Abstract
Objective. To determine the features predictive of atherosclerotic cardiovascular damage in patients with SLE.
Methods. SLE LUMINA (LUpus in MInorities: NAture vs nurture) patients (n = 637), aged ⩾16 years, disease duration ⩽5 years at baseline (T0), of African–American, Hispanic and Caucasian ethnicity were studied. Atherosclerotic cardiovascular damage was defined by the following items of the SLICC Damage Index (SDI) cardiovascular domain: angina or coronary artery by pass surgery, myocardial infarction and/or congestive heart failure; factors associated with its occurrence were examined by univariable and multivariable regression analyses.
Results. Forty-three (6.8%) of 637 patients developed cardiovascular damage over a mean ± s.d. total disease duration of 6.6 ± 3.6 years. Nearly 90% of the patients were women with a mean ± s.d. age of 36.5 (12.6) years; all ethnic groups were represented. By multivariable analyses, after adjusting for the cardiovascular manifestations present, age [odds ratio (OR) = 1.06; 95% CI 1.03, 1.09], male gender (OR = 3.57; 95% CI 1.35, 9.09) SDI at baseline (OR = 1.28; 95% CI 1.09, 1.50) and CRP levels [highest tertile (OR = 2.63; 95% CI 1.17, 5.91)] were associated with the occurrence of cardiovascular damage, whereas the number of years of education was negatively associated with such outcome (OR = 0.85; 95% CI 0.74, 0.94).
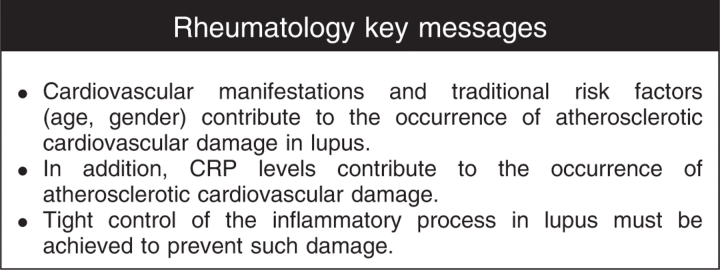
Conclusions. Our data suggest that atherosclerotic cardiovascular damage in SLE is multifactorial; traditional (age, gender) and disease-related factors (CRP levels, SDI at baseline) appear to be important contributors to such an occurrence. Tight control of the inflammatory process must be achieved to prevent it.
Keywords: Lupus, Cardiovascular, Atherosclerosis, Damage, Ethnicity, C-reactive protein
Introduction
The bimodal pattern of mortality in SLE with an early peak due to active disease and infections and a late one due to atherosclerotic heart disease was first recognized by Urowitz et al. [1] >30 years ago. Whereas the impact of infections and active disease on mortality has diminished dramatically over the years, this has not been the case for atherosclerotic heart disease [2]. One of the most remarkable characteristics of cardiovascular involvement in SLE is the occurrence of myocardial infarction in young women as reported by Manzi et al. [3] 12 years ago. In addition, fatal myocardial infarction has been described to occur three times more frequently in patients with SLE than in age- and gender-matched control subjects [4].
Traditional risk factors such as those described in the Framingham study (diabetes, hypertension, tobacco use, hyperlipidaemia and sedentary lifestyle) are common in SLE patients [5], but they alone fail to explain the increased frequency of cardiovascular disease in SLE patients [6]. In fact, Rahman et al. [7] have shown that non-lupus patients with coronary heart disease (CHD) have a higher mean number of individual risk factors compared to SLE patients. Searching for additional predictors of vascular events, we have previously found longer disease duration, elevated CRP levels and the presence of aPL to be associated with the occurrence of these events in patients from LUMINA (LUpus in MInorities, NAture vs nurture), a multiethnic lupus cohort [8].
Some specific items of the cardiovascular domain of the damage index [as measured by the SLICC Damage Index (SDI)] [9, 10] have been examined before (e.g. coronary artery disease) and found to be associated with the use of glucocorticoids [11, 12]. We have now expanded these observations to include all items in the cardiovascular damage domain that could be, to some extent, associated with atherosclerotic heart disease. We hypothesized that such damage in the cardiovascular system will be associated not only with preceding cardiovascular manifestations, but also with disease activity in general; furthermore, we also hypothesized that the deleterious effect of glucocorticoids will be evident and that medications such as HCQ may, in contrast, be protective.
Patients and methods
Patients
As has been previously reported, LUMINA is a longitudinal study of outcome in patients with SLE from three different ethnic groups residing in the USA [13]. This cohort was established in 1994 as a collaborative effort between the University of Alabama at Birmingham (UAB), the University of Texas Health Science Center at Houston (UTH) and the University of Puerto Rico Medical Sciences Campus (UPR) in order to elucidate the underlying causes of the discrepant outcomes observed in SLE patients from different ethnic groups. The LUMINA study has been approved by the Institutional Review Board of the participating institutions according to the Declaration of Helsinki for research in humans.
