Abstract
The majority of anuran amphibians (frogs and toads) use acoustic communication to mediate sexual behavior and reproduction. Generally, females find and select their mates using acoustic cues provided by males in the form of conspicuous advertisement calls. In these species, vocal signal production and reception are intimately tied to successful reproduction. Research with anurans has demonstrated that acoustic communication is modulated by reproductive hormones, including gonadal steroids and peptide neuromodulators. Most of these studies have focused on the ways in which hormonal systems influence vocal signal production; however, here we will concentrate on a growing body of literature that examines hormonal modulation of call reception. This literature suggests that reproductive hormones contribute to the coordination of reproductive behaviors between signaler and receiver by modulating sensitivity and spectral filtering of the anuran auditory system. It has become evident that the hormonal systems that influence reproductive behaviors are highly conserved among vertebrate taxa, thus studying the endocrine and neuromodulatory bases of acoustic communication in frogs and toads can lead to insights of broader applicability to hormonal modulation of vertebrate sensory physiology and behavior.
Keywords: anurans, arginine vasotocin, auditory system, gonadal steroids, sensory physiology
The most conspicuous and extensively studied behavior of anuran amphibians (frogs and toads) is their acoustic communication. In the vast majority of anurans, vocal signaling mediates sexual behavior: sexually active males produce courtship calls to attract females. In these species the tie between vocal communication and reproduction is unambiguous.
Reproduction in frogs occurs seasonally and, as is generally true for vertebrates, is regulated by steroid hormones and peptide neuromodulators (reviewed in Yamaguchi and Kelley, 2002). Given the role of acoustic signaling as the predominant mediator of sexual interaction in frogs, it is not surprising that their vocal communication systems are strongly influenced by hormonal systems. In anurans, seasonal changes in hormone levels correlate with seasonal changes in calling behavior (Varriale et al., 1986; Itoh et al., 1990; Itoh and Ishii, 1990; Gobbetti et al., 1991; Harvey et al., 1997; Polzonetti-Magni et al., 1998). In addition, endocrine state and/or peptide neuromodulator systems have been shown to impact the initiation of vocal signaling (Wada et al., 1976; Wada and Gorbman, 1977; Wetzel and Kelley, 1983; Penna et al., 1992; Boyd, 1994; Marler et al., 1995; Propper and Dixon, 1997; Solis and Penna, 1997; Semsar et al., 1998; Burmeister and Wilczynski, 2001), the characteristics of the calls that are produced (Marler et al., 1995; Chu et al., 1998; Klomberg and Marler, 2000; Kime et al., 2007), how vocal signals are received (Miranda and Wilczynski, this issue, Yovanof and Feng, 1983; Penna et al., 1992; Miranda, 2007), and which behaviors they induce (Schmidt, 1983; Boyd, 1994). Research in amphibians has also revealed that many of the hormones that modulate vocal communication are the same as those found in other vertebrate taxa, indicating that hormonal mechanisms influencing reproductive behaviors are evolutionarily conserved. Thus, studying the hormonal bases of acoustic communication in frogs presents a useful model for investigations of the action of these systems on vertebrate sensory physiology and behavior.
Frog acoustic communication
In the vast majority of vocal frog species, males are the sole callers. Under the classic paradigm, male frogs form lek-like aggregations during the reproductive season in which they produce a species-typical advertisement call that functions primarily to attract females for breeding, as well as to repel conspecific males. The production and reception of the advertisement call serve to coordinate the reproductive behaviors of the two sexes. Females use the acoustic cues provided by these calls to guide them toward sexually active males (i.e., engage in phonotaxis) and to choose among potential mates. Although this dimorphic call production pattern holds across thousands of species, there are some instances of female vocalization; these are most often “reciprocal” calls (Duellman and Trueb, 1986; Roy et al., 1995) produced in response to the advertisement calls of males (Given, 1987; Márquez and Verrell, 1991; Bush, 1997; Orlov, 1997; Schlaepfer and Figeroa-Sandi, 1998; Tobias et al., 1998). Despite these exceptions, the vast majority of study of the hormonal underpinnings of sexually specific behavior in frogs has focused on the male production of, and the female response to, advertisement signals.
Chemical control systems
There are two hormonal systems that are widely recognized to influence reproductive behaviors, including acoustic communication, in anurans. The first is a neuromodulator system comprised of arginine vasotocin (AVT), a peptide hormone produced by neurons in the telencephalon and diencephalon. The second is an endocrine system consisting of the suite of steroid hormones (e.g., testosterone, dihydrotestosterone, estrogen) produced by the gonads and regulated by a signaling cascade beginning with another neuropeptide produced in the basal forebrain, gonadotropin releasing hormone (GnRH). Below, we will give a brief summary of the primary features of these two systems.
Arginine vasotocin
The neuropeptide AVT is the homologue of the mammalian arginine vasopressin (AVP), and functions primarily as a systemic regulator of osmolarity and blood pressure. The peptide is released into general circulation by neurosecretory cells in the preoptic area (POA) or, in mammals, in the anterior hypothalamic nuclei. Additional extrahypothalamic, telencephic AVT-containing neurons have been found in the anuran brain that do not appear to be neurosecretory cells, and are likely to instead play a neuromodulatory role within the CNS (for review, see Wilczynski and Chu, 2001). These neuromodulatory cell populations provide a mechanism by which AVT can affect behavior by acting directly within the brain, in addition to its canonical systemic effects. Via neuromodulatory activity, AVT and AVP have been shown to affect a wide range of social behaviors among vertebrate groups, including aggression, territoriality, courtship behaviors, and mating (reviewed in Goodson and Bass, 2001; Yamaguchi and Kelley, 2002; Marler et al., 2003; Wilczynski et al., 2005). In frogs, there is substantial evidence that AVT administration impacts production of advertisement and territorial calls by males (Marler et al., 1995; Propper and Dixon, 1997; Chu et al., 1998; Semsar et al., 1998), and some indication that it enhances sexual receptivity in females (Boyd, 1994). Local concentrations of the peptide and its m-RNA have been located in regions of the frog brain involved with acoustic signal production; thus, it is probable that the peptide is implicated in the modulation of acoustic communication behavior (reviewed in Emerson and Boyd, 1999; Moore and Rose, 2002).
