Abstract
Bleeding or inflammation in early pregnancy may result in pregnancy loss or defective implantation. Their effect on HOX gene expression in first trimester decidua is unknown. Bleeding results in thrombin generation, although infection or inflammation results in production of cytokines typified by Interleukin-1β (IL-1β). First trimester decidual cells were pretreated with 17β estradiol (E2), medroxyprogesterone acetate (MPA) or both and subsequently treated with thrombin or IL-1β. Affymetrix microarray analysis was used to assess the expression of all HOX genes and confirmed using real-time RT–PCR. E2 or MPA treatment resulted in significant increases in HOXA10 and HOXA11. Subsequent treatment with thrombin resulted in diminished expression of HOXA10 and HOXA9. Treatment with IL-1β resulted in decreased expression of HOXA1, 3, 9, 10 and 11. HOXA10 expression was reduced by 70% after thrombin treatment (P = 0.018) and by 90% after IL-1β treatment (P = 0.004). HOXA11 mRNA expression was decreased by 88% after IL-1β treatment (P < 0.001), but not by thrombin treatment. Decidua was collected at the time of elective termination of pregnancy (n = 10) or surgical treatment of spontaneous pregnancy loss (n = 10). Real-time PCR and western analysis demonstrated decreased HOXA10 and HOXA11 RNA and protein expression in the decidua of spontaneous pregnancy loss compared with that of viable pregnancies. In conclusion, multiple HOX genes are expressed in decidual cells and inhibited by thrombin and IL-1β. Since HOXA10 and HOXA11 are known to be necessary for successful pregnancy, these findings suggest a molecular mechanism by which bleeding or inflammation may affect pregnancy outcome.
Keywords: HOX, IL-1β, Thrombin, decidual hemorrhage, pregnancy loss
Introduction
Embryonic loss in the first trimester occurs in 15–30% of all pregnancies (Wilcox et al., 1999). Although many losses are likely due to embryonic genetic anomalies, others are due to abnormalities in the uterine environment. Endometrial/decidual infection or bleeding has been associated with miscarriage and inflammatory cell infiltrates have been associated with recurrent pregnancy loss (Dosiou and Giudice, 2005; Christiasen et al., 2006; Lambropoulou et al., 2006). Bleeding and inflammation may result from pregnancy loss, as well as contribute to it; here we model the effect of these conditions on the uterine environment. Levels of inflammatory cytokines, typified by interleukin-1β (IL-1β), are increased in inflamed decidua (Huang et al., 2006; Ng et al., 2006; Arcuri et al., 2007). Similarly pregnancy loss is associated with subchorionic bleeding and thrombophilia, suggesting a role for local thrombin generation (Raziel et al., 2001; Gracia et al., 2005; Masini et al., 2009). The effect of these molecules on endometrial/decidual development is not known.
Endometrium is an extremely dynamic tissue, undergoing sequential developmental changes in preparation for implantation during each menstrual cycle (Noyes et al., 1953). The successful implantation of the blastocyst and initiation of pregnancy requires optimal development of uterine endometrial receptivity (Finn and Martin, 1974; Navot et al., 1986; Kodaman and Taylor, 2004). In many ways, cyclic endometrial development in the adult can be considered analogous to embryonic development. Many of the genes traditionally thought of as regulators of embryonic development are also used to regulate endometrial development (Taylor, 2000). HOX genes, essential regulators of morphogenesis and tissue differentiation in the embryo, are also essential for endometrial development, endometrial receptivity and decidualization (McGinnis and Krumlauf, 1992; Lim et al., 1999; Taylor, 2002; Lu et al., 2008). Coordinated expression of multiple HOX genes likely directs this development analogous to the way in which HOX genes direct embryonic development.
During embryonic morphogenesis and differentiation HOX genes assign unique spatial developmental identities to segments of previously uniform axes. In mammals, 39 HOX (human)/Hox (murine) genes reside in four separate chromosomal linkage groups, designated Hoxa, b, c and d, each of which has parallel and overlapping expression domains (McGinnis and Krumlauf, 1992). The genes of the Hoxa cluster, Hoxa9, Hoxa10 and Hoxa11, are expressed in localized areas of the paramesonephric duct destined to become the oviduct, the uterus or both the lower uterine segment and cervix, respectively (Taylor et al., 1997). Hoxa10 and Hoxa11 gene expression is necessary for endometrial development, allowing uterine receptivity to implantation. Female Hoxa10 (−/−) or Hoxa11 (−/−) homozygous mutant mice have uterine factor infertility (Hsieh-Li et al., 1995; Rijli et al., 1995; Satokata et al., 1995; Benson et al., 1996). Although these mice ovulate normally and produce viable preimplantation embryos, they are unable to support implantation. Embryos from Hoxa10 (−/−) mice successfully implant in pseudo-pregnant wild type surrogates, however, wild-type embryos do not implant in Hoxa10- or Hoxa11-deficient uterus. In addition to regulating the embryonic development of the uterus (Taylor et al., 1997), Hoxa10 and Hoxa11 have specific roles in endometrial development in the adult. Blocking maternal Hoxa10 expression in the adult uterus of wild-type mice with antisense messengers blocks implantation, demonstrating the necessity of adult uterine Hox expression for successful pregnancy (Bagot et al., 2000). HOXA10 and HOXA11 are regulated by estrogen and progesterone in the adult human endometrium, where their expression rises dramatically in the midsecretory phase, at the time of implantation, and remains elevated throughout the rest of the secretory phase (Taylor et al., 1998, 1999a; Sarno et al., 2005).