The LUMINA cohort is comprised of patients of Hispanic (from Texas and Puerto Rico), African–American and Caucasian background who meet at least four of the updated and revised ACR criteria for SLE [14, 15], are ⩾16 years of age and have a disease duration of ⩽5 years. Each LUMINA patient has a baseline or enrolment visit (T0); follow-up visits are conducted every 6 months for the first year (T0.5 and T1, respectively), and yearly thereafter. At each visit, every patient completes an interview, a physical examination and laboratory tests. Additional clinical information covering the period between visits as well as data for missed study visits are obtained, whenever possible, by review of all available medical records.
Disease duration was defined as the period covering the interval between diagnosis (TD) and T0, whereas duration of follow-up was defined as the period between T0 and the last visit (TL). TL for those patients who had developed cardiovascular damage was truncated at the time it first occurred, whereas for those who did not develop cardiovascular damage, TL was the time of their last visit.
Variables
The LUMINA database includes variables from the following domains: socio-economic–demographic, clinical, immunological, genetic and behavioural and psychological [16]. All variables with the exception of the antibody and genetic data (only obtained at T0) were ascertained at T0 and at every subsequent visit. Only the variables included in this study will be described.
Cumulative atherosclerotic cardiovascular damage, our primary end-point, was defined as per the following items of the corresponding domain of the SDI [9, 10] if one or more of these manifestations lasting at least 6 months occurred: (i) angina or coronary artery bypass surgery, (ii) heart failure and (iii) myocardial infarction. Of note, as per the SDI scoring instructions, myocardial infarction was included regardless of duration; a score of 2 is given if a second myocardial infarction >6 months apart occurs. According to the SDI, these manifestations are clinically defined rather than depending on extensive ancillary work up.
The socio-economic–demographic domain variables included were age, gender, ethnicity, education, poverty (as defined by the US federal government adjusted for the number of subjects in the household) [17], health-related behaviours (smoking and drinking, both defined categorically per self-report), marital status and health insurance. With the exception of health insurance, which was obtained either at TD or T0, all other variables were obtained at T0.
Variables from the clinical domain were the number of ACR criteria at T0, onset type [acute (accrual of four ACR criteria within a month) vs insidious, if otherwise], follow-up time (T0–TL, as defined), disease manifestations, disease activity and damage, immunological variables and medications. Cumulative clinical manifestations were examined as per the ACR classification criteria for SLE [14, 15]. Other manifestations considered to be important were also recorded; they include cardiopulmonary manifestations, independent of their duration (endocarditis, mitral valve prolapse, angina, conduction defects, cardiomyopathy, congestive heart failure, hypertension, pneumonitis, pulmonary haemorrhage, pulmonary hypertension and interstitial lung disease), obesity (per BMI ⩾30 kg/m2), claudication, cerebrovascular accidents and thrombotic events. The definitions used for all these manifestations were all clinically based as per our established glossary.
Comorbidities included were diabetes mellitus [fasting plasma glucose ⩾126 mg/dl and/or random glucose (or 2-h value in a oral glucose tolerance test) ⩾200 mg/dl, physician diagnosed and/or patient self-reported intake of pharmacological treatment (insulin and/or oral hypoglycaemic agent)], hypertension, regardless of cause defined as systolic blood pressure ⩾140 mmHg and/or a diastolic blood pressure ⩾90 mmHg on two or more occasions and/or patient self-reported intake of anti-hypertensive medications; if agents are taken for proteinuria [angiotensin-converting enzyme (ACE) inhibitors or ACE receptor blockers], and/or for RP; hypertension is not recorded as present.
Laboratory variables included were non-fasting serum lipoproteins [total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides and low-density lipoprotein (LDL) cholesterol calculated using the Friedewald formula] and serum CRP, measured as high-sensitivity CRP (hs-CRP; high >9 mg/l or the highest tertile for the distribution of our patients’ values) by immunometric assay (Immulite 2000; Diagnostic Products, Los Angeles, CA, USA) using patients' sera obtained at T0.
Disease activity was assessed using the SLAM-revised (SLAM-R) [18, 19] at all visits; for this study the SLAM-R score at TD was included. Overall damage was ascertained with the SDI [9, 10] at T0 and at every subsequent visit; for this study the SDI score at T0 was considered, excluding the items for the cardiovascular domain. The SDI documents cumulative and irreversible damage in 12 different organ systems regardless of its cause (disease activity, medications or intercurrent illnesses). To be scored, each manifestation must be present at least 6 months unless otherwise noted in the instructions accompanying this instrument, as already noted for myocardial infarction. The total SDI score is the sum of these items with a maximum possible score of 46. For the purpose of these analyses, all cardiovascular domain items were excluded from the SDI.
The following autoantibodies were obtained: ANA (by IF using HEp-2 cell line), anti-dsDNA [by IF against Crithidia luciliae (normal less than 1 : 10)], anti-Smith, anti-RNP, anti-La and anti-Ro (by CIE against human spleen and calf thymus extract) [20, 21], IgG and IgM aPL (abnormal >13 GPL U/ml and/or >13 MPL U/ml, by ELISA technique) [22] and the LAC (Staclot Test Diagnostica Stago 92600, Asnières-Sur-Seine, France) [23]. Patients were considered to be aPL positive if they exhibited abnormal levels of IgM and/or IgG aPL antibodies (>13 U/ml GPL and/or >13 IgM U/ml MPL, respectively) and/or LAC positivity. All antibodies were obtained at T0 except for LAC and anti-DNA, which were assessed at each visit.