Gonadal steroids
In accordance with the widely accepted vertebrate model, steroid hormones in anurans play critical roles in the organization and differentiation of sexually dimorphic body structures during development. In addition, gonadal steroids are key regulators of the expression of sex-specific mature reproductive behaviors. For example, in frogs, advertisement calling is androgen dependent (Wada et al., 1976; Wada and Gorbman, 1977; Wetzel and Kelley, 1983; Burmeister and Wilczynski, 2001) and the neural pathway subserving vocal production concentrates sex steroids (Kelley et al., 1975; Morrell et al., 1975; Kelley, 1981). Regulation of these steroids occurs through the hypothalamic-pituitary-gonadal, or reproductive (Vilain and McCabe, 1998), axis (HPGA). At the apex of the HPGA are the GnRH-releasing neurons, which in amphibians, including frogs, are distributed along the midline of the basal forebrain (Rastogi et al., 1998; Burmeister and Wilczynski, 2005). The secretion of GnRH stimulates the release of pituitary gonadotropins [e.g., luteinizing hormone (LH)], which in turn regulate the synthesis and release of steroid hormones from the gonads. Thus, plasma levels of gonadal steroids are ultimately regulated by the GnRH neuronal population, making these cells a primary site for regulation of reproductive physiology and behavior (Gore, 2002).
Gonadotropins exert their effects by binding to a transmembrane receptor, for example, the luteinizing hormone receptor (LHR). LHR was traditionally thought to be expressed only in the gonads, in accord with the role of gonadotropins in stimulating the release of gonadal steroids. However, LHR has since been found in the neural tissues of birds, mammals and frogs (Lei et al., 1993; You et al., 2000; Rao et al., 2004; Yang et al., 2007). These data suggest that gonadotropins may act directly in the brain to regulate reproductive behaviors, in addition to inducing steroid hormone production (Lei and Rao, 2001). Indeed, recent research suggests that gonadotropin works within the CNS of male Xenopus laevis (the South African clawed frog) to induce advertisement calling (Yang et al., 2007). This suggests a neuromodulatory role in the control of acoustic communication in this species (Yang et al., 2007).
Hormonal modulation of acoustic signal reception
As in any communication system, acoustic communication consists of two primary units, a signaler and a receiver. The principal elements involved in communication differ between these two units: for the signaler, it is the sound-production apparatus, and for the receiver, the auditory apparatus. On the receiving end, there is evidence from numerous vertebrate species, including humans, that acoustic perception is influenced by circulating hormones (Hinde and Steele, 1964; Komisaruk et al., 1972; Wright and Crow, 1973; Elkind-Hirsch et al., 1992; Penna et al., 1992; Sisneros et al., 2004; Walpurger et al., 2004; Maney et al., 2006; Lynch and Wilczynski, 2008). The modification of acoustic signal processing by hormones may facilitate communication during periods of reproductive behavior. Studying endocrine-based modulation of acoustic perception in animals such as frogs, however, is quite difficult, particularly when using a behavioral approach. Signal perception is only apparent when the focal individual expresses an overt response to acoustic stimuli. If no behavioral response is induced by an applied stimulus, it is impossible to determine if the signal was perceived. Perhaps as a result of these challenges, the bulk of research on the role of hormones in the modulation of frog acoustic communication has focused on the signaler. Herein, however, we will focus on the smaller, but rapidly growing, body of literature that examines the way in which hormones modulate the reception of vocalizations by the frog auditory system.
Female phonotaxis
One of the most evident behaviors observed during anuran acoustic communication is female phonotaxis towards the source of the conspecific advertisement call. Thus, phonotaxis provides an excellent behavioral assay for explorations of the impact of hormones on acoustic signal reception and sexual motivation in frogs. For example, studies of female túngara frogs (Physalaemus pustulosus) have demonstrated that maximal receptivity (i.e., positive phonotactic response to any conspecific male signal) and permissiveness (i.e., phonotactic selectivity assessed by a response to a synthesized call known to be less attractive than the conspecific call) correspond to peaks in circulating levels of the steroid hormones estrogen and progesterone (Lynch and Wilczynski, 2005). In addition, injection with human chorionic gonadotropin (hCG), which is an agonist at LHRs, thus inducing release of gonadal hormones, also increases receptivity and permissiveness in this species (Lynch et al., 2006). A subsequent study demonstrated that female túngara frogs injected with estradiol show a similar likelihood of phonotaxis, and similar call preferences, as non-manipulated females in breeding condition. These results suggest that estradiol fluctuations are primarily responsible for the changes in female sexual behavior seen over the breeding cycle (Chakraborty and Burmeister, 2008). Taken together, these studies indicate that positive phonotaxis and the ability to discriminate calls covary with circulating gonadal hormone levels in female túngara frogs, and suggest that estradiol plays a principal role in this hormonal effect. In addition to the studies on P. pustulosus, work with other frog species have shown that female receptivity and phonotactic efficiency is enhanced by injections of both hCG and AVT (Diakow, 1978; Diakow and Nemiroff, 1981; Raimondi and Diakow, 1981; Picker, 1983; Schmidt, 1983; 1985; Boyd, 1992; 1994).