Several human conditions associated with decreased implantation including hydrosalpinx and uterine leiomyomas demonstrate diminished endometrial HOXA10 and HOXA11 expression (Daftary and Taylor, 2002; Cermik et al., 2003; Rackow and Taylor, 2009). Both decidual infection and bleeding are known to interrupt pregnancy; however, the molecular mechanisms by which these disorders impact decidual support of pregnancy are not well characterized. Here we investigated the expression and regulation of HOX genes, essential mediators of decidualization, in first trimester human decidual cells. We found that several HOX genes are expressed at high level in these cells. The expression of those HOX genes most closely associated with embryo implantation are altered after treatment with IL-1β or thrombin. We also identify diminished decidual HOX gene expression in human pregnancy loss.
Materials and Methods
Collection and culture of first trimester decidual cells
Decidua was collected after informed consent at the time of elective termination of pregnancy or at the time of surgery for missed abortion. The Yale University School of Medicine Human Investigations Committee approved this protocol. Decidua was obtained electively from 23 individuals in the first trimester, 10 at the time of surgical intervention for spontaneous pregnancy loss and 13 undergoing elective first trimester termination. Tissue samples were divided in three for use in cell culture, real-time PCR or western analysis. For use in cell culture first trimester decidual stromal cells from these samples were isolated and purified to homogeneity on a Percoll gradient and passaged until >99% free of CD45+ cells as determined by fluorescent activated cell sorting analysis as previously described (Koopman et al., 2003). The presence of decidual cells was confirmed as previously described and by detection of prolactin expression (Koopman et al., 2003). Cells were grown to confluence in serum containing media. At confluence, the cells were primed with either 10−8 M 17β estradiol (E2) or 10−8 M E2+10−7 M medroxyprogesterone acetate (MPA) for 7 days to simulate the steroidal milieu of pregnancy, and then cultured in serum-free defined medium containing the corresponding steroids with either 2.5 U/ml of thrombin or 1 ng/ml of IL-1β. After 24 h, total RNA was isolated (RNeasy Mini Kit, Qiagen, MD, USA).
RNA isolation and microarray analysis
Microarray analysis was used to assess the expression of all HOX genes in response to treatment. First trimester decidual cells from three patients were grown to confluence in Falcon T-25 flasks. The human Affymetrix array was used in triplicate for each experimental condition. Each in vitro experimental condition was repeated three times and in triplicate; each repetition used cells obtained from separate individuals. Cells were harvested with QIAzol™ lysis reagent (Qiagen, Valencia, CA, USA) and used to prepare total RNA. According to the manufacturer's instruction, 100 µg of total RNA was precipitated using RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and used as a template for cDNA synthesis. A T7-(dT)24 oligo-primer was used to synthesize double-stranded cDNA by the One-Cycle cDNA Synthesis Kit (Affymetrix Inc., Santa Clara, CA, USA) which was subsequently purified using Sample Cleanup Module (Affymetrix Inc., Santa Clara, CA, USA) and ethanol precipitation. Then GeneChip IVT Labeling Kit (Affymetrix Inc., Santa Clara, CA, USA) was used to generate biotinylated cRNA. Additional cRNA purification was carried out using Sample Cleanup Module prior to the fragmentation of biotinylated cRNAs with 5× fragmentation buffer (Tris 200 mM, pH 8.1, KOAc 500 mM, MgOAc 150 mM). The chemically fragmented cRNAs were then hybridized on Affymetrix HG_U133 Plus 2.0 human chips in GeneChip Hybridization Oven 640 (Affymetrix, Santa Clara, CA, USA), screening for approximately 47 400 human genes and expression sequence tags, followed by fluorescence labeling with Fluidics Station 450 (Affymetrix, Santa Clara, CA, USA) and optical scanning with Affymetrix GeneChip Scanner 3000 by W.M. Keck Foundation Biotechnology Resource Laboratory at Yale University.
Raw data without normalization generated from Affymetrix GeneChip Operating Software Version 1.1.1 (GCOS 1.1.2) (Affymetrix, Santa Clara, CA, USA) were analyzed by GeneSpring software 7.2 (Agilent Technologies, Palo Alto, CA, USA). The gene readouts were normalized to the fiftieth percentile of the distribution of all measurements in each chip. Per gene normalization was performed using the median value of each gene throughout different chips in the same experimental condition. Nonparametric testing assuming unequal variance was applied to test statistical significance. Fold ratios were derived from comparing normalized data between control and treatment groups. HOX genes up- or down-regulated more than 2.0-fold by E2+MPA versus E2+MPA+IL-1β or thrombin were filtered.
Real-time polymerase chain reaction
HOX genes identified as expressed and regulated by IL-1β or thrombin in first trimester decidual cells by microarray analysis were confirmed using real-time RT–PCR. HOX gene expression after IL-1β or thrombin exposure were evaluated in cultured first trimester decidual cells. HOX gene expression in women undergoing surgical procedures was evaluated in surgical specimens of decidual tissue.
mRNA levels were evaluated by quantitative real-time RT–PCR and normalized to β-actin using the Roche LightCycler as previously described (Rackow and Taylor, 2009). Briefly, the reactions were carried out using the LightCycler RNA Master SYBR Green I kit. Reaction conditions included 1.0 µg of RNA, 2 mM Mn[OAc]2, respectively, 150 nM of each primer, and 1× RNA Master SYBR Green, for a final reaction volume of 20 µl. Primer sequences for each gene are as follows:
HOXA1 5′-AGTTGGAGAGTACGGCTACCTG-3′ and 5′-TGCAGGGATGCAGCGATCTCCAC-3′
HOXA3 5′-GGCTATCTGAACTCTATGCATTCG-3′ and 5′-AGGCCATGAGCGTGCGGGTCATA-3′
HOXA9 5′-CTGTTCAACATGTACCTCACCA-3′ and 5′-CACTCGTCTTTTGCTCGGTC-3′
HOXA10, 5′-AGGTGGACGCTGCGGCTAATCTCTA-3′ and 5′-GCCCCTTCCGAGAGCAGCAAAG-3′(46);
HOXA11 5′-GTACTTACTACGTCTCGGGTCCAG-3′ and 5′-AGTCTCTGTGCACGAGCTCCT-3′
β-actin, 5′-CGTACCACTGGCATCGTGAT-3′ and 5′-GTGTTGGCGTACAGGTCTTTG-3′.
