Abstract
Early administration of surfactant to preterm babies with respiratory distress syndrome saves lives and decreases morbidity such as pneumothorax. Surfactant administration shortly after birth to intubated babies less than 30 weeks gestation decreases pulmonary air leak, chronic lung disease and mortality. Some preterm babies may be born in hospitals with a transport team hours away. Surfactant administration may cause transient bradycardia or hypoxemia and may rapidly improve lung function. As preterm babies born outside of tertiary care centres will benefit from early administration of surfactant, every peripheral hospital performing deliveries should develop a plan in association with physicians in referral hospitals to provide this potentially life saving therapy.
Keywords: Community hospitals, Respiratory distress syndrome, Surfactant, Transport
Abstract
L’administration précoce de surfactant exogène aux prématurés souffrant d’un syndrome de détresse respiratoire sauve des vies et réduit la morbidité, telle que les pneumothorax. L’administration de surfactant exogène peu après la naissance à des bébés intubés nés à moins de 30 semaines d’âge gestationnel diminue les fuites d’air dans les poumons, les maladies pulmonaires chroniques et les décès. Certains prématurés peuvent naître dans un hôpital disposant d’une équipe de transport située à une distance de plusieurs heures. L’administration de surfactant exogène peut causer une bradycardie ou une hypoxémie transitoire et améliorer rapidement la fonction pulmonaire. Puisque les prématurés nés à l’extérieur des centres de soins tertiaires bénéficieront de l’administration précoce de surfactant exogène, chaque hôpital périphérique procédant à des accouchements devrait mettre un plan sur pied, en association avec les médecins des hôpitaux d’aiguillage, afin de prodiguer ce traitement susceptible de sauver des vies.
Respiratory distress syndrome (RDS) remains a major cause of morbidity and mortality in preterm infants despite the increased use of antenatal steroids. Although efforts are made to transfer mothers with threatened preterm labour to tertiary care centres, approximately 20% of Canadian preterm neonates are delivered in community hospitals (1). Surfactant administration is often postponed in these infants until the arrival of the transport team. Evidence indicates that surfactant should be administered to affected neonates as early as possible, but the large distances to referral hospitals in Canada can result in substantial delays. Early surfactant therapy should be considered in these peripheral centres as long as competent personnel are available. Treated babies still need continuing care in specialized neonatal units.
INDICATIONS FOR SURFACTANT THERAPY
The incidence of RDS increases with decreasing gestational age. The disorder is caused by both structural and biochemical immaturity of the lungs. Premature babies often fail to produce surfactant, which results in increased alveolar surface tension and progressive atelectasis. Affected infants present with signs of respiratory distress, including tachypnea, grunting and retractions. The diagnosis is confirmed by radiological findings of a generalized reticulogranular (‘ground glass’) appearance of the lungs with air bronchograms.
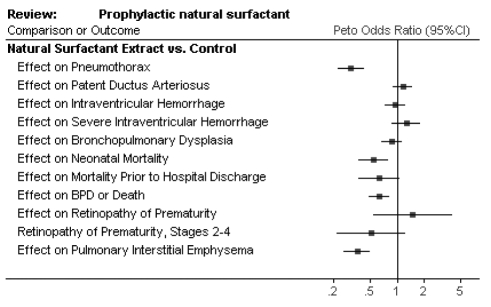
The use of exogenous surfactant received Food and Drug Administration approval in 1990 and has been the mainstay of RDS treatment. Epidemiological studies have shown an overall decrease in mortality and morbidity of very low birth weight infants since the introduction of surfactant therapy (2). Large randomized controlled trials have confirmed the benefits of surfactant administration with a meta-analysis (3) supporting a significant reduction in pneumothorax (RR=0.35, 95% CI 0.26 to 0.49) and neonatal mortality (RR=0.60, 95% CI 0.44 to 0.83). This implies that for every 100 preterm infants (less than 30 weeks gestation) treated with surfactant, there will be 15 fewer pneumothoraces and seven fewer neonatal deaths (Figure 1).
Figure 1.
Benefits of surfactant treatment. Reproduced with permission from reference 3. BPD Bronchopulmonary dysplasia
Surfactant is a well-established treatment for RDS and should be a supplement to, but not a substitute for, maternal antenatal corticosteroids. There is also evidence to support its use in other neonatal respiratory disorders such as meconium aspiration syndrome, pneumonia and pulmonary hemorrhage. In these diseases, there is a dysfunction or inactivation of endogenous surfactant. While research is ongoing, the absolute indications for administration in these situations is unclear. Future research may define the relative roles of early nasal continuous positive airway pressure and early surfactant administration, which currently necessitates intubation.