Phonotactic behavior is a motor response driven by the reception of acoustic signals, thus the coincident modulation of phonotaxis and hormone levels suggests that hormones may act directly on acoustic processing in female frogs. Only a few studies have tested this possibility. Yovanov and Feng (1983) found that auditory evoked potentials recorded from the midbrain (i.e., torus semicircularis) of female leopard frogs (Lithobates pipiens, formerly Rana pipiens) in response to tones representing frequencies contained in the conspecific advertisement call increased in amplitude after injection with the estrogen estradiol. The behavioral implications of this result, however, are unclear because estrogens have not been shown to promote female receptiveness in this species (Diakow et al., 1978). In female green treefrogs (Hyla cinerea), implantation with testosterone significantly increases midbrain auditory thresholds for frequencies corresponding to the male advertisement call, but not for frequencies outside these spectral bands (Miranda and Wilczynski, this issue, Miranda, 2007). Thus, testosterone apparently decreases the female treefrogs’ sensitivity to the conspecific call. This result may be explained by examining the pattern of natural testosterone fluctuation in P. pustulosus. Female túngara frogs have the highest circulating testosterone concentrations prior to the expression of maximal receptivity and permissiveness (Lynch et al., 2005; Lynch and Wilczynski, 2005). If this pattern holds in female H. cinerea, the reduction of sensitivity to the spectral range of the advertisement call during periods of high circulating testosterone may help inhibit premature mating behavior that precedes peak reproductive readiness (Miranda, 2007). Altogether, these electrophysiological studies, along with others showing seasonal fluctuations in neural activity in the auditory midbrain of both sexes of fire-bellied toads (Bombina bombina), grass frogs (Rana temporaria) and the gray tree frog (Hyla chrysoscleis) (Walkowiak, 1980; Hillery, 1984) provide intriguing evidence of the complex influence of sex hormones on sensitivity and spectral filtering of the anuran auditory system.
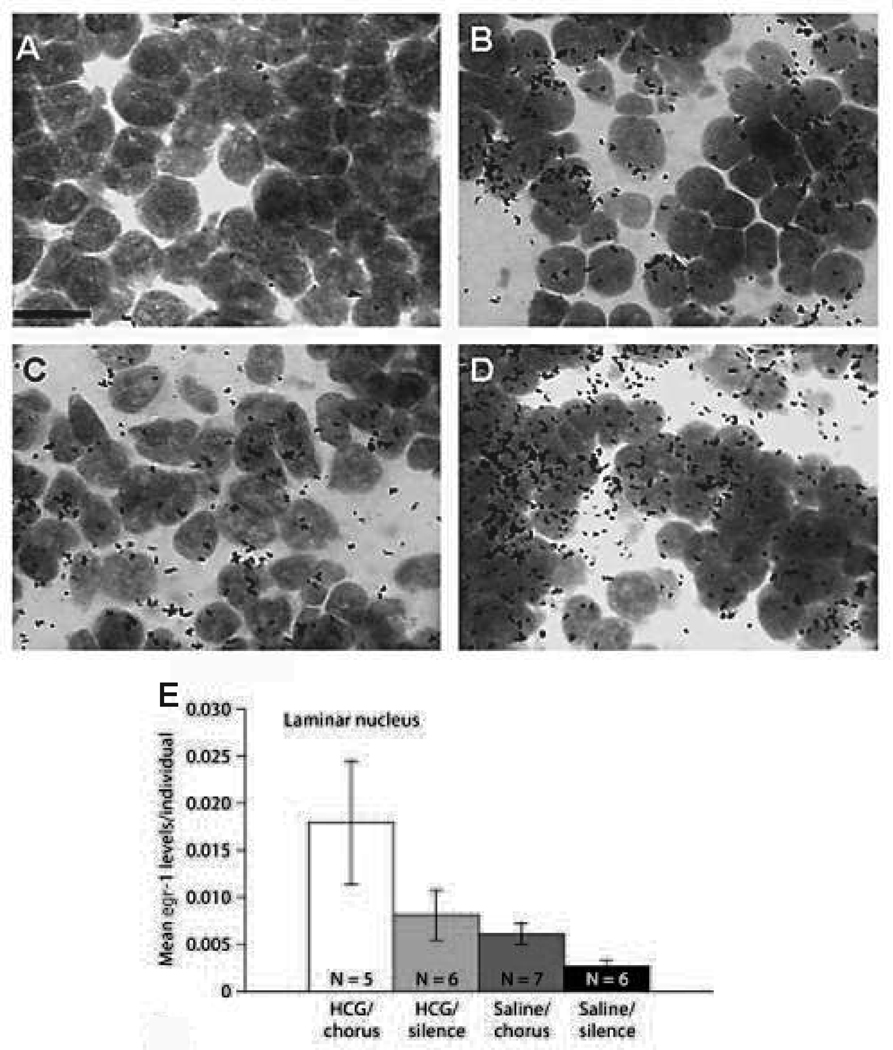
In a recent study, Lynch and Wilczynski (2008) took a different approach to investigating hormone-induced modulation of auditory signal processing. Instead of employing electrophysiological techniques, the investigators used in-situ hybridization to measure levels of expression of the activity-dependent immediate early gene egr-1 (early growth response 1) in the auditory midbrain of female túngara frogs injected with hCG and/or exposed to recordings of the conspecific mating chorus. Immediate early genes, such as egr-1, are commonly used in communication studies (Hoke et al., 2004; Mello et al., 2004; Hoke et al., 2005; Burmeister et al., 2008) because the location and abundance of egr-1 mRNA in the auditory midbrain serves as an indicator of patterns of neuronal activity induced by the processing of auditory signals. Lynch and Wilczynski (2008) found that injection of hCG significantly increased egr-1 induction in one of the nuclei (the laminar nucleus, see below) of the auditory midbrain in response to chorus exposure (figure 1). These data suggest that elevated gonadotropin levels enhance the stimulatory effect of the conspecific calls. It remains to be determined whether this enhancement is due to the downstream effects of hCG application (i.e., the induced release of gonadal hormones which influence midbrain activity via steroid receptors) or the direct action of the gonadotropin through LHRs on forebrain neurons which, in turn, modulate midbrain activity (Lynch and Wilczynski, 2008). The results of this study indicate that in sexually receptive females, higher concentrations of circulating hormones may increase the responsiveness of particular central auditory nuclei to conspecific signals. Future experiments employing this technique, which can provide a brain-wide view of neuronal activity modulation by hormones and sound, will provide an additional source of information about the mechanisms by which hormones work within the CNS to modulate acoustic processing.
