Abstract
Purpose
Taxanes have effects on angiogenesis causing difficulties in separating biologic effects of chemotherapy from those due to angiogenesis inhibitors. This randomized phase II trial was designed to evaluate the additional biomarker effect on angiogenesis when bevacizumab is added to docetaxel.
Experimental Design
Patients with inoperable breast cancer were randomized to either 2 cycles of preoperative docetaxel (D) 35 mg/m2 IV weekly for 6 weeks, followed by a 2 week break; or docetaxel with bevacizumab 10 mg/kg IV every other week for a total of 16 weeks (DB). Plasma and serum markers of endothelial damage, DCE-MRI and tumor microvessel density were assessed prior to treatment and at the end of each preoperative cycle.
Results
49 patients were randomized (DB = 24; D = 25). There was no difference in overall clinical response, progression-free survival or overall survival. VEGF increased during treatment; more so with DB (P < 0.0001). VCAM-1 also increased (P < 0.0001); more so with DB (P = 0.069). ICAM increased (P = 0.018) and E-selectin decreased (P = 0.006) overall. Baseline levels of VCAM-1 and E-selectin correlated with clinical response by univariate analysis. DCE-MRI demonstrated a greater fall in tumor perfusion calculated by IAUC90 in DB (P = 0.024). DCE-MRI also demonstrated an overall decrease in tumor volume (P=0.012).
Conclusion
Bevacizumab plus docetaxel caused a greater increase in VEGF and VCAM-1, and a greater reduction in tumor perfusion by DCE-MRI compared with docetaxel. Clinical outcomes of inoperable breast cancer were predicted by changes in VCAM-1 and E-selectin.
INTRODUCTION
Numerous studies have demonstrated that therapeutic disruption of nascent vasculature is effective in mediating tumor regression (1). A primary target for anti-angiogenic therapy is vascular endothelial growth factor (VEGF), a potent and specific regulator of tumor angiogenesis and endothelial cell survival (2-8). VEGF also induces vascular permeability, which is a critical step in promoting tumor growth, and induces the expression of adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) and intracellular adhesion molecule-1 (ICAM-1) on endothelial cells (9-11). VCAM-1 is a type-1 transmembrane glycoprotein important for cell adhesion needed for metastasis and is expressed on endothelial cells in response to VEGF stimulation or inflammation (12). VCAM-1 is also present in a soluble form and may function as attractants for endothelial cells (13). Similar to VCAM-1, ICAM-1 is a member of the immunoglobulin-like adhesion molecules and high circulating levels have been associated with a greater propensity for metastatic dissemination (14, 15). E-selectin is expressed on endothelial membranes as well as secreted, is upregulated by inflammation, and is associated with the development of metastasis (16). In addition, fluctuations in E-selectin levels may be a more specific marker of breast cancer than other malignancies (17). Expression of these endothelial adhesion molecules appear to correspond with clinical disease status, and thus can be investigated as a surrogate marker for disease response [18, 19].
Bevacizumab (rhuMAbVEGF, Avastin; Genentech, San Francisco, CA) is a humanized monoclonal antibody with binding specificity for VEGF. Clinical trials investigating the effect of this antibody with or without chemotherapy in the treatment of locally-advanced or metastatic breast cancer have been reported with modest clinical results (20-24). This supports the need to document the direct biologic consequence of bevacizumab therapy on breast cancer in vivo in order to differentiate its effects from that of chemotherapy. Wedam, et. al., examined tumor biopsy specimens in 21 patients with inflammatory and locally advanced breast cancer treated with bevacizumab (22). An analysis of breast cancer tissue sampled pre- and post-therapy revealed a median decrease of 66.7% in phosphorylated VEGFR2 (P = .004) and a median increase of 128.9% in tumor apoptosis (P = .0008). There were no significant changes in microvessel density (MVD) or VEGF-A expression. DCE-MRI, parameters reflected significantly reduced tumor blood perfusion following bevacizumab administration. A single-arm study of combination bevacizumab and docetaxel in metastatic breast cancer revealed a significant association between the cell adhesion molecule, E-selectin, and clinical response (23).
