two fold expansion that peaked at day 3 (Figure 1A). The OT-I T cells contracted modestly following the peak and were at levels similar to that seen in negative control B6 mice by day 6, and essentially undetectable by day 22. The response to OVA protein in K14-mOVA was in stark contrast to the response in K14-OVAp mice, where OT-I T cells expanded at least 100 fold and peaked at day 6 (Figure 1A).
Figure 1. Distinct outcomes of OT-I T cells adoptively transferred into K14-OVAp and K14-mOVA mice.
Naïve OT-I T cells were adoptively transferred into K14-OVAp, K14-mOVA, or B6 mice. Lymph nodes and spleen were harvested and analyzed by flow cytometry. (A) Fold expansion of 5 × 104 OT-I in different hosts at varying timepoints after adoptive transfer. The total number of OT-I was calculated from LN and spleen. Data shown is the average of at least 2 mice per group per timepoint. Inset depicts CFSE dilution in OT-I in K14-mOVA and B6 mice at day 4. (B) Increasing the number of adoptively transferred OT-I in K14-mOVA did not increase expansion observed at day 6. Data are from least 4 mice per group compiled from two experiments. (C) Phenotyptic analysis of LN cells 1 or 2 days after adoptive transfer of 1 × 106 naïve OT-I. Adoptively transferred OT-I (CD8a+, Thy1.1+) were gated and analyzed for CD25 (IL-2Rα), 4-1BB, CD54, and CD27. Data shown are representative of cells in both LN and spleen, in at least 4 mice per group from two experiments. (D) Intracellular staining for cleaved caspase 3 on OT-I T cells recovered from the lymph nodes at day 2 following adoptive transfer. Data shown are from a total of 4 mice per group in two experiments.
Experiments in the K14-mOVA mouse developed by Stephen Katz showed that OT-I T cells responded to transgenic ovalbumin protein expressed in the epidermis and underwent robust expansion that peaked around day 6 (24). In their system, high numbers of OT-I T cells (5–10 × 106) were adoptively transferred into K14-mOVA mice. To determine if we could induce OT-I expansion in our K14-mOVA model by transferring increasing numbers of OT-I T cells, we adoptively transferred 5×104, 5×105, or 5×106 naïve OT-I T cells into B6 and K14-mOVA mice. In contrast to the response observed in the Katz K14-mOVA mouse model, adoptively transferred OT-I T cells did not expand or induce autoimmunity in these K14-mOVA hosts, even at high numbers. In fact, the total number of OT-I T cells present at day 6 post adoptive transfer remained similar regardless of the number of OT-I adoptively transferred (Figure 1C).
To better understand the differential expansion outcomes in K14-OVAp and K14-mOVA mice, we analyzed the phenotype of OT-I T cells in each host at early timepoints in the response. In both mouse models, OT-I T cells in skin draining lymph nodes experienced antigen, as shown by upregulation of CD69 at day 1 and CD44 by day 2 (Figure 1D). However, differences were observed in surface expression of key cytokine receptors and co-stimulatory molecules. Although CD25 (IL-2Rα) had been upregulated on OT-I in both hosts at day 1, by day 2 it was downregulated on OT-I T cells in K14-mOVA hosts. Additionally, at day 2, OT-I T cells in K14-mOVA hosts had lower expression of 4-1BB, CD27, and CD54 (ICAM-1) than in K14-OVAp hosts (Figure 1D). Interestingly, OT-I T cells in both hosts retained expression of CD49d, the α4 integrin, a marker of skin homing T cells. Furthermore, the loss of CD25 and therefore IL-2 signals on OT-I in K14-mOVA mice, and the rapid contraction of this population suggested that the OT-I T cells might be dying in their response to K14-mOVA. We addressed this possibility by using intracellular staining for a cleaved and hence activated form of caspase 3, an effector caspase important for apoptosis. We observed a significant increase in the percentage of OT-I T cells with active caspase 3 at day 2 in K14-mOVA hosts (Figure 1E). These data suggested that OT-I T cells in K14-mOVA mice experienced the OVA antigen and divided, but did not accumulate because of loss of survival signals and subsequent apoptosis.