Figure 1.
A–D Four photomicrographs representing the quantitative difference in egr-1 mRNA expression in the laminar nucleus (LN) of Physalaemus pustulosus. A Egr-1 mRNA in saline/silence condition. B Egr-1 expression in the saline/chorus condition. C Egr-1 expression in the human chorionic gonadotropin (hCG)/silence condition. D Egr-1 expression in the hCG/chorus condition. The area within the field of view covered by black spots (silver grains) was counted to determine the amount of egr-1 expression. Scale bar = 1 µm. E Mean egr-1 mRNA levels in the LN of females treated with hCG or saline then exposed to natural mate choruses or silence (modified and reprinted with permission from S. Karger AG, Basel).
Modulation of auditory signal processing
How do hormones influence the reception and processing of acoustic signals? This is a complex question, but some progress has been made in understanding hormonal modulation of activity in the frog central auditory system. The midbrain torus semicircularis (TS) is an auditory nucleus that is considered to be the amphibian homolog of the mammalian inferior colliculus (Wilczynski and Endepols, 2007). The TS is the primary auditory processing center in the frog CNS, so it is an obvious starting point for investigating the influence of sex hormones on auditory processing. This nucleus integrates ascending auditory pathways, receives both ascending and descending auditory inputs, and serves as a sensorimotor integrator (Wilczynski, 1988; Wilczynski and Endepols, 2007). The TS is comprised of several sub-nuclei of which the two most widely studied are the principal nucleus and the laminar nucleus (LN). The LN has extensive connections to premotor and motor areas, and is therefore likely to bear significant responsibility for audiomotor integration (Endepols and Walkowiak, 2000). In addition, this nucleus has been found to contain cells that concentrate both androgens and estrogens (Kelley et al., 1975; Morrell et al., 1975; Kelley et al., 1978; Kelley, 1980; di Meglio et al., 1987). Thus, the LN potentially represents a key access site through which gonadal hormones can influence the processing of communication signals, including the modulation of motor correlates of signal reception, such as phonotaxis. To date, however, relatively little is known about the direct impact of hormone action on the processing of communication signals in the TS.
Upstream from the TS, forebrain limbic areas have also been implicated as possible CNS centers for hormonal modulation of acoustic processing. The ventral hypothalamus (VH) and the POA are the two forebrain nuclei known to be important in the control of GnRH secretion (Ball, 1981) and, like the LN, have been shown to concentrate steroid hormones (Endepols and Walkowiak, 1999). Projections from these forebrain areas into the TS could be another mechanism by which TS activity is modulated by hormonal secretion. Interestingly, robust ascending auditory connections from the TS to the VH and POA via thalamic relay nuclei have been characterized in anurans (Neary, 1988; Wilczynski et al., 1993). This anatomical pathway provides a means by which acoustic stimuli could influence endocrine state by altering the secretion of GnRH, and thus influencing the activity of the HPGA axis. Indeed, POA and other hypothalamic neurons have been found to respond to auditory stimulation (Wilczynski and Allison, 1989; Allison, 1992; Hoke et al., 2005), and it is known that the reception of communication signals induces steroid hormone production in male (Burmeister and Wilczynski, 2000; 2001; Chu and Wilczynski, 2001; Burmeister and Wilczynski, 2005) and female frogs (Lynch and Wilczynski, 2006).
In a recent study Kime et al., (2007) found that systemic AVT administration caused male túngara frogs to alter their calling activity in a manner that mimicked changes induced by exposure to a conspecific chorus; this correlation led the authors to hypothesize that AVT release may be stimulated by the receipt of conspecific vocalizations, and play a role in mediating social regulation of the frogs’ acoustic communication behavior (Kime et al., 2007). The mechanisms by which AVT may act within the frog brain to modulate communication behavior are as yet uncharacterized.
Taken together, this group of studies suggests that for both gonadal steroids and AVT, important incurrent pathways exist in which reception of conspecific vocal signals impact the release of hormones which, in turn, modulate the activity of the auditory system to affect appropriate sexual behavior during the breeding season. We must await further study before being able to describe the subtle interplay between external stimuli and hormonally modulated central integration that leads to acoustically triggered physiological and behavioral response.
Conclusion
Hormones play a vital role in regulating the sexual behavior of vertebrates, from frogs to humans, and hormonal systems are highly conserved across vertebrate taxa. Acoustic communication is the primary mediator of sexual behavior in the majority of anuran species, thus it provides an excellent system within which to explore the diversity of ways in which hormones work to ensure appropriate and successful reproductive activity. To coordinate breeding, both signalers and receivers are under selection to produce appropriate and temporally coordinated behaviors during the reproductive season. Although the majority of research in anurans has focused on hormonal influences on vocal signal production, intriguing evidence is emerging for a potent role of hormones in regulating signal reception and processing. By exploring the mechanisms by which hormonal activity within the auditory system of frogs connects the external social environment with internal physiological state, we have the potential to learn a great deal about the most basic processes that underlie vertebrate social behavior.