There is evidence to suggest that docetaxel administered on a weekly basis has potent anti-angiogenic effects in addition to its cytotoxic anti-tumor activity (25, 26). These anti-angiogenic effects of docetaxel increase synergistically with the addition of bevacizumab (27). These findings raise the question of the added benefit of bevacizumab to the anti-angiogenic effects of docetaxel in the treatment of breast cancer. In an attempt to target tumor vasculature and differentiate the anti-angiogenic behavior of chemotherapy from that of a targeted anti-angiogenesis inhibitor, we designed a phase II randomized trial of weekly docetaxel versus weekly docetaxel combined with bevacizumab in patients with inoperable breast cancer. We hypothesized that the patients receiving combination therapy with docetaxel and bevacizumab would experience a greater effect on angiogenesis exhibited by a greater reduction in tumor microvascular density, more significant alterations in circulating markers of endothelial adhesion, and a larger decrease in tumor perfusion as measured by DCE MRI than those receiving docetaxel alone.
PATIENTS AND METHODS
Patients
Men and women ≥ 18 years old were eligible if they had newly-diagnosed, histologically-confirmed, inoperable adenocarcinoma of the breast as confirmed by consensus decision of the multidisciplinary Case Comprehensive Cancer Center Tumor Board. Those with Stage IV breast cancer were eligible if they presented with a concurrent locally-advanced breast cancer. No prior treatment for breast cancer was allowed and measurable disease was required. Other eligibility criteria included: ECOG performance status ≤ 2, normal organ and bone marrow function as described elsewhere (28), and a calculated left ventricular ejection fraction (LVEF) ≥ 45%. Patients were excluded from participation if they had New York Heart Association classification III or IV heart disease, CNS metastases, major surgery within 28 days of the start of therapy, or a history of thromboses. Full-dose anticoagulation was not permitted. Informed consent was obtained prior to the patient undergoing any investigational procedure. In 2005, bevacizumab was found to be of therapeutic benefit for metastatic breast cancer (24), and trastuzumab was determined to be efficacious in the treatment of adjuvant disease (29). These findings changed practice patterns and limited subsequent trial accrual, resulting in early study termination after enrollment of 49 of 60 planned patients.
Treatment
Patients were randomized to receive two pre-operative 8-week cycles of either docetaxel (D) alone or the combination of docetaxel and bevacizumab (DB). In both arms, docetaxel was given as 6 weekly one-hour IV infusions of 35 mg/m2, followed by a 2-week break (30). Dexamethasone was administered per standard prescribing recommendations to reduce the incidence and severity of hypersensitivity reactions and fluid retention. Cytokine support was not permitted. Bevacizumab (NSC 7048565) was supplied by the Cancer Therapy Evaluation Program/National Cancer Institute and was administered to patients receiving the combination arm (DB) at a dose of 10 mg/kg IV every other week throughout the 2 cycles of treatment (8 doses). Bevacizumab was administered immediately prior to docetaxel. The first dose of bevacizumab was given over 90 minutes; subsequent infusion times were sequentially reduced to 30 minutes if no adverse reaction occurred.
Four to six weeks after the completion of pre-operative therapy, patients considered operable underwent definitive surgery consisting of either a modified radical mastectomy or lumpectomy with a level I/II axillary lymph node dissection. Three to six weeks after surgery, patients who underwent mastectomy received radiation to the chest wall (50.4 Gy with an additional 10-14 Gy boost for those with close/positive margins or inflammatory breast cancer); those who had breast-conserving surgery received radiation therapy to the breast (45-50.4 Gy with a boost of an additional 16-18 Gy). All patients received supraclavicular nodal irradiation (45-50.4 Gy prescribed to a depth of 3 cm). Axillary irradiation (45 - 50.4 Gy) was added in patients who had an inadequate axillary node dissection (< 10 lymph nodes removed) or extensive axillary nodal involvement. For patients with gross residual disease in the axilla, a dose of up to 59.4 Gy was allowed. There was no elective treatment of the internal mammary lymph node chain.
Four weeks after completion of radiation therapy, patients received 4 cycles of standard adjuvant chemotherapy consisting of doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) (AC) given IV every 21 days. Cytokine support was permitted. Patients with hormone receptor positive disease subsequently received 5 years of standard dose tamoxifen or anastrozole (if postmenopausal).