The factors involved in expansion in K14-OVAp
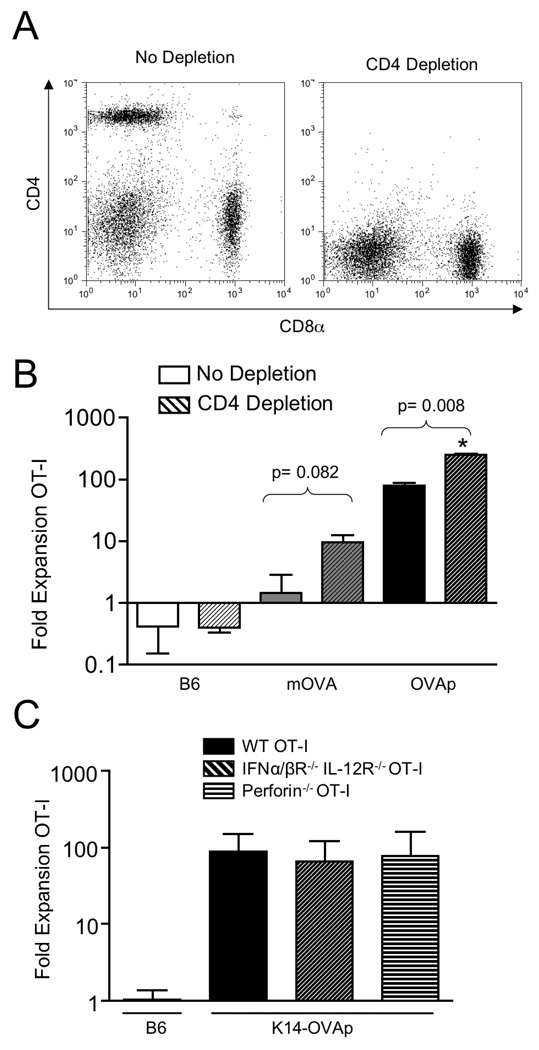
To understand the differential responses in K14-OVAp and K14-mOVA mice, we addressed the requirements for OT-I T cell expansion in both models. Importantly, the transgene in K14-OVAp encodes only an MHC class I epitope for recognition by CD8+ T cells. In K14-mOVA mice, the transgene encodes a fusion protein that includes both MHC class I and II epitopes, for presentation to both CD8+ and CD4+ T cells. Therefore it was possible that CD4+ T cells were important in the differential responses observed in K14-OVAp and K14-mOVA mice. CD4+ T cells can provide help in generating productive CD8+ T cell responses, but can also control these responses through the actions of CD4+ T regulatory cells (Tregs). We sought to understand the role of CD4+ T cells in these models by in vivo depletion using the CD4 specific complement-fixing antibody GK1.5. Depletion of CD4+ T cells was nearly 100% in both spleen (data not shown) and lymph node (Figure 2A). Adoptive transfer of OT-I T cells into CD4 depleted hosts resulted in a slightly increased response in both model systems, suggesting that CD4+ T cells normally limit both responses (Figure 2B). The increase observed in K14-OVAp mice indicated that the role of CD4 was not antigen-specific control through Tregs because the K14-OVAp transgene does not have a CD4+ T cell epitope. In addition blockade of Treg function using anti-CD25 antibody treatment did not affect the response in either model system (data not shown). These results might suggest a role for a non-antigen-specific regulatory CD4+ T cell population such as NKT cells, which nonetheless seem to be playing a similar role in both model systems.
Figure 2. The robust OT-I response in K14-OVAp does not require CD4 help or cytolytic effector function.
(A) Naïve OT-I T cells were adoptively transferred into K14-OVAp, K14-mOVA, or B6 mice that were treated with GK1.5 (to deplete CD4 T cells) or control antibody. Lymph nodes and spleen were harvested at day 6 and analyzed by flow cytometry. Staining of LN cells from GK1.5 treated mice with a non-competing CD4 antibody showed effective depletion of CD4 T cells from the lymphoid organs of recipient mice. Representative data are shown of 10 depleted mice. (B) Expansion of adoptively transferred OT-I in CD4 depleted B6, K14-mOVA, or K14-OVAp mice. Data shown include a total of at least 3 mice per group from two experiments. (C) Fold expansion of IFNα/βR−/− IL-12R−/− OT-I or Perforin−/− OT-I after adoptive transfer into K14-OVAp recipient mice. Data shown are representative of a total of 6 mice per group from two experiments.
OT-I T cells acquire cytolytic function in K14-OVAp mice (20). Thus it was important to determine if lytically active OT-I T cells induced tissue damage, leading to the release of more antigen, and producing an inflammatory loop that drives expansion of the antigen-specific T cells. To test this we adoptively transferred IFNα/βR−/− IL-12R−/− OT-I T cells. Such OT-I T cells cannot receive the “third signal” activation through type I Interferons or IL-12 that is required for full cytolytic effector function (36). In addition, we transferred perforin−/− OT-I that lack the perforin protein necessary for CD8+ T cell cytolytic activity. Both expanded normally (Figure 2C) indicating that T cell expansion in K14-OVAp does not require cytolysis of antigen expressing cells.