Reverse transcription was carried out for 30 min at 61°C, followed by initial denaturation at 95°C for 30 s and 45 cycles including denaturation at 95°C for 2 s, annealing at 65°C (HOXA3, 9, 10 and 11), 62°C (β-actin), or 58°C (HOXA1) for 5 s and elongation at 72°C for 14 s. A melting curve was created following the amplification to observe the specificity of the primers. Each experiment was repeated in triplicate and performed three times using cells from 10 different individuals. Comparisons between in vitro treatment groups were analyzed by ANOVA on ranks. Comparison of decidual HOX gene expression between 10 women experiencing spontaneous loss and 10 undergoing elective termination were performed using student's T test.
Western analysis
Western analysis was performed on 10 decidual samples obtained from women undergoing surgery for spontaneous pregnancy loss and 10 with a viable pregnancy. Protein was extracted from intact decidual tissue using Nuclear Extract Kit (Activemotif, CA, USA) according to manufacturer's instructions. Equal amounts of protein (60 µg) were electrophoresed through 4–15% polyacrylamide gels (Bio-Rad, CA, USA) at 160 V for 70 min and transferred onto Immun-Blot polyvindylidene difluoride membranes (Bio-Rad, CA, USA) in transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol) at 100 V for 1 h. After incubation in blocking buffer (1× PBS, 0.2% Tween 20, 5% milk), the membrane was incubated individually with either goat polyclonal HOXA10 antibody (sc-17159) (Santa Cruz, CA, USA) dilution 1:200, or goat polyclonal Actin antibody (sc-1615) (Santa Cruz, CA, USA) dilution 1:1000 overnight at 4°C. After washing, the membranes were incubated for 1 h with biotinylated horse anti-goat secondary antibody (Vector, CA, USA) diluted (3.5 µg/ml) in the blocking buffer. The membranes were incubated in ABC Elite (Vector, CA, USA), and then stained by DAB (Vector, CA, USA). Negative controls demonstrated lack of reactivity in tissues that do not express HOXA10 or HOXA11.
Results
Sex steroids increase HOX gene expression in decidual cells
We have previously reported regulation of several HOX genes in response to E2 and/or progesterone using endometrial stromal cells obtained from non-pregnant women (Taylor et al., 1999a; Sarno et al., 2005). Here we investigated if similar regulation persists into early pregnancy in decidual cells. Leukocyte-free first trimester human decidual cells were treated with either vehicle control or E2 with MPA. HOX genes affected by the treatment were analyzed by microarray. Several homeobox genes that have been previously reported to be expressed in the female reproductive tract were identified in the array. These include HOXA10 and HOXA11, that have previously been well characterized in endometrium. Additionally we identified the expression of HOXA1, 3 and 9 in first trimester decidual cells. HOXA1, 3, 9, 10 and 11 were found to be expressed consistently in all samples at significant levels.
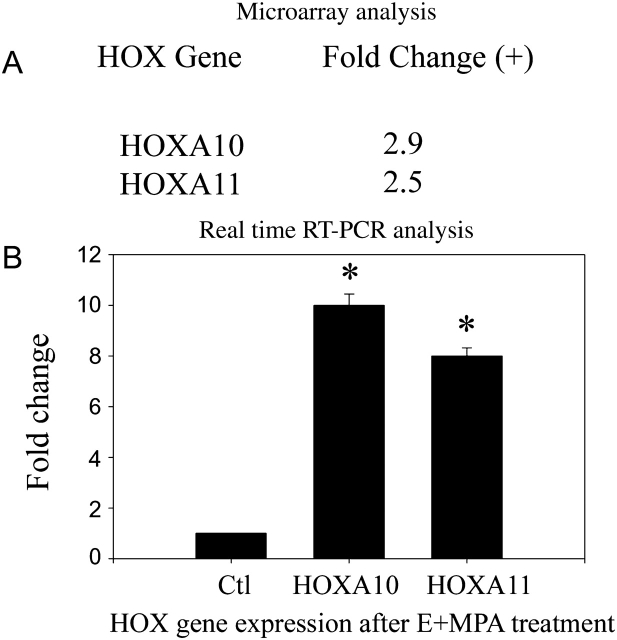
Sex steroid treatment resulted in increased expression of HOXA10. HOXA10 mRNA levels increased approximately 10-fold after treatment with E2+MPA. Similarly E2+MPA treatment resulted in an 8-fold increase in HOXA11 mRNA (Fig. 1). No significant changes were noted in HOXA1, 3 or 9.
Figure 1.
Sex steroid regulation of HOX genes in decidual cells.
(A) Microarray analysis showed HOXA10, and HOXA11 expression was increased in first trimester decidual cells treated with E2 and MPA. Microarray analysis revealed a statistically significant increase in HOX gene expression after treatment with E2+MPA (EM) compared with vehicle treated controls. Raw microarray data was normalized using the median value of each gene throughout different chips in the same experimental condition. Fold-ratios were derived from comparing normalized data between control and treatment groups. Genes up- or down-regulated more than 2.0-fold by E2+MPA (EM) were filtered. (B) Real-time RT–PCR analysis. Each experiment was repeated three times in triplicate. Each replicate consisted of cells obtained from a different subject. Shown are HOX mRNA levels normalized to actin. Fold changes from vehicle treated control are shown. (*) designates P < 0.01 compared with control using t test. The error bars are SEM.