Medication variables included were the cumulative exposure to HCQ, low-dose aspirin, warfarin, anti-platelets (clopidogrel, ticlopidine and dipyridamole), ACE inhibitors, statins, cyclophosphamide, AZA, mycophenolate mofetil and glucocorticoids (as prednisone equivalent). Glucocorticoid exposure (oral) was estimated either as a continuous variable [(weighted average glucocorticoid dose from TD to TL) calculated by multiplying the dose for each individual visit by the number of months in the interval between visits divided by the total follow-up time] or as a categorical variable [prednisone dose: low (<10 mg/day), medium (10–30 mg/day) and high (>30 mg/day)].
Statistical analyses
All variables described above were examined as a function of the presence of atherosclerotic cardiovascular damage using descriptive statistical tests, Student's t-tests and χ2 tests for continuous and categorical variables, respectively. Variables with a P ⩽ 0.10 in these analyses were entered into a multivariable logistic regression model. To improve the precision of the estimates a parsimonious or reduced model was done until no further variables were significant. Results are presented as odds ratios (ORs) with their corresponding 95% CIs. Statistical significance was defined as a P-value ⩽0.05. Analyses were performed using either SAS, version 9.1 (SAS Institute, Cary, NC, USA) or SPSS, version 15.0 (Chicago, IL, USA).
Results
At the time this study was conducted, the LUMINA cohort was constituted by a total of 637 patients, of whom 43 (6.8%) had developed one or more of the cardiovascular damage domain items being examined. As expected, patients were predominantly women (89.8%) of middle age (mean ± s.d. 36.5 ± 12.6 years). All ethnic groups were represented: there were 118 (18.5%) Texan Hispanics, 102 (16.0%) Puerto Rican Hispanics, 236 (37.1%) African–Americans and 181 (28.4%) Caucasians. The proportion of patients with cardiovascular damage, as defined, among these ethnic groups were 6.8, 1.0, 7.6 and 8.8%, respectively (P = 0.047). The mean age ± s.d. for patients who developed cardiovascular damage was 48.1 ± 15.7 years, whereas their disease duration at TL was 6.6 ± 3.6 years. Within the different items of the cardiovascular domain of the SDI, heart failure alone occurred in 42.0%, angina or coronary artery bypass surgery alone in 25.4% and myocardial infarction alone in 14.0%; heart failure and myocardial infarction occurred in 4.6% and heart failure and angina or coronary artery by pass surgery in 14.0%.
Univariable analyses
Socio-economic–demographic variables associated with the occurrence of cardiovascular damage were age and male gender. Although cardiovascular damage did not uniformly occur among the different ethnic groups, statistical significance was not reached. Smoking was more frequent in the patients who had developed cardiovascular damage; these patients had, on the average, fewer years of education than those who had not developed cardiovascular damage. These data are shown in Table 1. All other socio-economic–demographic variables examined were not significant in these analyses (data not shown).
Table 1.
Socio-economic–demographic variables and cardiovascular damage in LUMINA (patients by univariable analyses)a
| Cardiovascular damage |
|||
|---|---|---|---|
| Variable | Yes (n = 43) | No (n = 594) | P-value |
| Age, mean ± s.d., years | 48.1 ± 15.7 | 35.6 ± 12.0 | <0.0001 |
| Gender (male), % | 25.5 | 9.1 | 0.0006 |
| Ethnicity, % | |||
| Texan Hispanic | 18.6 | 18.5 | |
| Puerto Rican Hispanic | 2.3 | 17.0 | 0.0739 |
| African–American | 41.9 | 36.7 | |
| Caucasian | 37.2 | 27.8 | |
| Education, mean ± s.d., years | 11.2 ± 3.7 | 13.1 ± 3.0 | 0.0018 |
| Povertya, % | 39 | 33.2 | 0.4423 |
| Smoking, % | 23.8 | 12.9 | 0.0422 |
| Drinking, % | 7.1 | 10.2 | 0.5229 |
aAs per the US federal government guidelines, adjusted for the number of persons in the household.
Within the clinical features, serositis (pleuritis and/or pericarditis), cardiovascular (valvular heart disease, angina, conduction defects, cardiomyopathy, congestive heart failure and hypertension), renal (histopathological data included) and pulmonary manifestations (pneumonitis, pulmonary haemorrhage, interstitial lung disease and pulmonary arterial hypertension) were more frequent among patients who developed cardiovascular damage than among those who did not. Diabetes, claudication, cerebrovascular accidents, venous thrombosis and all other clinical variables examined were not found to be significant in these analyses. TD SLAM-R and T0 SDI (excluding the cardiovascular domain items) scores were higher among patients who developed cardiovascular damage, but the differences were statistically significant only for the SDI.
From the laboratory features, high levels of CRP were more frequent in patients who developed cardiovascular damage than among those who did not (Table 2). Neither of the autoantibodies nor the lipid profile variables examined were found to be associated with the occurrence of cardiovascular damage.