Study Evaluations
Toxicity evaluations were performed on each D, DB and AC treatment day. Disease response was assessed clinically after cycles 1 and 2 (C1 and C2) of pre-operative treatment (D or DB), and following completion of AC. RECIST criteria was used to determine disease response and toxicity assessments were determined using the National Cancer Institute Common Toxicity Criteria, version 2.0. LVEF was assessed at baseline, at the end of preoperative chemotherapy (after week 16), before AC and at completion of AC.
Biologic Correlates
Plasma levels of angiogenic cytokines (VEGF, bFGF) and serum markers of endothelial damage (E-selectin, VCAM-1, ICAM-1) were measured prior to beginning preoperative treatment (either D or DB), after completing C1 (week 8) and completing C2 (week 17) of pre-operative treatment, and at 3, 6 and 12 months after the completion of radiation therapy. The protocol for collection and measurement of these markers has been described previously (28). All samples were assayed in triplicate. Intra- and inter-assay coefficients of variation for VEGF were 3.3 and 7.4%; ICAM-1 3.3 and 6.3%; b-FGF 10.4 and 14.0%; E-selectin 3.6 and 7.2%; VCAM-1 5.9 and 12.4%. The minimum reportable value for VEGF was 6.4 pg/mL.
Tissue Correlates
Multiple 14-gauge core needle biopsies of the primary breast tumor were obtained for correlative studies before initiating treatment and after C1 (week 8). Additional tumor tissue was obtained at the time of definitive surgery (approximately week 20). Tissue samples were assessed for microvessel density (MVD) using techniques previously published (31). Three pathologists independently read each slide.
Imaging Correlates: DCE-MRI Tumor Perfusion Assessments
DCE-MRI was repeated three times for each subject: prior to the start of preoperative treatment, after C1, and after C2. Scans were obtained at 1.5T (Siemens Magnetom Symphony) with a bilateral breast-imaging coil. Tumors were located using images from the standard Siemens’ breast exam. DCE-MRI employed T1-w 2d FLASH (TR/TE: 43/4 msec, slices=5, thickness=8mm, gap=2mm, matrix=128×128). FOV between 300mm-350mm was kept constant within subjects. Pre-contrast T1 estimations used the dual flip angle approach (32): flip angles of 10° and 50° with five-point averaging. Dynamic acquisitions comprised 180 acquisitions at 4.8 sec intervals. A 30sec, 0.1mg/kg i.v. injection of Gadolinium-DTPA (Magnevist) was started between acquisition 3 and 4. Regions of interest were obtained from baseline-subtracted enhancement images from 70 seconds post- injection. Desired voxels were identified with a combination of thresholding and interactive boundary drawing, allowing delineation of tumors with complex spatial distributions. Gd-DTPA concentration vs. time curves were calculated per our prior methods (33). The accepted measure of tumor perfusion, the initial area under the curve for the first 90 seconds post-contrast (IAUC90) (31), along with the efflux rate constant kep (34) were obtained. Tumor volume was also calculated (number of ROI voxel×individual voxel volume).
Statistical Methods
The primary purpose of this randomized phase II trial was to determine the added effects on angiogenesis from the addition of bevacizumab to the effects on angiogenesis caused by docetaxel. The primary objective was to measure the difference in change in biologic parameters between the two treatment arms: tumor-associated biomarkers (MVD, VEGF, bFGF, E-selectin, ICAM-1 and VCAM-1) and tumor perfusion as determined by DCE-MRI. These parameters were assessed in two ways: first, parameters were compared as continuous variables between treatment groups by Student’s T-test and second, parameters were compared at two different time points by paired T-test. The association between baseline biomarkers and clinical response was evaluated by logistic regression in both univariate and multivariate analyses. T-test was used to compare the difference of continuous measurements between two groups after checking normality. The association between two factors was examined by chi-square test.
Progression-free survival (PFS) was defined as time from first day of treatment to the date of cancer recurrence, progression, or death, and censored at the date of last follow-up for those without disease progression and still alive. Overall survival (OS) was defined as time from the start of treatment to death, and censored at the time of last assessment for survivors. The probability of PFS and OS were estimated by Kaplan-Meier method (35) and the difference between treatment arms was examined by log-rank test. The predictive value of biomarkers on PFS was examined by Cox model (36) after checking proportional hazard assumption in univariate analysis followed by multivariate Cox regression. Temporal pattern of biological parameters was also summarized using scatter plot superimposed with lowess smoother (37). All tests were two-sided and a p-value ≤ 0.05 was considered statistically significant.