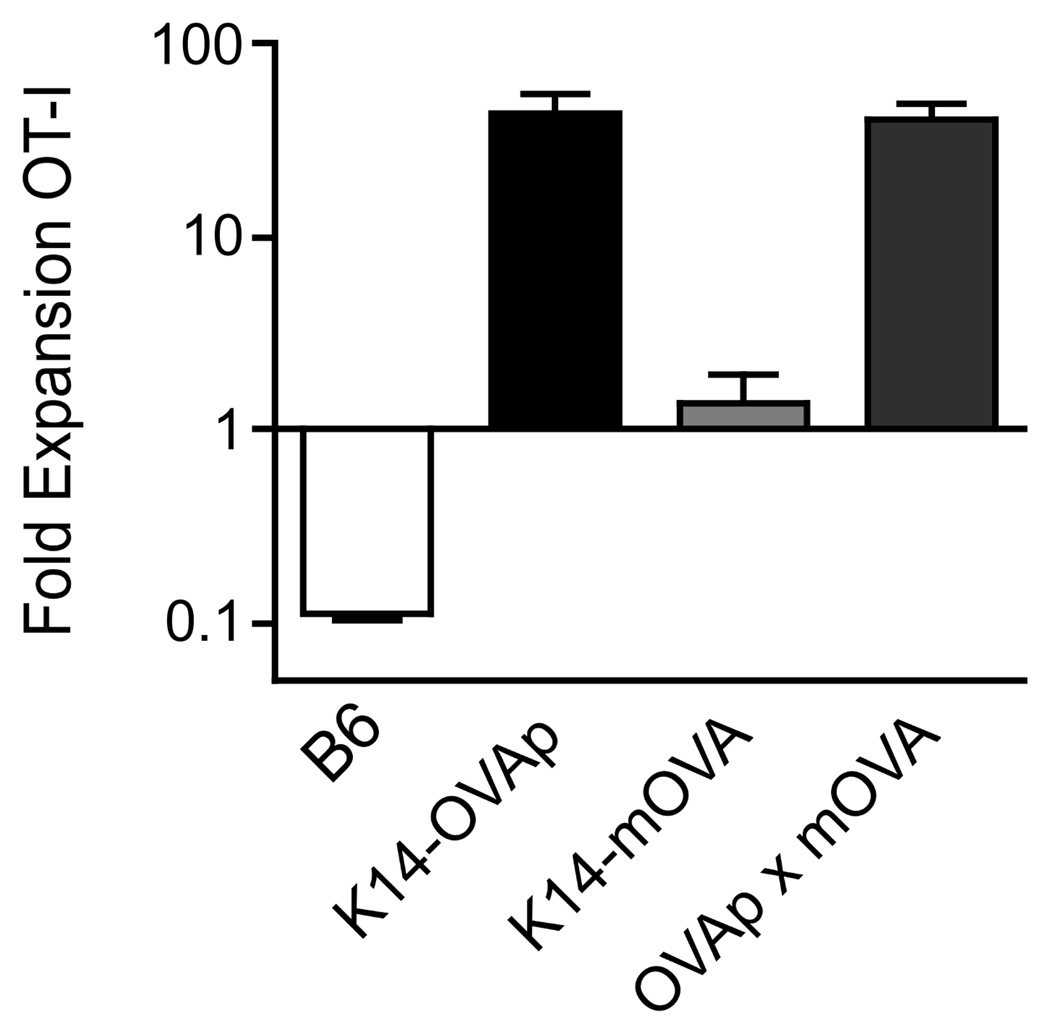
We had previously shown that the expansion of OT-I T cells in K14-OVAp mice does not occur when OVA protein antigen is expressed ubiquitously, as in K14-OVAp/Act-mOVA double transgenic mice (20). This suggests that ubiquitous antigen results in a dominant tolerance process. In order to determine if the K14-mOVA protein antigen induces a similar tolerance, we crossed K14-OVAp and K14-mOVA mice to make double transgenic mice expressing both OVAp and mOVA. Surprisingly, OT-I T cells still robustly expanded, similar to K14-OVAp single transgenic mice (Figure 3, note the log scale). This result suggests that K14-mOVA does not induce a dominant tolerance, and further supporting the idea that antigen-specific Tregs were not responsible for control of OT-I T cell response in K14-mOVA mice. Presentation of MHC class II epitopes from ovalbumin was observed in OVAp/mOVA double transgenic mice as well as mOVA single transgenics (data not shown), but this presumably either does not result in the induction of antigen-specific CD4+ Tregs, or such Tregs are insufficient to control the CD8 T cell tolerance.
Figure 3. OT-I expansion is dominant in K14-OVAp×K14-mOVA double transgenic mice.
Naïve OT-I T cells were adoptively transferred into B6, K14-OVAp, K14-mOVA, or K14-OVAp/K14-mOVA double transgenic mice. Lymph nodes and spleen were harvested at day 6 and analyzed by flow cytometry. Data shown are from a total of 5 mice per group from two experiments.
Radioresistant cells present OVA antigens in K14 transgenic mice
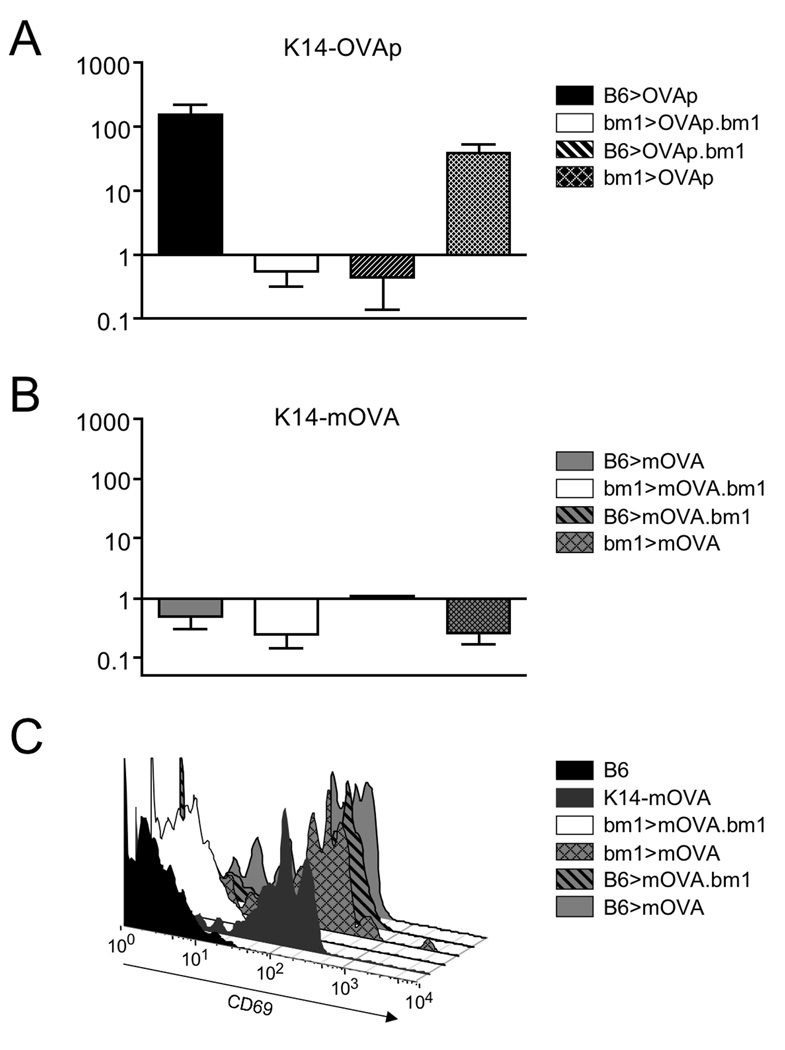
As expansion of OT-I T cells in K14-OVAp mice is dependent on antigen presentation by radioresistant cells, we sought to determine if the differential response observed in K14-mOVA was initiated by the same or different antigen presenting cells. To assess antigen presentation we created bone marrow chimeric mice taking advantage of H-2Kbm1 mice that have a mutation in the H-2Kb MHC class I molecule. The H-2Kbm1 mutant is not able to stimulate OT-I T cells in response to OVAp, allowing for the restriction of antigen presentation to radioresistant or radiosensitive cells. Bone marrow chimeras in which only radioresistant cells could present antigen (bm1>mOVA, bm1>OVAp), or only radiosensitive cells could present antigen (B6>mOVA.bm1, B6>OVAp.bm1), as well as control chimeras were used as