Table 2.
Clinical variables and cardiovascular damage in LUMINA patients by univariable analyses
| Cardiovascular damage |
|||
|---|---|---|---|
| Variable | Yes (n = 43) | No (n = 594) | P-value |
| Acute onset type, % | 9.3 | 15.3 | 0.2848 |
| Disease duration, mean ± s.d., years | 6.6 ± 3.8 | 5.8 ± 3.6 | 0.1944 |
| BMI, mean ± s.d. | 33.2 ± 18.8 | 28.4 ± 7.7 | 0.1248 |
| Cumulative disease manifestations, % | |||
| Malar rash | 51.2 | 62.2 | 0.1541 |
| Photosensitivity | 67.4 | 65.8 | 0.829 |
| Discoid rash | 14 | 17.2 | 0.5871 |
| Oral ulcers | 67.4 | 57.6 | 0.2052 |
| Arthritis | 81.4 | 79.6 | 0.7808 |
| Serositis | 69.8 | 49 | 0.0085 |
| Neurological disorder | 14 | 12.3 | 0.7492 |
| Renal disorder | 41.9 | 38.6 | 0.6672 |
| Renal disorder (histopathological data included) | 72.1 | 49.8 | 0.0048 |
| Haematological disorder | 86.1 | 80.1 | 0.3444 |
| Immunological disorder | 100 | 93.8 | 0.4741 |
| Cardiovascular manifestations, % | |||
| Valvular heart disease | 39.5 | 6.6 | <0.0001 |
| Angina | 23.3 | 0.7 | <0.0001 |
| Conduction defects | 16.3 | 3.2 | <0.0001 |
| Cardiomyopathy | 44.2 | 3.5 | <0.0001 |
| Congestive heart failure | 51.2 | 6.2 | <0.0001 |
| Hypertension | 81.4 | 57.1 | 0.0018 |
| Pulmonary manifestations, % | |||
| Pneumonitis | 23.3 | 11.6 | 0.0253 |
| Pulmonary haemorrhage | 11.6 | 2.2 | 0.0003 |
| Pulmonary arterial hypertension | 9.3 | 2.2 | 0.0052 |
| Interstitial lung disease | 14 | 6.7 | 0.0749 |
| Claudication | 0 | 1.2 | 0.4741 |
| Venous thrombosis | 14 | 8.9 | 0.2718 |
| Cerebrovascular accident | 2.3 | 3.2 | 0.7512 |
| Diabetes | 7 | 4.7 | 0.5059 |
| Medications, % | |||
| HCQ | 88.4 | 85.4 | 0.5866 |
| Cyclophosphamide | 32.6 | 26 | 0.3475 |
| AZA | 37.2 | 29.5 | 0.2876 |
| Mycophenolate mofetil | 5.7 | 10.8 | 0.342 |
| Low-dose aspirin | 39.5 | 22.7 | 0.0125 |
| Statins | 27.9 | 14 | 0.0133 |
| Warfarin | 23.3 | 8.9 | 0.0024 |
| Anti-platelets | 16.3 | 2.4 | <0.0001 |
| ACE inhibitors | 58.1 | 40.2 | 0.0214 |
| Weighted glucocorticoid, mean ± s.d. (as mg of prednisone) | 11.0 ± 13.6 | 8.9 ± 11.8 | 0.2659 |
| Glucocorticoids, as mg of prednisone | |||
| <10 | 46.5 | 40.4 | |
| 10–30 | 27.9 | 35.9 | 0.5651 |
| >30 | 25.6 | 23.7 | |
| SLAM-R, mean ± s.d. [at diagnosis (TD)] | 12.1 ± 5.6 | 10.9 ± 5.9 | 0.0794 |
| SDI, mean ± s.d. [at the baseline visit (T0)] | 3.9 ± 2.5 | 1.6 ± 2.0 | <0.0001 |
| CRP tertiles | |||
| Lower third | 26.2 | 34.3 | |
| Middle third | 14.3 | 34.2 | 0.0007 |
| Higher third | 59.5 | 31.6 | |
| ACR, mean ± s.d. [criteria number (TD)] | 4.5 ± 0.9 | 4.5 ± 0.8 | 0.5196 |
| ACR, mean ± s.d. [criteria number (T0)] | 5.7 ± 1.6 | 5.5 ± 1.3 | 0.3439 |
| Lipid profile | |||
| Cholesterol, mean ± s.d., mg | 175.8 ± 57.1 | 175.5 ± 64.9 | 0.9754 |
| Triglycerides, mean ± s.d., mg | 156.6 ± 74.3 | 143.5 ± 88.3 | 0.3522 |
| LDL cholesterol, mean ± s.d., mg | 107.4 ± 51.5 | 108.6 ± 55.1 | 0.8897 |
| HDL cholesterol, mean ± s.d., mg | 35.9 ± 16.6 | 37.3 ± 17.3 | 0.6199 |
As to the therapeutic variables, low-dose aspirin, anti-platelets, warfarin, statins and ACE inhibitors were more frequently used in those patients who developed cardiovascular damage but no significant differences were observed for glucocorticoids, cyclophosphamide, AZA, mycophenolate mofetil and HCQ. These data are also shown in Table 2.