RESULTS
Patient Characteristics
Between April 2002 and August 2005, 49 female patients were randomized to either D (n = 25) or DB (n = 24) (Table 1). Seventy-eight percent of patients were Caucasian and 22% African-American. The advanced nature of the study population is evidenced by 43% presenting with Stage III disease, 31% with inflammatory breast cancer (IBC), 12% having stage IV disease (also with inoperable local disease) and 39% having triple negative tumors. Groups were well-balanced in terms of clinical stage at presentation, however, a greater number of patients in the D arm had HER2 positive and hormone receptor negative disease.
Table 1. Patient Characteristics.
| Total | Docetaxel | Docetaxel + Bevacizumab | |
|---|---|---|---|
| No. enrolled | 49 | 25 | 24 |
| Median age (yrs) | 48 (22-79 yrs) | 46 | 48 |
| Caucasian | 38 (78%) | 20 | 18 |
| African-American | 11 (22%) | 5 | 6 |
| Clinical Stage IIB | 7 (14%) | 4 | 3 |
| Clinical Stage III | 21 (43%) | 10 | 11 |
| Clinical Stage IV | 6 (12%) | 3 | 3 |
| Subset Analysis | |||
| IBC | 15 (31%) | 8 | 7 |
| HER2+ | 11 (22%) | 9 | 2 |
| HR + | 23 (47%) | 8 | 15 |
| Triple Negative | 19 (39%) | 11 | 8 |
IBC - inflammatory breast cancer
HR - hormone receptor (ER or PR)
Toxicity
No unusual toxicity was evident during AC treatment; therefore the focus of this report is on the preoperative treatment. Both regimens were well tolerated. (Table 2). There were no episodes of uncontrolled hypertension, proteinuria, or thrombosis. The most notable grade 3/4 toxicities included neutropenia, stomatitis/pharyngitis and electrolyte abnormalities. The occurrence of grade 3 toxicity with DB was significantly higher compared to D (96% vs. 40%, p < 0.001). However, there was no significant difference in grade 4 toxicity between the two treatments (p = 0.667). One patient receiving DB died of complications from a perforated diverticuli, and another patient on DB experienced an upper GI bleed, which was controlled. Delayed wound healing, defined by an inability to start radiation therapy within 6 weeks of surgery, occurred in 8 patients, without a significant difference between treatment arms (DB = 5; D = 3; P = 0.691). Two patients experienced asymptomatic decreases in LVEF by 17%; one patient (DB) during preoperative treatment, and one patient (D) from baseline to end of AC (D). There were no episodes of congestive heart failure, or changes in LVEF to below the institutional upper limit of normal.
Table 2. Grade 3/4 Toxicity -Pre-operative Treatment Only.