recipients for OT-I T cell adoptive transfer. Confirming previous results, we observed expansion in both the K14-OVAp positive control chimera in which all cells can present and the chimera in which only radioresistant cells can present (Figure 4A). There was no appreciable expansion in the K14-OVAp chimera in which only bone marrow cells could present (B6>OVAp.bm1), yet antigen was still displayed as OT-I T cells in this chimera upregulated both CD69 and CD44 (data not shown). Therefore both radiosensitive and radioresistant APC present antigen, but only radioresistant APC drive the robust CD8 T cell response.
Figure 4. Both radioresistant and hematopoetic cells present antigen in K14-OVAp and K14-mOVA mice, but only radioresistant cells drive expansion in K14-OVAp.
Bone marrow chimeras were made using the indicated donors and hosts. Naïve OT-I T cells were adoptively transferred into K14-OVAp (A) and K14-mOVA (B) chimeras. Fold expansion of OT-I at day 6 is shown, and includes 2–6 mice per group from 2 experiments. (C) OT-I T cells adoptively were transferred into K14-mOVA chimeras and analyzed 2 days later. Data is representative of 3 chimeras per group from two experiments.
In contrast, OT-I T cell expansion was not observed in any of the K14-mOVA chimeras (Figure 4B). Nonetheless, antigen recognition was observed in all chimeras except the negative control (bm1>mOVA.bm1), as evidenced by upregulation of CD44 on OT-I T cells (Figure 4C). Thus both radioresistant and radiosensitive cells present antigen in the K14-mOVA model, but neither induce expansion of OT-I T cells.
Langerhans cells in presentation of K14 transgenic antigens
Langerhans cells are radioresistant dendritic cells that occupy the epidermis, and therefore reside in close proximity to the K14 transgene expressing keratinocytes. In radiation chimeras, LC remain of host origin for up to a year, despite total hematopoietic chimerism (22). Being radioresistant, LC were the presumed initiator of OT-I T cell expansion to K14-OVAp as well as the relevant radioresistant APC in K14-mOVA (20). We previously addressed the ability of LC to present the OVAp and mOVA antigens using an ear explant system (37). LC from skin of both K14-OVAp and K14-mOVA ears were able to stimulate OT-I T cell proliferation. Furthermore, E-cadherin+ DC (LC) isolated from the skin draining lymph nodes could stimulate OT-I in vitro (20).