IL-1β treatment represses the expression of multiple HOX genes
Leukocyte-free first trimester decidual cells were treated with IL-1β or vehicle control. HOX genes affected by the treatment were analyzed by microarray and confirmed using real-time RT–PCR. Cells were maintained in E2 and MPA as described to mimic the hormonal environment of pregnancy. Treatment of decidual cells with IL-1β at levels comparable to those seen with infection resulted in decreased expression of several HOX genes. As seen in Fig. 2, HOXA1, 3, 9, 10 and 11 expression were decreased by treatment with this inflammatory cytokine. RT–PCR analysis demonstrates that HOXA11, HOXA10, HOXA9 and HOXA3 expression were each decreased by 84–90% after IL-1β treatment (P > 0.01). HOXA1 expression was decreased to an undetectable level (>99%) after this treatment (P < 0.01).
Figure 2.
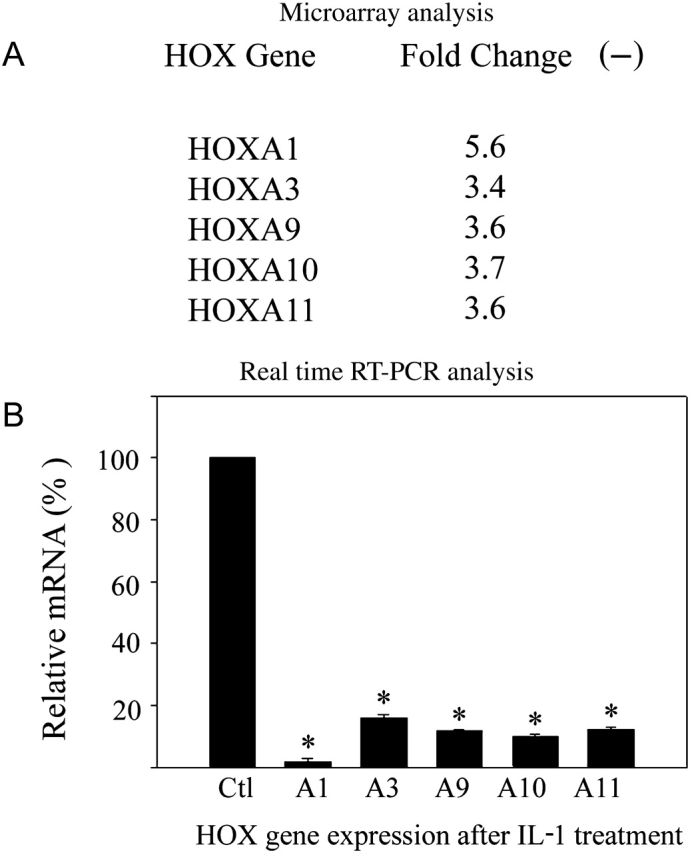
IL-1β regulation of HOX genes in decidual cells.
(A) Microarray analysis showed HOXA1, 3, 9, 10 and 11 expression were decreased in decidual cells treated with IL-1β. Microarray analysis revealed a statistically significant decrease in HOX gene expression in first trimester decidual cells treated with IL-1β (I) compared with E2+MPA (EM) vehicle treated controls. Raw microarray data was normalized using the median value of each gene throughout different chips in the same experimental condition. Fold-ratios were derived from comparing normalized data between control and treatment groups. Genes up- or down-regulated more than 2.0-fold by IL-1β are shown. All changes shown are statistically significant in comparison to EM treated controls. (B) Real-time RT–PCR analysis. IL-1β significantly decreased HOXA10 expression in first trimester decidual cells as determined by real-time RT–PCR. In E2+MPA (EM) vehicle control pretreated cells, 1 mg/ml IL-1β decreased HOXA1, HOXA3, HOXA9, HOXA10 and HOXA11 expression by 100, 84, 88, 90 and 88%, respectively. * = P < 0.001 compared with EM treated controls. The error bars are SEM.
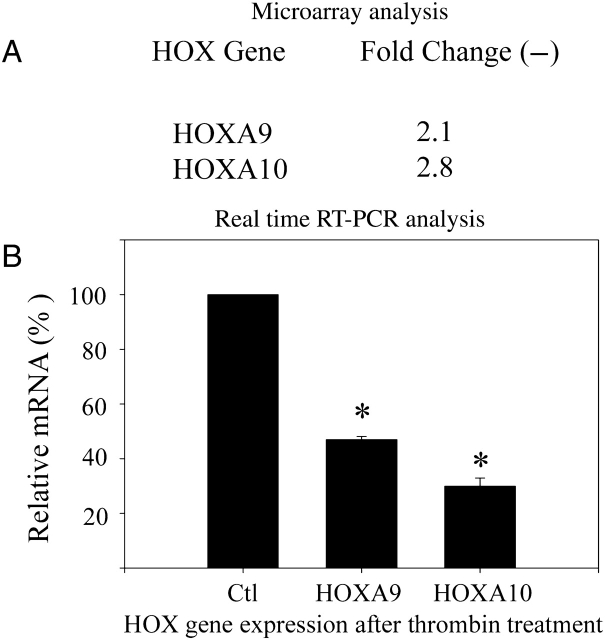
Thrombin treatment represses the expression of HOXA9 and HOXA10
The effect of thrombin on HOX mRNA expression in first trimester human decidual cells was evaluated by microarray analysis and quantitative real-time RT–PCR (Fig. 3). Addition of thrombin to cultures maintained in E2+MPA resulted in decreased expression of a limited number of HOX genes. Specifically HOXA9 and HOXA10 expression were significantly decreased. Expression of HOXA1, A3 and A11 were not altered. HOXA10 expression was decreased by 70% compared with treatment with E2+MPA alone (P = 0.018). HOXA9 expression was decreased by 53% by this treatment (P < 0.01).