| Grade 3 | Grade 4 | |||
|---|---|---|---|---|
| Hematologic | D | DB | D | DB |
| Leukocytopenia | 2 | 1 | ||
| Infection without neutropenia | 1 | 1 | ||
| Neutropenia | 1 | 1 | ||
| Anemia | 2 | |||
| Non-Hematologic | ||||
| Metabolic* | 3 | 1 | ||
| Hyperglycemia | 2 | 1 | ||
| Elevated transaminases | 1 | 1 | 1 | |
| Stomatitis | 4 | |||
| Anorexia/fatigue | 2 | 3 | ||
| Abdominal pain/nausea/vomiting/dehydration | 3 | 3 | ||
| Eye tearing | 1 | |||
| Sensory neuropathy | 1 | |||
| Myalgia | 1 | |||
| Melena/GI bleed | 1 | |||
| Perforated viscous** | 1 | |||
| Total | 10 | 23 | 4 | 2 |
D-docetaxel; DB-docetaxel and bevacizumab
Hypophosphatemia,hypouricemia,hyponatremia,hypokalemia
One treatment-related death No episodes of uncontrolled hypertension or proteinuria
Response to Therapy
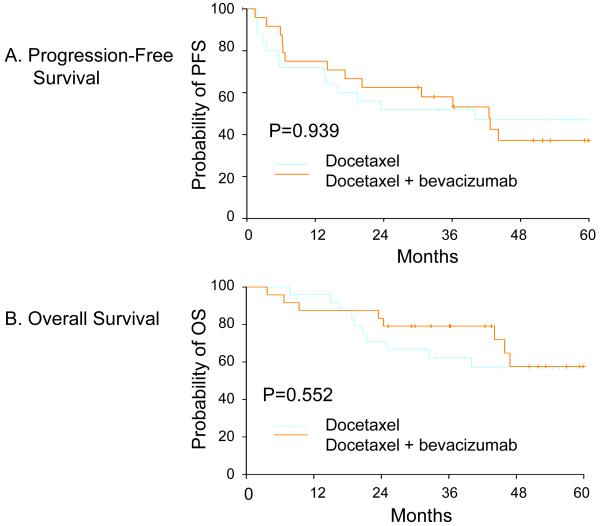
Surgical operability after D or DB constituted a clinical response. The overall clinical response rate was 78% with nineteen patients in each treatment arm becoming operable (Table 3). Three patients in each treatment arm had a clinical response, but remained inoperable. Three patients receiving D and 1 patient receiving DB developed progressive disease. One patient experienced a treatment-related death in the DB arm during neoadjuvant chemotherapy. Of the 38 patients who underwent surgery, the median number of pathologically-positive axillary lymph nodes (ALN) was 1 (range 0-20); 43% of patients had negative ALN (no difference between treatment groups). Two patients treated with D had residual ductal carcinoma in situ, consistent with a pathologic CR (38). The overall clinical response rates for the patients with IBC did not differ with respect to treatment assignment: 7 of the 16 patients became operable after protocol treatment, all achieving a partial pathologic response. There was no difference between the two groups in either 60-month PFS (D = 47.3%, DB = 37.2%, P = 0.939) or OS (D = 57.1%, DB = 57.6%, P = 0.583) (Figure 1). The median OS has not been reached.
Table 3. Clinical Outcomes.
| Outcome | Docetaxel N = 25 | Docetaxel and bevacizumab N = 24 |
|---|---|---|
| Surgical candidate | 19 (76%) | 19 (79%) |
| Breast conserving surgery | 7 (37%) | 1 (5%) |
| Modified radical mastectomy | 12 (63%) | 18 (95%) |
| Inadequate disease response | 3 | 3 |
| Disease progression on therapy | 3 | 1* |
| Delayed wound healing post-surgery | 3 (16%) | 5 (26%) |
Plus one treatment related death
Figure 1. Kaplan-Meier Outcome Curves.
Panel A shows the Kaplan-Meier estimation of progression-free survival (PFS) in months demonstrated by the two treatment groups: docetaxel (D) vs. docetaxel + bevacizumab (DB). The probability of PFS with DB treatment was consistently larger up to 3 years than with D treatment, though the difference was not statistically significant. Panel B shows the Kaplan-Meier estimation of overall survival (OS) in months demonstrated by the two treatment groups: docetaxel (D) vs. docetaxel + bevacizumab (DB). The median OS has not been reached in either treatment group, but again, there was no statistically significant difference in OS.
Biologic and Tissue Correlates
Plasma VEGF levels rose sharply from baseline to the end of C2 and with DB compared with a minimal increase in plasma VEGF levels with D (P < 0.0001). Plasma VEGF levels remained higher with DB than with D at 3 months post-radiation therapy (P = 0.011) (Figure 2A). There was no significant change in bFGF levels. Overall, there was a significant gradual increase in VCAM-1 levels from pre-treatment to the end of C2 (P < 0.0001). There was a trend for a greater increase in VCAM-1 levels among patients receiving DB compared to D, which did not reach statistical significance (P = 0.069) and completely resolved by 3 months post-radiation therapy (P = 0.382) (Figure 2B). ICAM levels gradually increased (P = 0.018) (Figure 2C) and E-selectin gradually decreased (P = 0.006) from pre-treatment to the end of C2 (Figure 2D), however there were no significant differences between treatment arms at any time points. Too few patients were available for analysis of biologic correlates at 6 and 12 months post radiation therapy. No significant changes were observed in tumor MVD assessment (data not shown).