Acknowledgments
We are grateful to Dr. Walt Wilczynski for his insightful comments on an earlier version of this manuscript. Financial support was provided by grants from the Paul S. Veneklasen Research Foundation and NIDCD (no. DC-00222) to P.M.N., and a GAANN Fellowship to V.S.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Victoria S. Arch, Email: varch@ucla.edu.
Peter M. Narins, Email: pnarins@ucla.edu.
References
- Allison JD. Acoustic modulation of neural activity in the preoptic area and ventral hypothalamus of the green treefrog (Hyla cinerea) J. Comp. Physiol. A. 1992;171:387–395. doi: 10.1007/BF00223968. [DOI] [PubMed] [Google Scholar]
- Ball JN. Hypothalamic control of the pars distalis in fishes, amphibians, and reptiles. Gen. Comp. Endocrinol. 1981;44:135–170. doi: 10.1016/0016-6480(81)90243-4. [DOI] [PubMed] [Google Scholar]
- Boyd SK. Sexual differences in hormonal control of release calls in bullfrogs. Horm. Behav. 1992;26:522–535. doi: 10.1016/0018-506x(92)90019-r. [DOI] [PubMed] [Google Scholar]
- Boyd SK. Arginine vasotocin facilitation of advertisement calling and call phonotaxis in bullfrogs. Horm. Behav. 1994;28:232–240. doi: 10.1006/hbeh.1994.1020. [DOI] [PubMed] [Google Scholar]
- Burmeister SS, Wilczynski W. Social signals influence hormones independently of calling behavior in the treefrog (Hyla cinerea) Horm. Behav. 2000;38:201–209. doi: 10.1006/hbeh.2000.1605. [DOI] [PubMed] [Google Scholar]
- Burmeister SS, Wilczynski W. Social context influences androgenic effects on calling in the green treefrog (Hyla cinerea) Horm. Behav. 2001;40:550–558. doi: 10.1006/hbeh.2001.1723. [DOI] [PubMed] [Google Scholar]
- Burmeister SS, Wilczynski W. Social signals regulate gonadotropin-releasing hormone neurons in the green treefrog. Brain Behav. Evol. 2005;65:26–32. doi: 10.1159/000081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister SS, Mangiamele LA, Lebonville CL. Acoustic modulation of immediate early gene expression in the auditory midbrain of female túngara frogs. Brain Res. 2008;1190:105–114. doi: 10.1016/j.brainres.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Bush SL. Vocal behavior of males and females in the Majorcan midwife toad. J. Herpetol. 1997;31:251–257. [Google Scholar]
- Chakraborty M, Burmeister SS. Estradiol induces sexual behavior in female frogs. Horm. Behav. 2008 doi: 10.1016/j.yhbeh.2008.09.001. In press. [DOI] [PubMed] [Google Scholar]
- Chu J, Wilczynski W. Social influences on androgen levels in the Southern leopard frog, Rana sphenocephala. Gen. Comp. Endocrinol. 2001;121:66–73. doi: 10.1006/gcen.2000.7563. [DOI] [PubMed] [Google Scholar]
- Chu J, Marler CA, Wilczynski W. The effects of arginine vasotocin on the calling behavior of male cricket frogs in changing social contexts. Horm. Behav. 1998;34:248–261. doi: 10.1006/hbeh.1998.1479. [DOI] [PubMed] [Google Scholar]
- di Meglio M, Morrell JI, Pfaff DW. Localization of steroid-concentrating cells in the central nervous system of the frog Rana esculenta. Gen. Comp. Endocrinol. 1987;67:149–154. doi: 10.1016/0016-6480(87)90142-0. [DOI] [PubMed] [Google Scholar]
- Diakow C. Hormonal basis for breeding behavior in female frogs: vasotocin inhibits release call of Rana pipiens. Science. 1978;199:1456–1457. doi: 10.1126/science.305115. [DOI] [PubMed] [Google Scholar]
- Diakow C, Nemiroff A. Vasotocin, prostaglandin, and female reproductive behavior in the frog, Rana pipiens. Horm. Behav. 1981;15:86–93. doi: 10.1016/0018-506x(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Diakow C, Wilcox JN, Woltmann R. Female frog reproductive behavior elicited in the absence of the ovaries. Horm. Behav. 1978;11:183–189. doi: 10.1016/0018-506x(78)90047-8. [DOI] [PubMed] [Google Scholar]
- Duellman WE, Trueb L. Biology of Amphibians. New York: McGraw-Hill; 1986. [Google Scholar]
- Elkind-Hirsch KE, Stoner WR, Stach BA, Jerger JF. Estrogen influences auditory brainstem responses during the normal menstrual cycle. Hear. Res. 1992;60:143–148. doi: 10.1016/0378-5955(92)90016-g. [DOI] [PubMed] [Google Scholar]
- Emerson SB, Boyd SK. Mating vocalizations of female frogs: Control and evolutionary mechanisms. Brain Behav. Evol. 1999;53:187–197. doi: 10.1159/000006594. [DOI] [PubMed] [Google Scholar]
- Endepols H, Walkowiak W. Influence of descending forebrain projections on processing of acoustic signals and audiomotor integration in the anuran midbrain. Eur. J. Morph. 1999;37:182–184. doi: 10.1076/ejom.37.2.182.4753. [DOI] [PubMed] [Google Scholar]
- Endepols H, Walkowiak W. Integration of ascending and descending inputs in the auditory midbrain of anurans. J. Comp. Physiol. A. 2000;186:1119–1133. doi: 10.1007/s003590000159. [DOI] [PubMed] [Google Scholar]
- Given MF. Vocalizations and acoustic interactions of the carpenter frog, Rana virgatipes. Herpetologica. 1987;43:467–481. [Google Scholar]