Figure 3.
Thrombin regulation of HOX genes in decidual cells.
(A) Microarray analysis showed HOXA9 and HOXA10 expression was decreased in decidual cells treated with thrombin. Microarray analysis revealed a statistically significant decrease in HOX gene expression in first trimester decidual cells treated with thrombin compared with E2+MPA (EM) vehicle treated controls. Raw microarray data was normalized using the median value of each gene throughout different chips in the same experimental condition. Fold-ratios were derived by comparing normalized data between control and treatment groups. Genes up- or down-regulated more than 2.0-fold by 2.5 U/ml thrombin compared with EM controls are shown. (*) indicates P < 0.01 for each comparison. (B) Real-time RT–PCR analysis. Thrombin significantly decreased HOXA9 and HOXA10 expression in decidual cells in vitro as determined by real-time RT–PCR. (A) In E2+MPA (EM) pretreated cells, thrombin decreased HOXA9 and HOXA10 expression by 53 and 70%, respectively. * = P < 0.001 compared with EM vehicle treated controls. The error bars are SEM.
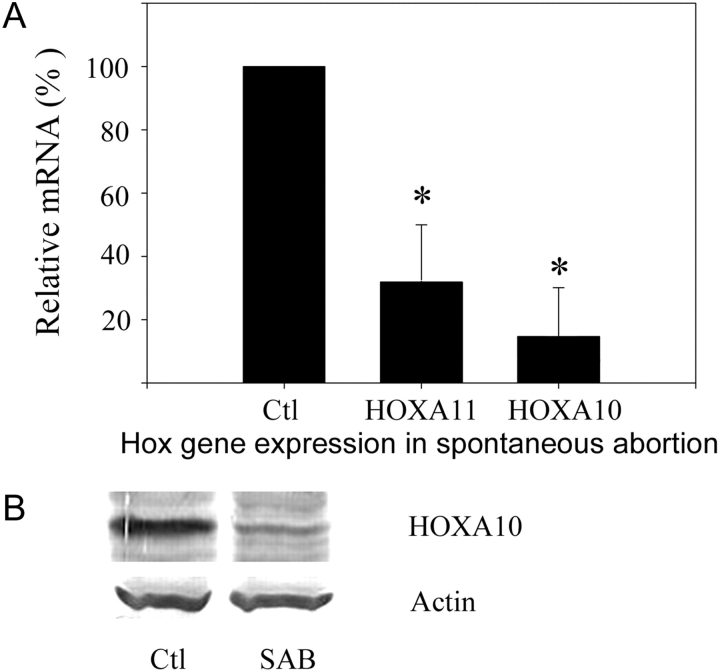
Decreased decidual HOXA10 and HOXA11 expression in spontaneous miscarriage
Decidua obtained from women undergoing surgical treatment for either elective termination of pregnancy (n = 10) or spontaneous miscarriage (n = 10) was analyzed for HOXA10 and HOXA11 expression by real-time PCR. Samples were matched for gestational age. The level of expression for each HOX gene in viable pregnancies was considered the control group and set at 100%. Expression of HOXA10 and HOXA11 in the decidua associated with spontaneous pregnancy loss was decreased to 12 and 27%, respectively, compared with that of a viable pregnancy (P < 0.001). Each of the decidua obtained from spontaneous pregnancy losses expressed less HOXA10 than any of the decidua obtained at the time of elective termination. Similarly, HOXA10 and HOXA11 protein expression was consistently decreased in decidua obtained from women with spontaneous loss as demonstrated in Fig. 4B. A representative western blot demonstrating decreased HOXA10 expression is shown.
Figure 4.
Decidual HOX gene expression in spontaneous pregnancy loss.
(A) Real-time RT–PCR analysis showed that decidual HOXA10 and HOXA11 expression was decreased in spontaneous miscarriage compared with that of elective termination of pregnancy. Decidua obtained from women undergoing surgical treatment for either elective termination of pregnancy (here considered the control group) or spontaneous miscarriage was analyzed for HOXA10 and HOXA11 expression by real-time PCR. Expression of HOXA10 and HOXA11 in the decidua associated with spontaneous pregnancy loss was decreased to 12 and 27%, respectively, compared the expression of each gene in the decidua of a viable pregnancy (each assigned a value of 100%). * = P < 0.001 expression in decidua of spontaneous loss compared with viable pregnancy controls. (B) Western blot analysis demonstrates decreased HOXA10 protein expression in the decidua obtained from spontaneous pregnancy loss compared with that obtained at the time of termination of pregnancy. Representative data is shown. SAB = spontaneous miscarriage.