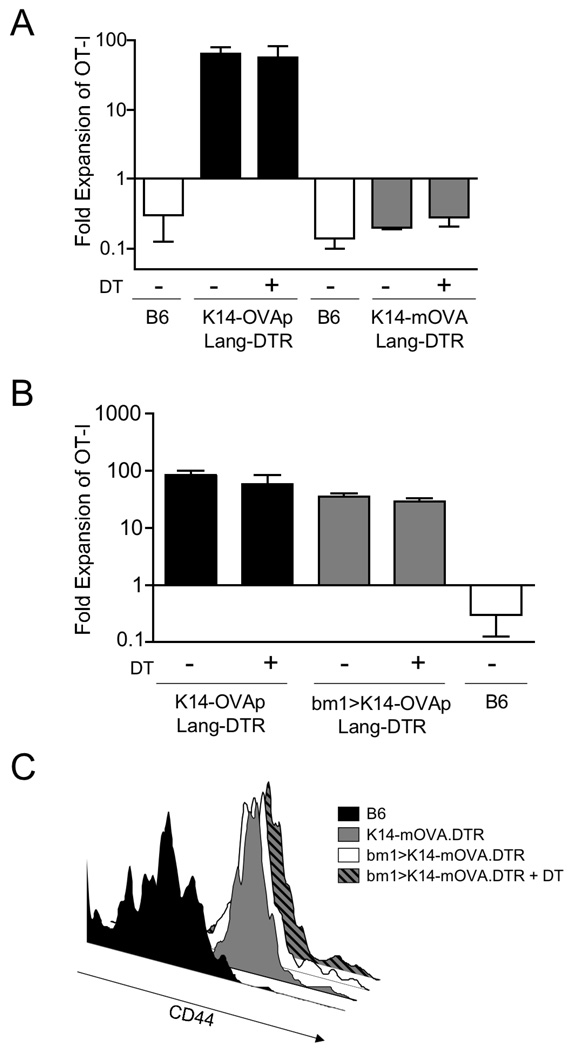
Since the publication of our initial analysis, inducibly LC deficient mice were developed, that allow for direct assessment of the role of LC in vivo (31). We crossed both K14-OVAp and K14-mOVA mice to Langerin-DTREGFP knockin mice that express the diphtheria toxin receptor within the endogenous mouse langerin locus. Diphtheria toxin treatment effectively ablates nearly 100% of LC in the epidermis (38) and this was confirmed in our hands (data not shown). Surprisingly, treatment of K14-OVAp Lang-DTR and K14-mOVA Lang DTR mice with diphtheria toxin prior to adoptive transfer of OT-I T cells did not affect OT-I expansion in K14-OVAp or K14-mOVA (Figure 5A). Combined, these results suggested that LC present OVA antigens in both strains, but are not the relevant APC for induction of the distinct expansion responses observed.
Figure 5. Langerhans cells can present K14 antigens, but are not required for activation in either K14-OVAp or K14-mOVA mice.
(A) Naïve OT-I T cells were adoptively transferred into K14-OVAp/Lang-DTR and K14-mOVA/Lang-DTR mice that had been treated with 1µg diphtheria toxin i.p. at day −4, −1 and +2 relative to adoptive transfer. Lymph nodes and spleen were harvested at day 6 and analyzed by flow cytometry. Depletion of LC did not affect expansion in K14-OVAp or K14-mOVA. Data shown include 3–6 mice per group from two experiments. (B–C) Bone marrow chimeras were made using the H-2Kbm1 bone marrow donors and K14-OVAp/Lang-DTR and K14-mOVA/Lang-DTR recipients. 1µg diphtheria toxin (DT) was administered i.p. on day −4, −1 and +2 relative to adoptive transfer of OT-I. (B) Naïve OT-I T cells were adoptively transferred into DT treated or control K14-OVAp/Lang-DTR mice and chimeras. Lymph nodes and spleen were harvested at day 6 and analyzed by flow cytometry. Data shown includes 3–6 mice per group in two experiments. (C) Naïve OT-I T cells were adoptively transferred into DT treated or control K14-mOVA/Lang-DTR mice and chimeras. Lymph nodes and spleen were harvested at day 3 and analyzed by flow cytometry. Histograms are representative of at least 4 mice per group from two experiments.
Radioresistant cells present K14 transgenic antigens and can induce activation and expansion of OT-I T cells in K14-OVAp. Although LC are a radioresistant APC and could present OVA, they are not required for antigen presentation or OT-I T cell expansion. However, it is possible that the timecourse of LC depletion did not affect LC or LC precursors that could be returning to the epidermis after depletion, and could then present the OVA antigen. So to further study the response in LC depleted hosts, we measured OT-I expansion in chimeras where the marrow was H-2K bm1 and the host was Lang-DTR. In this system, returning LC (which are bone marrow derived) would be H-2Kbm1 and unable to present the OVA antigen to OT-I T cells. Even under these conditions, OT-I T cells robustly expanded (Figure 5B). This is quite surprising, since almost all radioresistant DC in the skin (dermis and epidermis) express Langerin. Fewer than 2% of Langerin negative DC in the dermis are host derived (data not shown), consistent with the observation that skin migrating DC in the SDLN are predominantly bone marrow derived, except those that express Langerin (38). Thus, in the chimeras shown in Figure 5B, presentation by skin migratory DC is largely eliminated. Altogether, these data suggest that a radioresistant, non-skin derived DC drives the CD8 T cell response in these mice, which is quite robust at least in the case of peptide antigen.
Discussion
This study has provided characterization of the distinct responses that occur in K14-OVAp and K14-mOVA mice. Whereas OT-I T cells drive a pathogenic, sometimes lethal, autoimmune response in K14-OVAp mice, in K14-mOVA they undergo abortive activation and are eventually deleted. The response observed in this K14-mOVA model contrasts previous reports of autoimmunity in the Katz K14-mOVA mice (24). Differences in the transgenic construct may account for the different T cell responses observed in these models. The Katz K14-mOVA transgenic ovalbumin is localized to membranes by fusion to the platelet-derived growth factor receptor (PDGFR), as opposed to the transferrin receptor fusion protein used in these studies. These different membrane localization domains may result in different subcellular compartmentalization of the transgene. In addition, the effects of differences in level of expression or stability of the messenger RNA encoding the two antigens are unknown. Although these factors may be affecting the outcome in K14-mOVA transgenics, it is more likely that the experimental design of the adoptive transfer led to the disparate results. In the study by Katz and colleagues, large numbers of purified OT-I (5–10 × 106) were adoptively transferred into K14-mOVA mice. They were not further selected for naïve OT-I T cells by depletion of CD44high T cells. This is of primary importance as memory T cells respond immediately upon antigen recognition (39). The importance of this was directly demonstrated in a study using K5-mOVA mice (40). In K5-mOVA mice, naïve OT-I T cells underwent clonal deletion, but memory OT-I T cells expanded and induced autoimmunity, particularly at high doses (2–4 × 106). Therefore, memory phenotype OT-I T cells can expand and induce disease, regardless of peripheral tolerance mechanisms that would suppress activation of naïve T cells.