- Gobbetti A, Zerani M, Bolelli GF, Botte V. Seasonal changes in plasma prostaglandin F2α and sex hormones in the male water frog, Rana esculenta. Gen. Comp. Endocrinol. 1991;82:331–336. doi: 10.1016/0016-6480(91)90307-r. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res. Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Gore A. Gonadotropin-releasing hormone (GnRH) neurons: gene expression and neuroanatomical studies. Prog. Brain Res. 2002;141:193–208. doi: 10.1016/S0079-6123(02)41094-1. [DOI] [PubMed] [Google Scholar]
- Harvey LA, Propper CR, Woodley SK, Moore MC. Reproductive endocrinology of the explosively breeding desert spadefoot toad, Scaphiopus couchii. Gen. Comp. Endocrinol. 1997;105:102–113. doi: 10.1006/gcen.1996.6805. [DOI] [PubMed] [Google Scholar]
- Hillery CM. Seasonality of two midbrain auditory responses in the treefrog, Hyla chrysoscelis. Copeia. 1984;1984:844–852. [Google Scholar]
- Hinde RA, Steele E. Effect of exogenous hormones on the tactile sensitivity of the canary brood patch. J. Endocrinol. 1964;30:355–359. doi: 10.1677/joe.0.0300355. [DOI] [PubMed] [Google Scholar]
- Hoke KL, Ryan MJ, Wilczynski W. Social cues shift functional connectivity in the hypothalamus. Proc. Natl. Acad. Sci. U. S. A. 2005;102:10712–10717. doi: 10.1073/pnas.0502361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke KL, Burmeister SS, Fernald RD, Rand AS, Ryan MJ, Wilczynski W. Functional mapping of the auditory midbrain during mate call reception. J. Neurosci. 2004;24:11264–11272. doi: 10.1523/JNEUROSCI.2079-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Ishii S. Changes in plasma levels of gonadotropins and sex steroids in the toad, Bufo japonicus, in association with behavior during the breeding season. Gen. Comp. Endocrinol. 1990;80:451–464. doi: 10.1016/0016-6480(90)90194-q. [DOI] [PubMed] [Google Scholar]
- Itoh M, Inoue M, Ishii S. Annual cycle of pituitary and plasma gonadotropins and plasma sex steroids in a wild population of the toad, Bufo japonicus. Gen. Comp. Endocrinol. 1990;78:242–253. doi: 10.1016/0016-6480(90)90011-a. [DOI] [PubMed] [Google Scholar]
- Kelley DB. Auditory and vocal nuclei in the frog brain concentrate sex hormones. Science. 1980;207:553–555. doi: 10.1126/science.7352269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DB. Locations of androgen concentrating cells in the brain of Xenopus laevis: autoradiography with 3H-dihydrotestosterone. J. Comp. Neurol. 1981;199:221–231. doi: 10.1002/cne.901990206. [DOI] [PubMed] [Google Scholar]
- Kelley DB, Morrell JI, Pfaff DW. Autoradiographic localization of hormone concentrating cells in brain of an amphibian, Xenopus laevis 1. Testosterone. J. Comp. Neurol. 1975;164:47–61. doi: 10.1002/cne.901640105. [DOI] [PubMed] [Google Scholar]
- Kelley DB, Lieberburg I, McEwen BS, Pfaff DW. Autoradiographic and biochemical studies of steroid hormone-concentrating cells in the brain of Rana pipiens. Brain Res. 1978;140:287–305. doi: 10.1016/0006-8993(78)90461-4. [DOI] [PubMed] [Google Scholar]
- Kime NM, Whitney TK, Davis ES, Marler CA. Arginine vasotocin promotes calling behavior and call changes in male túngara frogs. Brain Behav. Evol. 2007;69:254–265. doi: 10.1159/000099613. [DOI] [PubMed] [Google Scholar]
- Klomberg KF, Marler CA. The neuropeptide arginine vasotocin alters male call characteristics involved in social interactions in the grey treefrog, Hyla versicolor. Anim. Behav. 2000;59:807–812. doi: 10.1006/anbe.1999.1367. [DOI] [PubMed] [Google Scholar]
- Komisaruk BR, Adler NT, Hutchison J. Genital sensory field: enlargement by estrogen treatment in female rats. Science. 1972;178:1295–1298. doi: 10.1126/science.178.4067.1295. [DOI] [PubMed] [Google Scholar]
- Lei ZM, Rao CV. Neural actions of luteinizing hormone and human chorionic gonadotropin. Semin. Reprod. Med. 2001;19:103–109. doi: 10.1055/s-2001-13917. [DOI] [PubMed] [Google Scholar]
- Lei ZM, Rao CV, Kornyei JL, Licht P, Hiatt ES. Novel expression of human chorionic gonadotropin/luteinizing hormone receptor gene in brain. Endocrinology. 1993;132:2262–2270. doi: 10.1210/endo.132.5.8477671. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Wilczynski W. Gonadal steroids vary with reproductive stage in a tropically breeding female anuran. Gen. Comp. Endocrinol. 2005;143:51–56. doi: 10.1016/j.ygcen.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Wilczynski W. Social regulation of plasma estradiol concentration in a female anuran. Horm. Behav. 2006;50:101–106. doi: 10.1016/j.yhbeh.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KS, Wilczynski W. Reproductive hormones modify reception of species-typical communication signals in a female anuran. Brain Behav. Evol. 2008;71:143–150. doi: 10.1159/000111460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KS, Rand AS, Ryan MJ, Wilczynski W. Plasticity in female mate choice associated with changing reproductive states. Anim. Behav. 2005;69:689–699. [Google Scholar]