Conclusion
The expression of HOX genes in the developing female reproductive tract and adult uterine endometrium has been well characterized (Taylor et al., 1997, 1998, 1999a; Sarno et al., 2005; Kwon and Taylor, 2004; Daftary and Taylor, 2006). HOX genes impart developmental identity to the paramesonephric duct and subsequently two HOX genes are necessary for endometrial differentiation in each menstrual cycle (Taylor et al., 1997; Block et al., 2000). HOXA10 and HOXA11 are required for endometrial receptivity to the implanting blastocyst (Hsieh-Li et al., 1995; Rijli et al., 1995; Satokata et al., 1995; Bagot et al., 2000). Hoxa10 (−/−) or Hoxa11 (−/−) mice are sterile; they produce viable embryos but have a uterine environment that cannot support early embryo development or implantation of even wild type embryos. These genes are expressed in a menstrual cycle specific fashion in human endometrium (Taylor et al., 1998, 1999a, b). Increased expression of HOXA10 and HOXA11 in the secretory phase is thought to regulate the window of endometrial receptivity in human endometrium. Several human disorders associated diminished implantation are noted to demonstrate decreased endometrial expression of these two genes (Taylor et al., 1999b; Taylor, 2000; Daftary and Taylor, 2002; Cermik et al., 2003; Browne and Taylor, 2006; Rackow and Taylor, 2009); these include, endometriosis, hydrosalpinx, polycystic ovary syndrome and myomata. HOX genes are likely to have a continued role in the regulation of decidual cell growth and differentiation in pregnancy.
Here we show that the expression of the two HOX genes known to be necessary for the establishment of pregnancy also continues into the first trimester of pregnancy. It is possible that the persistent expression of these genes maintain the differentiated state of the endometrial stromal/decidual cells. We have also identified the expression of additional HOX A cluster genes in the decidua, since expression of HOXA1, HOXA3 and HOXA9 was detected in these cells. HOXA9 expression has been previously detected in the developing Müllerian tract, where it is typically associated with development of the Fallopian tubes or oviducts. Since targeted disruption of Hoxa9 does not result in adverse pregnancy outcomes, the functional role of this gene in the decidua is likely redundant. The expression of HOXA1 and HOXA3 has not previously been reported in the reproductive tract. Typically these genes have been associated with development of anterior structures in early development. The role of HOXA1 and HOXA3 in early pregnancy will be the subject of further study. Interestingly, we did not detect the expression of genes in the HOX C or D clusters in pregnancy. Previously we have reported that HOXC10, HOXC11, HOXD10 and HOXD11 are all expressed in the endometrium (Akbas and Taylor, 2004). However, unlike the HOX A cluster genes, HOX C and D genes were expressed primarily in the proliferative phase endometrium and thought to regulate endometrial growth and propagation rather than the differentiated state associated with endometrial receptivity. Consistent with this role, we would not expect to find expression of HOX C or D genes in decidual cells. The findings reported here further support the theory that HOX C and D cluster genes regulate proliferation in the reproductive tract, although HOX A cluster genes support the differentiated and functional state.
Diminished expression of the genes that maintain the differentiated phenotype of the endometrium/decidua may lead to withdrawal of support of early pregnancy. HOXA10 regulates decidualization, including stromal cell responsiveness to progesterone and prostaglandins (Lim et al., 1999; Daftary et al., 2002; Gao et al., 2002; Kim et al., 2003; Troy et al., 2003; Daftary and Taylor, 2004, 2006; Lu et al., 2008). HOX gene products are transcription factors that regulate structural and regulatory genes associated with the establishment and maintenance of pregnancy. For example, HOXA10 is known to regulate the expression of integrins, insulin-like growth factor binding protein-1, prostaglandin receptors, as well as other transcriptional regulators (Lim et al., 1999; Gao et al., 2002; Daftary et al., 2002; Taylor, 2002; Kim et al., 2003; Troy et al., 2003; Vitiello et al., 2008). Without these important mediators of decidual function, maintenance of pregnancy could be jeopardized. Diminished HOX gene expression may be a molecular mechanism by which IL-1β and thrombin lead to adverse pregnancy outcomes. Pregnancy loss is characterized by reduced expression of these two genes, perhaps contributing to miscarriage.
Inflammation and hemorrhage in early pregnancy is associated with miscarriage (Gracia et al., 2005; Challis et al., 2009; Weiss et al., 2009). Although bleeding is clearly a result of pregnancy loss, it frequently precedes loss; subchorionic hemorrhage can be observed in early pregnancy in the presence of a viable embryo, yet increases the risk for subsequent pregnancy loss. Decidual hemorrhage results in intense local thrombin generation (Lockwood et al., 1993, 2001). Thrombin binding to protease-activated receptor-1 stimulates numerous molecular responses apart from those associated with thrombosis. Here we show that thrombin administration to first trimester decidual cells results in decreased expression of HOXA9 and HOXA10. It is likely that diminished expression of HOXA10 results in withdrawal of decidual support of pregnancy leading to miscarriage. Although both inflammatory cytokines and thrombin reduce HOX gene expression in these cells, thrombin affects only a limited subset of those HOX genes altered by IL-1β. Both may trigger withdrawal of decidual support of pregnancy through a common molecular mechanism involving HOX genes, however, the effect of inflammation or infection may be more profound.
Here we demonstrate the regulated expression of several HOX genes in decidual cells obtained from first trimester of pregnancy. HOX genes likely continue to regulate the differentiated developmental identity of these cells, as has been described in stromal cells of non-pregnant endometrium. Genes of the HOXA, but not HOX C or D, cluster appear to serve this role. Pregnancy loss is characterized by significantly decreased expression of HOXA10 and A11. IL-1β and thrombin may disrupt HOX gene regulation, resulting in loss of appropriate transcriptional control needed to maintain the differentiated decidual phenotype. Profound repression of critical HOX gene expression may represent a common mechanism by which bleeding or infection may result in first trimester pregnancy loss.
Funding
Grant support: hD36887(HST), U54 HD052668(HST) and HL07004(CJL).