Our study also examined the role of differences that exist between K14-OVAp and K14-mOVA mouse models. We were unable to identify gross differences in which APC presentation antigen in these strains. LC present antigen in both strains, yet LC depletion did not impair or alter the outcome of T cell activation. It is possible that different levels of antigen are displayed by APC in K14-OVAp versus K14-mOVA. We attempted to study this using a specific antibody to the OVAp/Kb complex (41). However complex was not detectable on DC from either strain, even when gating on LC (data not shown), suggesting that the level of presentation in both strains is below the sensitivity of the antibody. Another key difference may be the presence of MHC class II epitopes for recognition by CD4+ T cells in K14-mOVA, but not K14-OVAp mice. This difference could be relevant because of the MHC class II restricted CD4+ Tregs, that might suppress the response in K14-mOVA. However, our data did not support a role for CD4+ T cell suppression of OT-I T cell expansion in K14-mOVA. Nonetheless, it did implicate a non-antigen-specific limitation of OT-I T cell responses in both models. We presume that this control may be mediated NKT cells that express CD4. In addition, OT-I T cells expanded in K14-mOVA / K14-OVAp double transgenic mice, which indicates that an antigen-specific Treg population, if it does exist in K14-mOVA mice, is not capable of suppressing the response in K14-OVAp. Finally, it was possible that LC were the predominant antigen presenting cell in K14-OVAp, but were unable to present the antigen in K14-mOVA, resulting in different T cell outcomes. To address this we used inducibly LC deficient mice and showed that although LC can present the OVA antigen in both K14-OVAp and K14-mOVA, they are not required for antigen presentation in either model. Ultimately, LC deficiency did not affect the robust expansion of OT-I T cells in K14-OVAp. Together these results suggest that other radioresistant antigen presenting cells are responsible for the dramatic activation of CD8 T cells in K14-OVAp mice.
Radioresistant cells that may play an important role in this response are the basal keratinocytes of stratified epithelia, which are known to express the human keratin-14 promoter. Initial antigen presentation in the lymph node would activate T cells and direct them to these tissues (such as the epidermis) where they would inflict tissue damage and release more antigen. The antigen release into the system could then perpetuate the productive T cell response and subsequent autoimmunity. We have shown here that the OT-I T cells do not need to acquire cytolytic activity to undergo the robust expansion observed in K14-OVAp mice. However, CD8+ T cells may still induce tissue damage in these sites via cytokine production (TNFα and IFNγ) (42) and killing of tissue cells that present antigen via ligation of death receptors such as Fas (43). While this is formally possible, it seems unlikely as treatment of K14-OVAp mice with an SIP agonist that blocks lymphocyte egress from lymph nodes did not diminish the OT-I expansion observed (data not shown). Additionally, we have observed that although the response in the lymphoid tissue of K14-OVAp can be distinguished from K14-mOVA as early as day 2 (Figure 1), OT-I T cells are not observed in the skin until day 6 (data not shown). This timecourse suggests that activated OT-I T cells do not need to traffic to the skin to induce the expansion observed, but we cannot exclude the possibility that there are undetectable numbers of OT-I T cells in the skin at early timepoints that mediate this response.
A remaining question is the identity of the radioresistant APC that drives expansion of low numbers naïve OT-I T cells in the K14-OVAp mouse model. This is of primary interest because of the apparent ability of this radioresistant population to induce a productive immune response to a self antigen, at least in peptide form. The activation of naïve T cell responses is generally thought to be mediated by hematopoietic professional APC, such as dendritic cells (44), as opposed to presentation by the tissue stroma, which results in T cell tolerance. It is possible that there is another radioresistant hematopoietic APC, in addition to LC, that can potently stimulate T cells, but has yet to be identified. Such cells might not be recoverable using traditional DC releasing digestion with collagenase D. Alternatively, recent reports of promiscuous expression of tissue-restricted antigens by lymph node stromal cells provide another possible antigen presenting cell population (45–47). In all of these models, peripheral tolerance was mediated by antigen expressed by radioresistant stromal cells in the lymph node. These cells were also recently shown to produce IL-7, a cytokine that is essential for T cell homeostasis (48). Thus lymph node stromal cells may be specialized for interaction with T cells.
Whether lymph node stromal cells present K14 derived antigens or not, we were unable to define differences in the APC that present antigen in K14-OVAp versus K14-mOVA mice. Thus it remains of interest to determine the mechanism behind the distinct responses observed. Based on the results in other models, one would predict that presentation of self-antigens by these fibroblastic reticular cells would result in T cell tolerance. The expansion of OT-I T cells observed in K14-OVAp mice would not fit this model. The use of a peptide mini-gene construct in K14-OVAp might allow higher levels of antigen presentation on rare radioresistant APC than in K14-mOVA mice. This together with limited antigen presentation on tolerogenic DC could result in the robust immune promoting properties observed.