Multivariable analyses
After adjusting for the cardiovascular manifestations significant in the univariable analyses (conduction defects, congestive heart failure, valvular disease, cardiomyopathy and angina), the following variables were significantly associated with cardiovascular damage in the reduced logistic regression model: age (OR = 1.06; 95% CI 1.03, 1.09), male gender (OR = 3.57; 95% CI 1.35, 9.09), CRP levels [highest tertile (OR = 2.63; 95% CI 1.17, 5.91)] and disease damage (excluding cardiovascular domain items) (OR = 1.28; 95% CI 1.09, 1.50), whereas the number of years of education (OR = 0.84; 95% CI 0.74, 0.94) was negatively associated with the occurrence of cardiovascular damage. Pulmonary haemorrhage was of borderline statistical significance (OR = 5.02; 95% CI 0.96, 26.15). Given that none of the medications of interest (glucocorticoids and HCQ) were significant in the univariable analyses, all other medications significant in the univariable analyses were not included in the multivariable model presented, as their association was probably directly related to the outcome being examined. These data are depicted in Table 3.
Table 3.
Variables associated with the occurrence of cardiovascular damage in LUMINA (patients by multivariable logistic regression analyses)a
| Full model |
Reduced model |
|||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Age, years | 1.07 | 1.04, 1.11 | <0.0001 | 1.06 | 1.03, 1.09 | <0.0001 |
| Gender (male) | 3.23 | 1.07, 9.09 | 0.0349 | 3.57 | 1.35, 9.09 | 0.0098 |
| Ethnicity | 0.97 | 0.62, 1.51 | 0.8912 | |||
| Education, years | 0.85 | 0.74, 0.97 | 0.0178 | 0.84 | 0.74, 0.94 | 0.0035 |
| Smoking | 2.78 | 0.98, 7.88 | 0.0522 | |||
| Serositis | 1.37 | 0.52, 3.62 | 0.5199 | |||
| Interstitial lung disease | 0.28 | 0.04, 1.80 | 0.1778 | |||
| Pneumonitis | 1.32 | 0.42, 4.09 | 6.349 | |||
| Pulmonary hypertension | 2.69 | 0.37, 19.50 | 0.3274 | |||
| Pulmonary haemorrhage | 5.48 | 0.82, 36.65 | 0.0791 | 5.02 | 0.96, 26.15 | 0.0555 |
| Hypertension | 2.08 | 0.68, 6.49 | 0.2067 | |||
| Renal disorderb | 1.27 | 0.46, 3.53 | 0.6509 | |||
| SLAM-R at diagnosis | 1.01 | 0.94, 1.09 | 0.7543 | |||
| SDI at baseline | 1.23 | 1.03, 1.48 | 0.0264 | 1.28 | 1.09, 1.50 | 0.003 |
| CRP tertiles | ||||||
| Low | Reference group |
|||||
| Medium | 0.36 | 0.09, 1.36 | 0.1302 | |||
| High | 1.89 | 0.69, 5.16 | 0.2172 | 2.63 | 1.17, 5.91 | 0.0197 |
aAdjusted for cardiovascular manifestations, as noted in the text. bHistopathological data included.
Discussion
We have now examined the factors associated with the occurrence of primary atherosclerotic cardiovascular damage in a large multiethnic cohort. The overall rate of cardiovascular damage as defined at a mean follow-up of 6.6 years was 6.8% in our cohort, an intermediate rate when compared with data from the published literature, 5.0–16.4% albeit at 10 years of disease duration [24, 25]; however, it should be noted that we have used a more restrictive definition of cardiovascular damage than other authors. These discrepant rates probably relate to differences in the ethnic composition, geographic distribution, age and duration of follow-up of the patients studied as well as the analytical methods used in the published studies and the study being reported.
After adjusting for the presence of cardiovascular manifestations, male gender, older age, damage at baseline and high CRP levels were found to be associated with the occurrence of cardiovascular damage, as defined. In contrast, the number of years of education was negatively associated with such occurrence. Some traditional risk factors associated with coronary heart disease such as smoking, obesity, hypertension and diabetes as well as aPL antibodies were not found to be significant in these analyses for which a clear explanation does not emerge (see below).
Elevated serum level of CRP, an independent and powerful marker of cardiovascular events in the general population [26–30] as well as in SLE patients, has been described to be associated with overall damage by us and others [8, 31–33]. However, in the study by Lee et al. [33], the specific association with the cardiovascular domain of the damage index was not found to be significant. CRP levels are, by and large, not particularly elevated in patients with SLE. Nevertheless, we have previously reported a modest association between CRP levels and the cardiovascular domain of the SDI, but adjusted analyses taking into account other possible confounders were not conducted at that time. Given that CRP plays a pivotal role in the development of thrombosis and atherogenesis as demonstrated in laboratory animals [34, 35] and, as suggested by data from the general population, elevated CRP levels in the SLE patient may indicate an increased risk for atherosclerotic cardiovascular damage; these findings had obvious implications for patient management. Although disease activity was not found to be associated with the cardiovascular damage domain items examined, damage which is importantly affected by disease activity was found to be associated. Nevertheless, our findings relative to CRP strongly support the role of inflammation in the occurrence of atherosclerotic cardiovascular damage in lupus, which is consistent with the data from the general population. Within our existent database elements, we failed to identify any other marker of atherosclerotic cardiovascular damage that could be regarded as being lupus-specific [36], and other markers which have recently been identified at the population level and for some of them in patients with SLE as well, such as CD40 ligand, soluble interadhesion molecule 1 (s-1CAM1), pregnancy-associated plasma protein and soluble VEGF receptor (sFlt-1) [37–40].