References
- Akbas GE, Taylor HS. HOXC and HOXD gene expression in human endometrium: lack of redundancy with HOXA paralogs. Biol Reprod. 2004;70:39–45. doi: 10.1095/biolreprod.102.014969. [DOI] [PubMed] [Google Scholar]
- Arcuri F, Buchwalder L, Toti P, Cintorino M, Tosi P, Lockwood CJ, Rybalov B, Schatz F. Differential regulation of colony stimulating factor 1 and macrophage migration inhibitory factor expression by inflammatory cytokines in term human decidua: implications for macrophage trafficking at the fetal-maternal interface. Biol Reprod. 2007;76:433–439. doi: 10.1095/biolreprod.106.054189. [DOI] [PubMed] [Google Scholar]
- Bagot CN, Troy PJ, Taylor HS. Alteration of maternal Hoxa10 expression by in vivo gene transfection affects implantation. Gene Ther. 2000;7:1378–1384. doi: 10.1038/sj.gt.3301245. [DOI] [PubMed] [Google Scholar]
- Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- Block K, Kardana A, Igarashi P, Taylor HS. In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing mullerian system. FASEB J. 2000;14:1101–1108. doi: 10.1096/fasebj.14.9.1101. [DOI] [PubMed] [Google Scholar]
- Browne H, Taylor HS. HOXA10 expression in ectopic endometrial tissue. Fertil Steril. 2006;85:1386–1390. doi: 10.1016/j.fertnstert.2005.10.072. [DOI] [PubMed] [Google Scholar]
- Cermik D, Selam B, Taylor HS. Regulation of HOXA10 expression by testosterone in vitro and in the endometrium of patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:238–243. doi: 10.1210/jc.2002-021072. [DOI] [PubMed] [Google Scholar]
- Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- Christiansen OB, Nielsen HS, Kolte AM. Inflammation and miscarriage. Semin Fetal Neonatal Med. 2006;11:302–308. doi: 10.1016/j.siny.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Daftary GS, Taylor HS. Hydrosalpinx fluid diminishes endometrial HOXA10 expression. Fertil Steril. 2002;78:577–580. doi: 10.1016/s0015-0282(02)03306-x. [DOI] [PubMed] [Google Scholar]
- Daftary GS, Taylor HS. Pleiotropic effects of Hoxa10 on the functional development of peri-implantation endometrium. Mol Reprod Dev. 2004;67:8–14. doi: 10.1002/mrd.20013. [DOI] [PubMed] [Google Scholar]
- Daftary GS, Taylor HS. Endocrine regulation of HOX genes. Endocr Rev. 2006;27:331–335. doi: 10.1210/er.2005-0018. [DOI] [PubMed] [Google Scholar]
- Daftary GS, Troy PJ, Bagot CN, Young SL, Taylor HS. Direct regulation of Beta-3 integrin subunit gene expression by HOXA10 in endometrial cells. Mol Endocrinol. 2002;16:571–579. doi: 10.1210/mend.16.3.0792. [DOI] [PubMed] [Google Scholar]
- Dosiou C, Giudice LC. Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocr Rev. 2005;26:44–62. doi: 10.1210/er.2003-0021. [DOI] [PubMed] [Google Scholar]
- Finn CA, Martin L. The control of implantation. J Reprod Fertil. 1974;39:195–206. doi: 10.1530/jrf.0.0390195. [DOI] [PubMed] [Google Scholar]
- Gao J, Mazella J, Tseng L. Hox proteins activate the IGFBP-1 promoter and suppress the fucntion of hPR in human endometrial cells. DNA Cell Biol. 2002;21:819–825. doi: 10.1089/104454902320908469. [DOI] [PubMed] [Google Scholar]
- Gracia CR, Sammel MD, Chittams J, Hummel AC, Shaunik A, Barhart KT. Risk factors for spontaneous abortion in early symptomatic first trimester pregnancies. Obstet Gynecol. 2005;106:993–999. doi: 10.1097/01.AOG.0000183604.09922.e0. [DOI] [PubMed] [Google Scholar]
- Hsieh-Li HM, Witte DP, Weinstein M, Branford W, Li H, Small K, Potter SS. Hoxa11 structure, extensive antisense transcription, and function in male and female fertility. Development. 1995;121:1373–1385. doi: 10.1242/dev.121.5.1373. [DOI] [PubMed] [Google Scholar]
- Huang SJ, Schatz F, Masch R, Rahman M, Buchwalder L, Niven-Fairchild T, Tang C, Abrahams VM, Krikun G, Lockwood CJ. Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. J Reprod Immunol. 2006;72:60–73. doi: 10.1016/j.jri.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Taylor HS, Akbas GE, Foucher I, Trembleau A, Jaffe RC, Fazleabas AT, Unterman TG. Regulation of insulin-like growth factor binding protein-1 promoter activity by FKHR and HOXA10 in primate endometrial cells. Biology of Reproduction. 2003;68:24–30. doi: 10.1095/biolreprod.102.009316. [DOI] [PubMed] [Google Scholar]
- Kodaman PH, Taylor HS. Hormonal regulation of implantation. Obstet Gynecol Clin North Am. 2004;31:745–766. doi: 10.1016/j.ogc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Koopman LA, Kopcow D, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, et al. Human decidual natrual killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1202. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HE, Taylor HS. The role of HOX genes in human implantation. Ann N Y Acad Sci. 2004;1034:1–18. doi: 10.1196/annals.1335.001. [DOI] [PubMed] [Google Scholar]
- Lambropoulou M, Tamiolakis D, Venizelos J, Liberis V, Galazios G, Tsikouras P, Karamanidis D, Petrakis G, Constantinidis T, Menegaki M, et al. Imbalance of mononuclear cell infiltrates in the placental tissue from foetuses after spontaneous abortion versus therapeutic termination from 8th to 12th weeks of gestational age. Clin Exp Med. 2006;6:171–176. doi: 10.1007/s10238-006-0111-x. [DOI] [PubMed] [Google Scholar]