- Lynch KS, Crews D, Ryan MJ, Wilczynski W. Hormonal state influences aspects of female mate choice in the túngara frog (Physalaemus pustulosus) Horm. Behav. 2006;49:450–457. doi: 10.1016/j.yhbeh.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. Eur. J. Neurosci. 2006;23:1523–1529. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- Marler CA, Chu J, Wilczynski W. Arginine vasotocin injection increases probability of calling in cricket frogs, but causes call changes characteristic of less aggressive males. Horm. Behav. 1995;29:554–570. doi: 10.1006/hbeh.1995.1286. [DOI] [PubMed] [Google Scholar]
- Marler CA, Bester-Meredith JK, Trainor BC. Paternal behavior and aggression: endocrine mechanisms and nongenomic transmission of behavior. Adv. Stud. Behav. 2003;32:263–323. [Google Scholar]
- Márquez R, Verrell P. The courtship and mating of the Iberian midwife toad Alytes cisternasii (Amphibia, Anura, Discoglossidae) J. Zool. 1991;225:125–139. [Google Scholar]
- Mello CV, Velho TAF, Pinaud R. Song-induced gene expression: A window on song auditory processing and perception. Ann. N. Y. Acad. Sci. 2004;1016:263–281. doi: 10.1196/annals.1298.021. [DOI] [PubMed] [Google Scholar]
- Miranda JA. Austin: University of Texas, Austin; 2007. Sex differences and hormone influences on auditory processing of communication signals in the green treefrog, Hyla cinerea, Neuroscience, Vol. Doctor of Philosophy; p. 120. [Google Scholar]
- Moore FL, Rose JD. Sensorimotor processing model: how vasotocin and corticosterone interact and control reproductive behaviors in an amphibian. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 2. San Diego: Academic Press; 2002. pp. 515–545. [Google Scholar]
- Morrell JI, Kelley DB, Pfaff DW. Autoradiographic localization of hormone concentrating cells in brain of an amphibian, Xenopus laevis 2. Estradiol. J. Comp. Neurol. 1975;164:63–77. doi: 10.1002/cne.901640106. [DOI] [PubMed] [Google Scholar]
- Neary TJ. Forebrain auditory pathways in ranid frogs. In: Fritszch B, Ryan MJ, Wilczynski W, Hetherington T, Walkowiak W, editors. The Evolution of the Amphibian Auditory System. New York: Wiley; 1988. pp. 233–252. [Google Scholar]
- Orlov N. Breeding behavior and nest construction in a Vietnam frog related to Rana blythi. Copeia. 1997:464–465. [Google Scholar]
- Penna M, Capranica RR, Somers J. Hormone-induced vocal behavior and midbrain auditory sensitivity in the green treefrog, Hyla cinerea. J. Comp. Physiol. A. 1992;170:73–82. doi: 10.1007/BF00190402. [DOI] [PubMed] [Google Scholar]
- Picker MD. Hormonal induction of the aquatic phonotactic response of Xenopus. Behaviour. 1983;84:74–90. [Google Scholar]
- Polzonetti-Magni AM, Mosconi G, Carnevali O, Yamamoto K, Hanaoka Y, Kikuyama S. Gonadotropins and reproductive function in the anuran amphibian, Rana esculenta. Biol. Reprod. 1998;58:88–93. doi: 10.1095/biolreprod58.1.88. [DOI] [PubMed] [Google Scholar]
- Propper CR, Dixon TB. Differential effects of arginine vasotocin and gonadotropin-releasing hormone on sexual behaviors in an anuran amphibian. Horm. Behav. 1997;32:99–104. doi: 10.1006/hbeh.1997.1408. [DOI] [PubMed] [Google Scholar]
- Raimondi D, Diakow C. Sex dimorphism in responsiveness to hormonal induction of female behavior in frogs. Physiol. Behav. 1981;27:167–170. doi: 10.1016/0031-9384(81)90316-4. [DOI] [PubMed] [Google Scholar]
- Rao CV, Zhou XL, Lei ZM. Functional luteinizing hormone/chorionic gonadotropin receptors in human adrenal cortical H295R cells. Biol. Reprod. 2004;71:579–587. doi: 10.1095/biolreprod.104.027300. [DOI] [PubMed] [Google Scholar]
- Rastogi RK, Meyer DL, Pinelli C, Fiorentino M, D'Aniello B. Comparative analysis of GnRH neuronal systems in the amphibian brain. Gen. Comp. Endocrinol. 1998;112:330–345. doi: 10.1006/gcen.1998.7144. [DOI] [PubMed] [Google Scholar]
- Roy D, Borah B, Sarma A. Analysis and significance of female reciprocal call in frogs. Curr. Sci. 1995;69:265–270. [Google Scholar]
- Schlaepfer MA, Figeroa-Sandi R. Female reciprocal calling in a Costa Rican leaf-litter frog, Eleutherodactylus podiciferus. Copeia. 1998;1998:1076–1080. [Google Scholar]
- Schmidt RS. Mating call phonotaxis in the female American toad: induction by hormones. Horm. Behav. 1983;17:94–102. [Google Scholar]
- Schmidt RS. Prostaglandin-induced mating call phonotaxis in female American toad: facilitation by progesterone and arginine vasotocin. J. Comp. Physiol. A. 1985;156:823–829. [Google Scholar]
- Semsar K, Klomberg KF, Marler C. Arginine vasotocin increases calling-site acquisition by nonresident male grey treefrogs. Anim. Behav. 1998;56:983–987. doi: 10.1006/anbe.1998.0863. [DOI] [PubMed] [Google Scholar]