Age and male gender are well-recognized risk factors for cardiovascular disease and our data corroborate these findings; furthermore, lupus has been found to be more severe in men than in women [41, 42] and late-onset lupus has been associated with damage accrual [43]; in addition, age has been associated with increasing rates of coronary calcifications, although they tend to occur at a younger age in lupus than in non-lupus patients [44].
We are somewhat puzzled by our inability to corroborate the previous effect of glucocorticoids in the cardiovascular system as well as to substantiate our hypothesis about the protective effect of HCQ in relation to such occurrence. Nevertheless, the published data to date, by and large, support the judicious use of glucocorticoids, while at the same time the liberal use of anti-malarials is being recommended [11,12,45–50].
Our study is not without limitations. First, our end point may not have been as narrow as needed to explore atherosclerotic cardiovascular damage given that we included heart failure; however, one of the most frequent, if not the most frequent cause of congestive heart failure is coronary heart disease [51] and thus we felt it was reasonable to include it; furthermore, exploring each individual item of the cardiovascular domain of the SDI was not possible given the relatively small number of individual events. Secondly, hyperhomocysteinaemia, insulin resistance and the presence of the metabolic syndrome were not included in our analyses since they are not elements of the LUMINA database; thus, we were unable to ascertain their possible contribution to the development of cardiovascular damage in our patients. Thirdly, Puerto Rican Hispanics have been followed up a shorter time than patients in the other ethnic groups and thus their contribution to the end point examined may not have been yet fully realized. Finally, aPL antibodies, which are considered of importance in SLE patients with cardiovascular disease, were ascertained only at T0 (with the exception of LAC), which may partially account for their lack of association with the cardiovascular damage items examined in our study.
Our data clearly suggest that rapid and energetic control of the inflammatory process in lupus patients is necessary to prevent the occurrence of cardiovascular damage given that elevated CRP levels are associated with its occurrence. Preventing damage in the cardiovascular system may exert a favourable impact on the long-term outcome of the disease.
Acknowledgements
The authors would like to acknowledge all LUMINA patients without whom this study would have not been possible, our supporting staff (Jigna Liu and Ellen Sowell at UAB, Carmine Pinilla-Díaz at UPR and Robert Sandoval at UTH) for their efforts in securing our patients’ follow-up and performing other LUMINA-related tasks and Ms Maria Tyson for her expert assistance in the preparation of this manuscript. We also gratefully acknowledge Gene V. Ball for his most helpful comments to an earlier version of this manuscript.
Funding: Supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases P01 AR49084, General Clinical Research Centers M01-RR02558 (UTH) and M01-RR00032 (UAB) and from the National Center for Research Resources (NCRR/NIH) RCMI Clinical Research Infrastructure Initiative (RCRII) 1P20RR11126 (UPR) and by the STELLAR (Supporting Training Efforts in Lupus for Latin American Rheumatologists) Program funded by Rheuminations, Inc. (UAB). The work of L.A.G. was also supported by Universidad de Antioquía, Medellín, Colombia.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Urowitz MB, Bookman AA, Koehler BE, Gordon DA, Smythe HA, Ogryzlo MA. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med. 1976;60:221–5. doi: 10.1016/0002-9343(76)90431-9. [DOI] [PubMed] [Google Scholar]
- 2.Bernatsky S, Boivin JF, Joseph L, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2550–7. doi: 10.1002/art.21955. [DOI] [PubMed] [Google Scholar]
- 3.Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham study. Am J Epidemiol. 1997;145:408–15. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 4.Rosner S, Ginzler EM, Diamond HS, et al. A multicenter study of outcome in systemic lupus erythematosus. II. Causes of death. Arthritis Rheum. 1982;25:612–17. doi: 10.1002/art.1780250602. [DOI] [PubMed] [Google Scholar]
- 5.Petri M, Spence D, Bone LR, Hochberg MC. Coronary artery disease risk factors in the Johns Hopkins Lupus Cohort: prevalence, recognition by patients, and preventive practices. Medicine (Baltimore) 1992;71:291–302. doi: 10.1097/00005792-199209000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–7. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Rahman P, Urowitz MB, Gladman DD, Bruce IN, Genest J., Jr Contribution of traditional risk factors to coronary artery disease in patients with systemic lupus erythematosus. J Rheumatol. 1999;26:2363–8. [PubMed] [Google Scholar]