TYPES OF SURFACTANT
Human surfactant is composed of phospholipids and proteins. The dominant phospholipid, dipalmitoylphos-phatidylcholine (DPPC) acts to reduce surface tension in the alveoli. DPPC is a poor surfactant on its own because it cannot adsorb to the air-water interface. The specific surfactant proteins (SPs) (SP-A, SP-B, SP-C and SP-D) facilitate this adsorption and also play roles in the host defence (4).
Natural surfactants are obtained from either bovine or porcine lungs. They contain both DPPC and the proteins SP-B and SP-C. Synthetic surfactants do not contain surfactant proteins. Several studies have shown that natural surfactants are superior to synthetic surfactants in the treatment of RDS, with a reduced risk of pneumothorax (RR=0.63, 95% CI 0.53 to 0.75) and death (RR=0.87, 95% CI 0.76 to 0.98) (5). Bovine lipid extract surfactant (BLES, BLES Biochemicals Inc, Canada) is the most commonly used surfactant in Canada. Newer synthetic surfactants containing protein analogues are currently in development and may eventually prove to be the safest and most effective option.
TIMING OF ADMINISTRATION
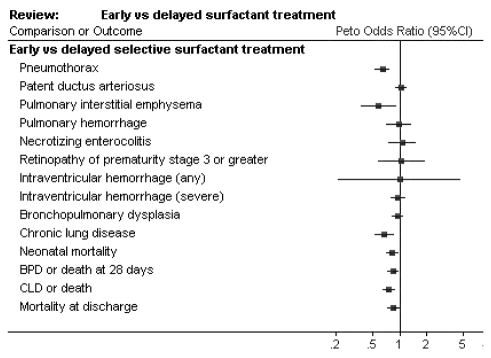
A major focus of surfactant research has been to determine the optimal timing of administration. Once symptoms of RDS have developed in a neonate, early administration of surfactant may decrease the need for ventilatory support and reduce barotrauma to the lungs. Indeed, early selective treatment results in decreased risk of pneumothorax (RR=0.70, 95% CI 0.59 to 0.82), neonatal mortality (RR=0.87, 95% CI 0.77 to 0.99) and chronic lung disease (RR=0.70, 95% CI 0.55 to 0.88) (6) (Figure 2). The largest study (7) looking at early versus late treatment of established RDS showed a poorer outcome for late treatment even when surfactant treatment was only delayed from 2 h to 3 h postnatal age. This has important implications for the preterm neonate born outside the tertiary care centre.
Figure 2.
Early versus delayed surfactant treatment. Reproduced with permission from reference 6. BPD Bronchopulmonary dysplasia; CLD Chronic lung disease
Prophylactic surfactant therapy for infants deemed to be at a significant risk for RDS is given within the first 15 min after birth, before signs of RDS develop. This strategy results in a further decrease in morbidity and mortality (8), but also requires the overtreatment of a certain proportion of infants. Studies show clinical benefit in infants born at less than 32 weeks gestation, but a gestational age cut-off of less than 30 weeks may be more appropriate for prophylactic therapy as this would result in less unnecessary therapy.
METHODS OF ADMINISTRATION
Surfactant can be administered through a feeding catheter inserted into the endotracheal tube or through a sideport adapter attached to the tube. The usual dose of BLES is 5 mL/kg. It needs to be thawed and this is usually accomplished by warming the vial in the hand for 10 min before drawing it up in a syringe. The surfactant is delivered in small aliquots to prevent reflux back up the tube. It can be distributed by intermittent manual ventilation or continued mechanical ventilation. During administration, correct placement of the endotracheal tube and horizontal positioning of the infant’s chest will result in the most even distribution of the surfactant in the two lungs. Suctioning is avoided if possible for 1 h to 2 h after the dose is given. Repeat doses of surfactant can be beneficial, but would generally be administered in a tertiary care centre.
RISKS OF SURFACTANT THERAPY
Surfactant instillation can cause hypoxemia and bradycardia. These episodes are generally transient and respond to a slower rate of surfactant infusion and to increased airway pressures and fraction of inspired oxygen. Rarely, the endotracheal tube can become blocked and require replacement. Studies have also shown an increased risk of pulmonary hemorrhage following surfactant therapy (RR=1.47, 95% CI 1.05 to 2.07), but the overall incidence remains low at 5% to 6% (9). Furthermore, mortality and long-term morbidity ascribed to pulmonary hemorrhage is not increased.