- Sisneros JA, Forlano PM, Deitcher DL, Bass AH. Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science. 2004;305:404–407. doi: 10.1126/science.1097218. [DOI] [PubMed] [Google Scholar]
- Solis R, Penna M. Testosterone levels and evoked vocal responses in a natural population of the frog Batrachyla taeniata. Horm. Behav. 1997;31:101–109. doi: 10.1006/hbeh.1997.1366. [DOI] [PubMed] [Google Scholar]
- Tobias ML, Viswanathan SS, Kelley DB. Rapping, a female receptive call, initiates male-female duets in the South African clawed frog. Proc. Natl. Acad. Sci. U. S. A. 1998;95:1870–1875. doi: 10.1073/pnas.95.4.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varriale B, Pierantoni R, Dimatteo L, Minucci S, Fasano S, Dantonio M, Chieffi G. Plasma and testicular estradiol and plasma androgen profile in the male frog Rana esculenta during the annual cycle. Gen. Comp. Endocrinol. 1986;64:401–404. doi: 10.1016/0016-6480(86)90075-4. [DOI] [PubMed] [Google Scholar]
- Vilain E, McCabe ERB. Mammalian sex determination: From gonads to brain. Mol. Gen. Metabol. 1998;65:74–84. doi: 10.1006/mgme.1998.2749. [DOI] [PubMed] [Google Scholar]
- Wada M, Gorbman A. Relation of mode of administration of testosterone to evocation of male sex behavior in frogs. Horm. Behav. 1977;8:310–319. doi: 10.1016/0018-506x(77)90005-8. [DOI] [PubMed] [Google Scholar]
- Wada M, Wingfield JC, Gorbman A. Correlation between blood level of androgens and sexual behavior in male leopard frogs, Rana pipiens. Gen. Comp. Endocrinol. 1976;29:72–77. doi: 10.1016/0016-6480(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Walkowiak W. The coding of auditory signals in the torus semicircularis of the fire-bellied toad and the grass frog: responses to simple stimuli and to conspecific calls. J. Comp. Physiol. 1980;138:131–148. [Google Scholar]
- Walpurger V, Pietrowsky R, Kirschbaum C, Wolf OT. Effects of the menstrual cycle on auditory event-related potentials. Horm. Behav. 2004;46:600–606. doi: 10.1016/j.yhbeh.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Wetzel DM, Kelley DB. Androgen and gonadotropin effects on male mate calls in South African clawed frogs, Xenopus laevis. Horm. Behav. 1983;17:388–404. doi: 10.1016/0018-506x(83)90048-x. [DOI] [PubMed] [Google Scholar]
- Wilczynski W. Brainstem auditory pathways in anuran amphibians. In: Fritszch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W, editors. The Evolution of the Amphibian Auditory System. New York: Wiley; 1988. pp. 209–231. [Google Scholar]
- Wilczynski W, Allison JD. Acoustic modulation of neural activity in the hypothalamus of the leopard frog. Brain Behav. Evol. 1989;33:317–324. doi: 10.1159/000115939. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Chu J. Acoustic communication, endocrine control, and the neurochemical systems of the brain. In: Ryan MJ, editor. Anuran Communication. Smithsonian Institution Press; 2001. pp. 23–35. [Google Scholar]
- Wilczynski W, Endepols H. Central auditory pathways in anuran amphibians: The anatomical basis of hearing and sound communication. In: Narins PM, Feng AS, Fay RR, Popper AN, editors. Hearing and Sound Communication in Amphibians: Springer Handbook of Auditory Research. Vol. 28. New York: Springer; 2007. pp. 221–249. [Google Scholar]
- Wilczynski W, Allison JD, Marler CA. Sensory pathways linking social and environmental cues to endocrine control regions of amphibian forebrains. Brain Behav. Evol. 1993;42:252–264. doi: 10.1159/000114159. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Lynch KS, O'Bryant EL. Current research in amphibians: Studies integrating endocrinology, behavior, and neurobiology. Horm. Behav. 2005;48:440–450. doi: 10.1016/j.yhbeh.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P, Crow RA. Menstrual cycle: effect on sweetness preferences in women. Horm. Behav. 1973;4:387–391. [Google Scholar]
- Yamaguchi A, Kelley DB. Hormonal mechanisms of acoustic communication. In: Megela-Simmons A, Popper AN, Fay RR, editors. Acoustic Communication. New York: Springer Verlag; 2002. pp. 275–323. [Google Scholar]
- Yang EJ, Nasipak BT, Kelley DB. Direct action of gonadotropin in brain integrates behavioral and reproductive functions. Proc. Natl. Acad. Sci. U. S. A. 2007;104:2477–2482. doi: 10.1073/pnas.0608391104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You S, Kim H, El Halawani ME, Foster DN. Three different turkey luteinizing hormone receptor (tLH-R) isoforms II: characterization of differentially regulated tLH-R messenger ribonucleic acid isoforms in the ovary. Biol. Reprod. 2000;62:117–124. doi: 10.1095/biolreprod62.1.117. [DOI] [PubMed] [Google Scholar]
- Yovanof S, Feng AS. Effects of estradiol on auditory evoked responses from the frog's auditory midbrain. Neurosci. Lett. 1983;36:291–297. doi: 10.1016/0304-3940(83)90015-0. [DOI] [PubMed] [Google Scholar]