- 8.Toloza SMA, Uribe AG, McGwin G, Jr, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA) XXIII: baseline predictors of vascular events. Arthritis Rheum. 2004;50:3947–57. doi: 10.1002/art.20622. [DOI] [PubMed] [Google Scholar]
- 9.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the systemic lupus international collaborating clinics/American College of Rheumatology Damage Index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–9. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 10.Gladman DD, Urowitz MB, Goldsmith CH, et al. The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:809–13. doi: 10.1002/art.1780400506. [DOI] [PubMed] [Google Scholar]
- 11.Zonana-Nacach A, Barr SG, Magder LS, Petri M. Damage in systemic lupus erythematosus and its association with corticosteroids. Arthritis Rheum. 2000;43:1801–8. doi: 10.1002/1529-0131(200008)43:8<1801::AID-ANR16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 12.Karp I, Abrahamowicz M, Fortin PR, et al. Recent corticosteroid use and recent disease activity: independent determinants of coronary heart disease risk factors in systemic lupus erythematosus? Arthritis Rheum. 2008;59:169–75. doi: 10.1002/art.23352. [DOI] [PubMed] [Google Scholar]
- 13.Alarcon GS, Friedman AW, Straaton KV, et al. Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. LUpus in MInority populations: NAture vs. Nurture. Lupus. 1999;8:197–209. doi: 10.1191/096120399678847704. [DOI] [PubMed] [Google Scholar]
- 14.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 16.Reveille JD, Moulds JM, Ahn C, et al. Systemic lupus erythematosus in three ethnic groups: I. The effects of HLA class II, C4, and CR1 alleles, socioeconomic factors, and ethnicity at disease onset. LUMINA Study Group. Lupus in minority populations, Nature versus Nurture. Arthritis Rheum. 1998;41:1161–72. doi: 10.1002/1529-0131(199807)41:7<1161::AID-ART4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 17.Commerce USDepartment of, Bureau of the Census. Series P-23, No. 28 and Series P-60, No. 68 and subsequent years. Housing and Household Economic Statistics Division: Washington, DC; 1995. Current population reports. [Google Scholar]
- 18.Liang MH, Socher SA, Larson MG, Schur PH. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum. 1989;32:1107–18. doi: 10.1002/anr.1780320909. [DOI] [PubMed] [Google Scholar]
- 19.Gladman DD, Goldsmith CH, Urowitz MB, et al. Sensitivity to change of 3 systemic lupus erythematosus disease activity indices: international validation. J Rheumatol. 1994;21:1468–71. [PubMed] [Google Scholar]
- 20.Aarden LA, De Groot ER, Feltkamp TE. Immunology of DNA. III. Crithidia luciliae, a simple substrate for the determination of anti-dsDNA with the immunofluorescence technique. Ann NY Acad Sci. 1975;254:505–15. doi: 10.1111/j.1749-6632.1975.tb29197.x. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton RG, Harley JB, Bias WB, et al. Two Ro (SS-A) autoantibody responses in systemic lupus erythematosus. Correlation of HLA-DR/DQ specificities with quantitative expression of Ro (SS-A) autoantibody. Arthritis Rheum. 1988;31:496–505. doi: 10.1002/art.1780310406. [DOI] [PubMed] [Google Scholar]
- 22.Harris EN. Special report. The Second International Anti-cardiolipin Standardization Workshop/the Kingston Anti-phospholipid Antibody Study (KAPS) Group. Am J Clin Pathol. 1990;94:476–84. doi: 10.1093/ajcp/94.4.476. [DOI] [PubMed] [Google Scholar]
- 23.Triplett DA, Barna LK, Unger GA. A hexagonal (II) phase phospholipid neutralization assay for lupus anticoagulant identification. Thromb Haemost. 1993;70:787–93. [PubMed] [Google Scholar]
- 24.Zonana-Nacach A, Camargo-Coronel A, Yanez P, et al. Measurement of damage in 210 Mexican patients with systemic lupus erythematosus: relationship with disease duration. Lupus. 1998;7:119–23. doi: 10.1191/096120398678919831. [DOI] [PubMed] [Google Scholar]
- 25.Gladman DD, Urowitz MB, Rahman P, Ibanez D, Tam LS. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol. 2003;30:1955–9. [PubMed] [Google Scholar]
- 26.Koenig W, Sund M, Frohlich M, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men. Results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–42. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 27.Folsom AR, Aleksic N, Catellier D, Juneja HS, Wu KK. C-reactive protein and incident coronary heart disease in the Atherosclerosis Risk In Communities (ARIC) study. Am Heart J. 2002;144:233–8. doi: 10.1067/mhj.2002.124054. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–3. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–8. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 31.Kao AH, Wasko MC, Krishnaswami S, et al. C-reactive protein and coronary artery calcium in asymptomatic women with systemic lupus erythematosus or rheumatoid arthritis. Am J Cardiol. 