There is often a rapid improvement in lung compliance and gas exchange following surfactant administration. Caregivers must be prepared to wean ventilation within minutes of dosing to avoid hyperventilation, overdistension and air leaks. Weaning is accomplished by monitoring chest excursion and oxygen saturation. Ventilatory changes often need to occur before blood gas results are available. To assist with weaning, a blood gas is usually measured at approximately 30 min after surfactant administration. With the use of natural surfactants, there is also a theoretical risk of sensitization or transmission of infectious agents, such as viruses and prions.
SURFACTANT USE IN COMMUNITY HOSPITALS
Very preterm infants born outside tertiary care centres are at increased risk for death and long-term disability (10). Regionalization of perinatal care in the 1970s was a major advancement in the management of prematurity, and every effort should be made to provide antenatal corticosteroids and to transfer high-risk mothers to perinatal centres before delivery. When maternal transfer is not possible, these newborns should be considered for early surfactant therapy.
Surfactant should be administered by experienced physicians, nurses or respiratory therapists. These individuals should be comfortable with neonatal intubation and ventilation. They must be prepared to respond to rapid changes in lung compliance and be aware of the potential complications of surfactant therapy. Physicians in referral centres should be consulted to discuss the appropriateness of surfactant therapy in individual cases. Babies treated with surfactant still need to be transferred to specialized neonatal care units for care of other problems of prematurity. Every community hospital performing deliveries should have its own plan for the management of unexpected preterm birth, including resuscitation and initial management of RDS. If centres plan to use surfactant, personnel may benefit from prior experience in a supervised setting. Surfactant can be life saving if given early and given properly.
REFERENCES
- 1.Lee SK, McMillan DD, Ohlsson A, et al. Variations in practice and outcomes in the Canadian NICU Network: 1996–1997. Pediatrics. 2000;106:1070–9. doi: 10.1542/peds.106.5.1070. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RM, Luby AM, Scanlon JW, Kellogg RJ. Effect of surfactant on morbidity, mortality, and resource use in newborn infants weighing 500 to 1500 g. N Engl J Med. 1994;330:1476–80. doi: 10.1056/NEJM199405263302102. [DOI] [PubMed] [Google Scholar]
- 3.Soll RF, Soll RF. The Cochrane Library. Vol. 4. Oxford: Update Software; 2001. Prophylactic natural surfactant extract for preventing morbidity and mortality in preterm infants (Cochrane Review) < www.nichd.nih.gov/cochraneneonatal/SOLL3/SOLL3.HTM> (Version current at January 17, 2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jobe AH, Ikegami M. Biology of surfactant. Clin Perinatol. 2001;28:655–69. doi: 10.1016/s0095-5108(05)70111-1. [DOI] [PubMed] [Google Scholar]
- 5.Soll RF, Blanco F. The Cochrane Library. Vol. 4. Oxford: Update Software; 2001. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome (Cochrane Review) < www.nichd.nih.gov/cochraneneonatal/SOLL2/SOLL.HTM> (Version current at January 17, 2004). [DOI] [PubMed] [Google Scholar]
- 6.Yost CC, Soll RF. The Cochrane Library. Vol. 4. Oxford: Update Software; 2001. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome (Cochrane Review) < www.nichd.nih.gov/cochraneneonatal/yost/yost.htm> (Version current at January 17, 2004). [DOI] [PubMed] [Google Scholar]
- 7.The OSIRIS Collaborative Group. Early versus delayed neonatal administration of a synthetic surfactant – the judgement of OSIRIS. Lancet. 1992;340:1363–9. [PubMed] [Google Scholar]
- 8.Soll RF, Morley CJ. The Cochrane Library. 4. Chichester, UK: John Wiley & Sons, Ltd; 2004. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants (Cochrane Review) < www.nichd.nih.gov/cochraneneonatal/SOLL4/SOLL.HTM> (Version current at January 17, 2004). [DOI] [PubMed] [Google Scholar]
- 9.Raju TN, Langenberg P. Pulmonary hemorrhage and exogenous surfactant therapy: A metaanalysis. J Pediatr. 1993;123:603–10. doi: 10.1016/s0022-3476(05)80963-1. [DOI] [PubMed] [Google Scholar]
- 10.Chien LY, Whyte R, Aziz K, Thiessen P, Matthew D, Lee SK for the Canadian Neonatal Network. Improved outcome of preterm infants when delivered in tertiary care centers. Obstet Gynecol. 2001;98:247–52. doi: 10.1016/s0029-7844(01)01438-7. [DOI] [PubMed] [Google Scholar]