- Lim H, Ma L, Ma WG, Maas RL, Dey SK. Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol Endocrinol. 1999;13:1005–1017. doi: 10.1210/mend.13.6.0284. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Nemerson Y, Krikun G, Hausknecht V, Markiewicz L, Alvarez M, Guller S, Schatz F. Steroid-modulated stromal cell tissue factor expression: a model for the regulation of endoemtrial hemostatis and menstruation. J Clin Endocrinol Metab. 1993;77:1014–1019. doi: 10.1210/jcem.77.4.8408448. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Krikun G, Schatz F. Decidual cell-expressed tissue factor maintains hemostasis in human endometrium. Ann NY Acad Sci. 2001;943:77–88. doi: 10.1111/j.1749-6632.2001.tb03793.x. [DOI] [PubMed] [Google Scholar]
- Lu Z, Hardt J, Kim JJ. Global analysis of genes regulated by HOXA10 in decidualization reveals a role in cell proliferation. Mol Hum Reprod. 2008;14:357–366. doi: 10.1093/molehr/gan023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini S, Ticconi C, Gravina P, Tomassini M, Pietropolli A, Forte V, Federici G, Piccione E, Bernardini S. Thrombin-activatable fibrinolysis inhibitor polymorphisms and recurrent pregnancy loss. Fertil Steril. 2009 doi: 10.1016/j.fertnstert.2008.07.015. PMID 18774564 [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Navot D, Laufer N, Kopolovic J, Rabinowitz R, Birkenfeld A, Lewin A, Granat M, Margalioth EJ, Schenker JG. Artificially induced endometrial cycles and establishment of pregnancies in the absence of ovaries. N Engl J Med. 1986;314:806–811. doi: 10.1056/NEJM198603273141302. [DOI] [PubMed] [Google Scholar]
- Ng YH, Zhu H, Pallen CJ, Leung PC, MacCalman CD. Differential effects of interleukin-1beta and transforming growth factor-beta1 on the expression of the inflammation-associated protein, ADAMTS-1, in human decidual stromal cells in vitro. Hum Reprod. 2006;21:1990–1999. doi: 10.1093/humrep/del108. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rcok J. Dating of the endometrial biopsy. Fertil Steril. 1953;1:3–5. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- Rackow BW, Taylor HS. Submucosal uterine leiomyoma have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2009 doi: 10.1016/j.fertnstert.2008.03.029. PMID 18555231. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raziel A, Kornberg Y, Friedler S, Schachter M, Sela BA, Ron-El R. Hypercoagulable thrombophilic defects and hyperhomocysteinemia in patients with recurrent pregnancy loss. Am J Reprod Immunol. 2001;45:65–71. doi: 10.1111/j.8755-8920.2001.450201.x. [DOI] [PubMed] [Google Scholar]
- Rijli FM, Matyas R, Pellegrini M, Dierich A, Gruss P, Dolle P, Chambon P. Cryptorchidism and homeotic transformations of spinal nerves and vertebrae in Hoxa-10 mutant mice. Proc Natl Acad Sci USA. 1995;92:8185–8189. doi: 10.1073/pnas.92.18.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarno JL, Kliman HJ, Taylor HS. HOXA10, Pbx2, and Meis1 protein expression in the human endometrium: formation of multimeric complexes on HOXA10 target genes. J Clin Endocrinol Metab. 2005;90:522–528. doi: 10.1210/jc.2004-0817. [DOI] [PubMed] [Google Scholar]
- Satokata I, Benson G, Maas R. Sexually dimorphic sterility phenotypes in Hoxa10 deficient mice. Nature. 1995;374:460–463. doi: 10.1038/374460a0. [DOI] [PubMed] [Google Scholar]
- Taylor HS. The role of HOX genes in human implantation. Hum Reprod Update. 2000;6:75–79. doi: 10.1093/humupd/6.1.75. [DOI] [PubMed] [Google Scholar]
- Taylor HS. Transcriptional regulation of implantation by HOX genes. Rev Endocr Metab Disord. 2002;3:127–132. doi: 10.1023/a:1015454828489. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Vandenheuvel GB, Igarashi P. A conserved Hox axis in the mouse and human female reproductive system - late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod. 1997;57:1338–1345. doi: 10.1095/biolreprod57.6.1338. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101:1379–1384. doi: 10.1172/JCI1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HS, Igarashi P, Olive DL, Arici A. Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J Clin Endocrinol Metab. 1999;a 84:1129–1135. doi: 10.1210/jcem.84.3.5573. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Bagot C, Kardana A, Olive D, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;b 14:1328–1331. doi: 10.1093/humrep/14.5.1328. [DOI] [PubMed] [Google Scholar]
- Troy PJ, Daftary GS, Bagot CN, Taylor HS. Transcriptional repression of peri-implantation EMX2 expression in mammalian reproduction by HOXA10. Mol Cell Biol. 2003;23:1–13. doi: 10.1128/MCB.23.1.1-13.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello D, Pinard R, Taylor HS. Gene expression profiling reveals putative HOXA10 downstream targets in the periimplantation mouse uterus. Reprod Sci. 2008;15:529–535. doi: 10.1177/1933719108316911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, Goldsmith LT, Taylor RN, Bellet D, Taylor HS. Inflammation in reproductive disorders. Reprod Sci. 2009;16:216–229. doi: 10.1177/1933719108330087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340:1796–1799. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]