Figure 2. Changes In Levels Of Angiogenic And Endothelial Markers Over Time.
All figures demonstrate a scatter plot of angiogenic and endothelial markers vs. time (days on study), superimposed with lowess smoother (locally weighted scatterplot smoothing) by treatment group; docetaxel (D) vs. docetaxel + bevacizumab (DB). The arrow in all figures denotes the end of Cycle 2 (C2).
Figure 2A - Overall, vascular endothelial growth factor (VEGF) levels increased with pre-operative treatment. There was no difference in VEGF levels pre-treatment, but at the end of C2, VEGF levels with DB were significantly larger than VEGF levels with D.
Figure 2B - Overall, there was a gradual increase in vascular cell adhesion molecule-1 (VCAM-1) levels with treatment. There was no difference in VCAM-1 levels pre-treatment, and only marginally different at the end of C2.
Figure 2C - Overall, there was an increase in intracellular adhesion molecule-1 (ICAM-1) with treatment. There was no difference in ICAM-1 levels pre-treatment or after C2.
Figure 2D - Overall, there was a decrease in E-selectin levels with treatment. There was no difference in E-selectin levels pre-treatment or at the end of C2.
Tumor Volume and Perfusion
Using DCE-MRI, there was a statistically significant decrease in overall IAUC90 after the completion of C1 and C2 (P = 0.007, P<0.0001, respectively) (Figure 3B). This reduction was greater among patients receiving DB compared to D (P = 0.024). There was also a statistically significant overall decrease in tumor volume in both groups following the completion of C1 and C2 (P = 0.004, P=0.012, respectively) (Figure 3A), with no difference between treatment arms.
Figure 3. Changes In DCE-MRI Analysis Of Tumor Volume And Tumor Perfusion.
Figure 3A: Bar plot of tumor volume (number of ROI voxel×individual voxel volume) by treatment; docetaxel (D) vs. docetaxel + bevacizumab (DB). Top figure demonstrates the mean ± SEM of all patients treated at baseline, end of cycle 1 (C1) and end of cycle 2 (C2). There was a significant decrease in tumor volume compared with baseline at both C1 and C2. Bottom figure demonstrates the mean ± SEM by treatment; D vs. DB, at baseline, C1 and C2. There was no difference in tumor volume between the two treatments at any time point.
Figure 3B: Bar plot of tumor IAUC90 (initial area under the curve for the first 90 seconds post-contrast) by treatment; D vs. DB. Top figure demonstrates all patients treated at baseline, end of C1 and end of C2. Compared with baseline, there was a significant decrease in IAUC90 at C1 and C2. Bottom figure demonstrates the mean ± SEM by treatment; D vs. DB, at baseline, C1, and C2. There was no difference in IAUC90 at baseline, DB had a significant low level of IAUC90 at C1 compared with D, and no significant difference at C2.
Use of Clinical and Biologic Correlatives to Predict Disease Response
On univariate analysis, baseline VCAM-1 and E-selectin levels were significant inverse predictors of clinical response (operability). (P = 0.033 and P= 0.035, respectively). The odds ratio of having a clinical response favored patients without IBC (Odds Ratio = 0.065; P = 0.002). In multivariate logistic regression analysis, only elevated VCAM-1 and presence of IBC showed a trend toward significance in predicting lower clinical response (P = 0.062 and 0.066, respectively).
DISCUSSION
The National Comprehensive Cancer Network (NCCN) guidelines support the use of preoperative systemic therapy for the treatment of locally-advanced (LABC) or inoperable breast cancer.1 This approach enables LABC to become a model for the investigation of novel anti-cancer agents by providing accessible tumor and blood specimens at various time points during systemic treatment. The goal of using this model is to gain information about biologic correlates that correspond to, or predict, a clinical outcome. We applied this principle to 49 patients with inoperable breast cancer to provide biologic evidence of inhibition of angiogenesis that may occur due to chemotherapy (docetaxel) or only when a specific anti-angiogenic agent (bevacizumab) is combined with chemotherapy (docetaxel). The hypothesis of this investigation was to show that changes in biologic correlatives, i.e., tumor blood perfusion as demonstrated by DCE-MRI, tumor microvessel density; and circulating markers of endothelial damage, would correspond with clinical disease response. We also hypothesized that the changes in these parameters would be greater among those patients treated with the combination of an anti-angiogenesis inhibitor and chemotherapy compared with chemotherapy alone.