This study ruled out an essential role for epidermal Langerhans cells in T cell expansion (in K14-OVAp mice) or tolerance (in K14-mOVA mice), using Lang-DTR mice. Lang-DTR mice have been used to study contact hypersensitivity, and acute LC depletion led to reduced CHS in at least one model (49, 50). However, we and others recently defined a novel population of dendritic cells in the dermis that also expresses a high level of Langerin (38, 51, 52). It is the dermal Langerin+ cells, not epidermal LC, that promote immune responses, both in the case of CHS (38), and epicutaneous immunization (53). Thus, the immune promoting effects of epidermal Langerhans cells remain to be demonstrated. In contrast, an immune suppressive role for epidermal Langerhans cells has been suggested from studies of CHS (54) and skin transplants (55). Overall, our work here is consistent with an emerging paradigm where unique DC subsets have the potential to drive distinct immune responses in the steady state.
Acknowledgements
The authors wish to thank Dan Kaplan, Liangchun Wang, and Steve Jameson for thoughtful discussion and advice, and XiaoJie Ding and Brian Goudy for excellent technical assistance.
Footnotes
Supported by NIH grants AI35296 and AI70380 to K.A.H.
Abbreviations used in this paper: DC, Dendritic cell; LC, Langerhans cell; EGFP, Enhanced Green Fluorescent Protein; DT, Diphtheria Toxin; DTR, Diphtheria Toxin Receptor
Disclosures: The authors declare no conflict of interest or financial interests
References
- 1.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 2.Kishimoto H, Sprent J. A defect in central tolerance in NOD mice. Nat Immunol. 2001;2:1025–1031. doi: 10.1038/ni726. [DOI] [PubMed] [Google Scholar]
- 3.Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–840. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 4.Zehn D, Bevan MJ. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 2006;25:261–270. doi: 10.1016/j.immuni.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohashi PS, Oehen S, Aichele P, Pircher H, Odermatt B, Herrera P, Higuchi Y, Buerki K, Hengartner H, Zinkernagel RM. Induction of diabetes is influenced by the infectious virus and local expression of MHC class I and tumor necrosis factor-alpha. J Immunol. 1993;150:5185–5194. [PubMed] [Google Scholar]
- 6.McGargill MA, Mayerova D, Stefanski HE, Koehn B, Parke EA, Jameson SC, Panoskaltsis-Mortari A, Hogquist KA. A spontaneous CD8 T cell-dependent autoimmune disease to an antigen expressed under the human keratin promoter. J Immunol. 2002;169:2141–2147. doi: 10.4049/jimmunol.169.4.2141. [DOI] [PubMed] [Google Scholar]
- 7.Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nature reviews. 2002;2:11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 8.Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 10.Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belz GT, Behrens GM, Smith CM, Miller JF, Jones C, Lejon K, Fathman CG, Mueller SN, Shortman K, Carbone FR, Heath WR. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196:1099–1104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pooley JL, Heath WR, Shortman K. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8− dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J Immunol. 2001;166:5327–5330. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- 15.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunological reviews. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 17.Wilson NS, El-Sukkari D, Belz GT, Smith CM, Steptoe RJ, Heath WR, Shortman K, Villadangos JA. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood. 2003;102:2187–2194. doi: 10.1182/blood-2003-02-0513. [DOI] [PubMed] [Google Scholar]
- 18.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Probst HC, Lagnel J, Kollias G, van den Broek M. Inducible transgenic mice reveal resting dendritic cells as potent inducers of CD8+ T cell tolerance. Immunity. 2003;18:713–720. doi: 10.1016/s1074-7613(03)00120-1. [DOI] [PubMed] [Google Scholar]
- 20.Mayerova D, Parke EA, Bursch LS, Odumade OA, Hogquist KA. Langerhans cells activate naive self-antigen-specific CD8 T cells in the steady state. Immunity. 2004;21:391–400. doi: 10.1016/j.immuni.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 21.Hemmi H, Yoshino M, Yamazaki H, Naito M, Iyoda T, Omatsu Y, Shimoyama S, Letterio JJ, Nakabayashi T, Tagaya H, Yamane T, Ogawa M, Nishikawa S, Ryoke K, Inaba K, Hayashi S, Kunisada T. Skin antigens in the steady state are trafficked to regional lymph nodes by transforming growth factor-beta1-dependent cells. Int Immunol. 2001;13:695–704. doi: 10.1093/intimm/13.5.695. [DOI] [PubMed] [Google Scholar]
- 22.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borkowski TA, Van Dyke BJ, Schwarzenberger K, McFarland VW, Farr AG, Udey MC. Expression of E-cadherin by murine dendritic cells: E-cadherin as a dendritic cell differentiation antigen characteristic of epidermal Langerhans cells and related cells. Eur J Immunol. 1994;24:2767–2774. doi: 10.1002/eji.1830241129. [DOI] [PubMed] [Google Scholar]
- 24.Shibaki A, Sato A, Vogel JC, Miyagawa F, Katz SI. Induction of GVHD-like skin disease by passively transferred CD8(+) T-cell receptor transgenic T cells into keratin 14-ovalbumin transgenic mice. J Invest Dermatol. 2004;123:109–115. doi: 10.1111/j.0022-202X.2004.22701.x. [DOI] [PubMed] [Google Scholar]