2008;102:755–60. doi: 10.1016/j.amjcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertoli AM, Vila LM, Reveill JD, Alarcon GS. Systemic lupus erythematosus in a multiethnic U.S. cohort (LUMINA): The value of high sensitivity C-reactive protein as a marker of disease activity and damage. J Rheumatol. 2008;35:2355–8. doi: 10.3899/jrheum.080175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SS, Singh S, Link K, Petri M. High-sensitivity c-reactive protein as an associate of clinical subsets and organ damage in systemic lupus erythematosus. Semin Arthritis Rheum. 2008;38:54. doi: 10.1016/j.semarthrit.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danenberg HD, Szalai AJ, Swaminathan RV, et al. Increased thrombosis after arterial injury in human C-reactive protein-transgenic mice. Circulation. 2003;108:512–15. doi: 10.1161/01.CIR.0000085568.13915.1E. [DOI] [PubMed] [Google Scholar]
- 35.Paul A, Ko KWS, Li L, et al. C-reactive protein accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:647–55. doi: 10.1161/01.CIR.0000114526.50618.24. [DOI] [PubMed] [Google Scholar]
- 36.Kiani AN, Mahoney JA, Petri M. Asymmetric dimethylarginine is a marker of poor prognosis and coronary calcium in systemic lupus erythematosus. J Rheumatol. 2007;34:1502–5. [PubMed] [Google Scholar]
- 37.Baldassarre D, Porta B, Camera M, et al. Markers of inflammation, thrombosis and endothelial activation correlate with carotid IMT regression in stable coronary disease after atorvastatin treatment. Nutr Metab Cardiovasc Dis. doi: 10.1016/j.numecd.2008.10.003. Advance Access published January 24, 2009, doi: 10.1016/j.numecd.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Consuegra-Sanchez L, Fredericks S, Kaski JC. Pregnancy-associated plasma protein-A (PAPP-A) and cardiovascular risk. Atherosclerosis. 2009;203:346–52. doi: 10.1016/j.atherosclerosis.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 39.Rho YH, Chung CP, Oeser A, et al. Novel cardiovascular risk factors in premature coronary atherosclerosis associated with systemic lupus erythematosus. J Rheumatol. 2008;35:1789–94. [PMC free article] [PubMed] [Google Scholar]
- 40.Ciferska H, Horak P, Hermanova Z, et al. The levels of sCD30 and of sCD40L in a group of patients with systemic lupus erythematodes and their diagnostic value. Clin Rheumatol. 2007;26:723–8. doi: 10.1007/s10067-006-0389-9. [DOI] [PubMed] [Google Scholar]
- 41.Andrade RM, Alarcon GS, Fernandez M, Apte M, Vila LM, Reveille JD. Accelerated damage accrual among men with systemic lupus erythematosus: XLIV. Results from a multiethnic US cohort. Arthritis Rheum. 2007;56:622–30. doi: 10.1002/art.22375. [DOI] [PubMed] [Google Scholar]
- 42.Garcia MA, Marcos JC, Marcos AI, et al. Male systemic lupus erythematosus in a Latin-American inception cohort of 1214 patients. Lupus. 2005;14:938–46. doi: 10.1191/0961203305lu2245oa. [DOI] [PubMed] [Google Scholar]
- 43.Bertoli AM, Alarcon GS, Calvo-Alen J, Fernandez M, Vila LM, Reveille JD. Systemic lupus erythematosus in a multiethnic US cohort. XXXIII. Clinical [corrected] features, course, and outcome in patients with late-onset disease. Arthritis Rheum. 2006;54:1580–7. doi: 10.1002/art.21765. [DOI] [PubMed] [Google Scholar]
- 44.Asanuma Y, Oeser A, Shintani AK, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2407–15. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 45.Petri M, Lakatta C, Magder L, Goldman D. Effect of prednisone and hydroxychloroquine on coronary artery disease risk factors in systemic lupus erythematosus: a longitudinal data analysis. Am J Med. 1994;96:254–9. doi: 10.1016/0002-9343(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 46.Wei L, MacDonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med. 2004;141:764–70. doi: 10.7326/0003-4819-141-10-200411160-00007. [DOI] [PubMed] [Google Scholar]
- 47.Alarcon GS, McGwin G, Jr, Bertoli AM, et al. Effect of hydroxychloroquine in the survival of patients with systemic lupus erythematosus. Data from LUMINA, a multiethnic US cohort (LUMINA L) Ann Rheum Dis. 2007;66:1168–72. doi: 10.1136/ard.2006.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanay A, Leibovitz E, Frayman A, Zimlichman R, Shargorodsky M, Gavish D. Vascular elasticity of systemic lupus erythematosus patients is associated with steroids and hydroxychloroquine treatment. Ann N Y Acad Sci. 2007;1108:24–34. doi: 10.1196/annals.1422.003. [DOI] [PubMed] [Google Scholar]
- 49.Petri M. Hydroxychloroquine: past, present, future. Lupus. 1998;7:65–67. doi: 10.1191/096120398678919886. [DOI] [PubMed] [Google Scholar]
- 50.Wallace DJ. Antimalarial agents and lupus. Rheum Dis Clin North Am. 1994;20:243–63. [PubMed] [Google Scholar]
- 51.Velagaleti RS, Vasan RS. Heart failure in the twenty-first century: is it a coronary artery disease or hypertension problem? Cardiol Clin. 2007;25:487–95. doi: 10.1016/j.ccl.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