- 25.Azukizawa H, Kosaka H, Sano S, Heath WR, Takahashi I, Gao XH, Sumikawa Y, Okabe M, Yoshikawa K, Itami S. Induction of T-cell-mediated skin disease specific for antigen transgenically expressed in keratinocytes. Eur J Immunol. 2003;33:1879–1888. doi: 10.1002/eji.200323630. [DOI] [PubMed] [Google Scholar]
- 26.Stoitzner P, Tripp CH, Douillard P, Saeland S, Romani N. Migratory Langerhans cells in mouse lymph nodes in steady state and inflammation. J Invest Dermatol. 2005;125:116–125. doi: 10.1111/j.0022-202X.2005.23757.x. [DOI] [PubMed] [Google Scholar]
- 27.Mayerova D, Wang L, Bursch LS, Hogquist KA. Conditioning of Langerhans cells induced by a primary CD8 T cell response to self-antigen in vivo. J Immunol. 2006;176:4658–4665. doi: 10.4049/jimmunol.176.8.4658. [DOI] [PubMed] [Google Scholar]
- 28.Stefanski HE, Mayerova D, Jameson SC, Hogquist KA. A low affinity TCR ligand restores positive selection of CD8+ T cells in vivo. J Immunol. 2001;166:6602–6607. doi: 10.4049/jimmunol.166.11.6602. [DOI] [PubMed] [Google Scholar]
- 29.Teasdale RD, D'Agostaro G, Gleeson PA. The signal for Golgi retention of bovine beta 1,4-galactosyltransferase is in the transmembrane domain. J Biol Chem. 1992;267:13113. [PubMed] [Google Scholar]
- 30.Williams IR, Rawson EA, Manning L, Karaoli T, Rich BE, Kupper TS. IL-7 overexpression in transgenic mouse keratinocytes causes a lymphoproliferative skin disease dominated by intermediate TCR cells: evidence for a hierarchy in IL-7 responsiveness among cutaneous T cells. J Immunol. 1997;159:3044–3056. [PubMed] [Google Scholar]
- 31.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Kieper WC, Jameson SC. Homeostatic expansion and phenotypic conversion of naive T cells in response to self peptide/MHC ligands. Proc Natl Acad Sci U S A. 1999;96:13306–13311. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikolic-Zugic J, Bevan MJ. Thymocytes expressing CD8 differentiate into CD4+ cells following intrathymic injection. Proc Natl Acad Sci U S A. 1988;85:8633–8637. doi: 10.1073/pnas.85.22.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu L, Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borkowski TA, Letterio JJ, Mackall CL, Saitoh A, Wang XJ, Roop DR, Gress RE, Udey MC. A role for TGFbeta1 in langerhans cell biology. Further characterization of the epidermal Langerhans cell defect in TGFbeta1 null mice. J Clin Invest. 1997;100:575–581. doi: 10.1172/JCI119567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch L, Ronchese F, Romani N. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci U S A. 2006;103:7783–7788. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annual review of immunology. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 40.Waithman J, Gebhardt T, Davey GM, Heath WR, Carbone FR. Cutting edge: Enhanced IL-2 signaling can convert self-specific T cell response from tolerance to autoimmunity. J Immunol. 2008;180:5789–5793. doi: 10.4049/jimmunol.180.9.5789. [DOI] [PubMed] [Google Scholar]
- 41.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 42.Rutigliano JA, Graham BS. Prolonged production of TNF-alpha exacerbates illness during respiratory syncytial virus infection. J Immunol. 2004;173:3408–3417. doi: 10.4049/jimmunol.173.5.3408. [DOI] [PubMed] [Google Scholar]
- 43.Allison J, Thomas HE, Catterall T, Kay TW, Strasser A. Transgenic expression of dominant-negative Fas-associated death domain protein in beta cells protects against Fas ligand-induced apoptosis and reduces spontaneous diabetes in nonobese diabetic mice. J Immunol. 2005;175:293–301. doi: 10.4049/jimmunol.175.1.293. [DOI] [PubMed] [Google Scholar]
- 44.Jung S, Strotmann R, Schultz G, Plant TD. TRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cells. American journal of physiology. 2002;282:C347–C359. doi: 10.1152/ajpcell.00283.2001. [DOI] [PubMed] [Google Scholar]
- 45.Lee JW, Epardaud M, Sun J, Becker JE, Cheng AC, Yonekura AR, Heath JK, Turley SJ. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 46.Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J Immunol. 2007;179:993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- 47.Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, Johannes KP, Su MA, Chang HY, Krummel MF, Anderson MS. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 49.Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, Clausen BE. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bennett CL, Noordegraaf M, Martina CA, Clausen BE. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J Immunol. 2007;179:6830–6835. doi: 10.4049/jimmunol.179.10.6830. [DOI] [PubMed] [Google Scholar]
- 51.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, Ochando J, Kissenpfennig A, Malissen B, Grisotto M, Snoeck H, Randolph G, Merad M. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L, Bursch LS, Kissenpfennig A, Malissen B, Jameson SC, Hogquist KA. Langerin expressing cells promote skin immune responses under defined conditions. J Immunol. 2008;180:4722–4727. doi: 10.4049/jimmunol.180.7.4722. [DOI] [PubMed] [Google Scholar]
- 54.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 55.Obhrai JS, Oberbarnscheidt M, Zhang N, Mueller DL, Shlomchik WD, Lakkis FG, Shlomchik MJ, Kaplan DH. Langerhans cells are not required for efficient skin graft rejection. J Invest Dermatol. 2008;128:1950–1955. doi: 10.1038/jid.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]