A total of 49 patients were treated on study. All patients presented with locally advanced disease such that there was a low likelihood of obtaining a complete pathologic response with preoperative single agent docetaxel. All patients also received postoperative doxorubicin and cyclophosphamide so as to not compromise their overall clinical outcome. Thirty-seven patients (76%) achieved a clinical response allowing for surgical resection. Those patients were evenly divided between the two treatment groups. Although clinical response has been shown to be an important indicator in phase II trial design investigating targeting agents, this study was not powered to compare differences in clinical outcomes between the two treatment arms: docetaxel vs. docetaxel plus bevacizumab (39). In addition, both agents may be targeting the tumor vasculature, thus resulting in an inferior anti-cancer effect, especially when compared with other taxanes combined with angiogenesis inhibitors (24, 27).
More importantly, this study adds to the understanding of the changes in tumor-associated biomarkers and their correlation with clinical disease response. Byrne and colleagues (12) found that baseline levels of VCAM-1 were higher among patients with breast cancer and corresponded to a poorer prognosis. This phenomenon was also demonstrated in a study of 92 patients with breast cancer versus 31 matched controls (14). These data correspond with our results on univariate and multivariate analysis showing that increased baseline levels of VCAM-1 was associated with a reduction in disease response. Although there was a trend in demonstrating a greater increase in VCAM-1 levels with combination treatment using docetaxel and bevacizumab compared to docetaxel alone, both treatment arms were associated with a significant increase in VCAM-1 levels during active therapy. This may be due to our enrollment of patients with advanced stage as corroborated in other studies, or may reflect a small sample size examined in our study, or it may simply be a tumor response to chemotherapy (40).
Of interest, our institution investigated treatment of inflammatory breast cancer using combination doxorubicin and SU5416, a VEGFR2 tyrosine kinase inhibitor, and found no correlation between VCAM-1 expression and clinical outcome, whereas increasing levels of ICAM-1 correlated with event-free survival (33). Other studies involving breast cancer patients have also found a correlation between higher levels of ICAM-1 and a worse prognosis, especially among patients with metastatic disease (41). Our current study did not demonstrate this association. One possible explanation is that ICAM-1 may be a more sensitive marker of advanced or aggressive disease, i.e. IBC. Another possibility is that different cancers, e.g., lung and thyroid may be associated with a different spectrum of secreted adhesion molecules (42, 43).
Our current study using docetaxel with or without bevacizumab demonstrated an association between reduction of E-selectin levels and clinical outcome by univariate analysis (P=0.035). These results are supported by prior studies using SU5416 and doxorubicin therapy for inflammatory breast cancer, and a study combining bevacizumab and docetaxel in patients with metastatic breast cancer (23, 33).
Based upon our results and those of other investigators, VCAM-1 and E-selectin may represent important surrogates for breast cancer response; however the changes in quantity may not be specific to the administration of targeted angiogenesis inhibitors. Although our study demonstrated higher VCAM-1 levels in the combination bevacizumab and docetaxel arm compared with docetaxel alone, there was no statistical difference in E-selectin or ICAM-1 levels with the addition of the angiogenesis inhibitor. This may be a consequence of the potency of docetaxel as a chemotherapeutic agent with anti-angiogenic properties.
We also noted an increase in plasma VEGF initially with combination docetaxel and bevacizumab therapy, but this rise in VEGF subsequently normalized after completion of DB to levels indistinguishable from those observed in the docetaxel alone cohort. This finding may either reflect a compensatory rise in serum VEGF because of sequestration by bevacizumab, or might relate to the fact that the ELISA assay measures both unbound and bevacizumab-bound VEGF, thereby reflecting a longer serum half-life of antibody-bound VEGF (44). Although several studies have demonstrated a correlation between elevated levels of circulating VEGF and an adverse prognosis among breast cancer patients, we did not find this correlation with our current study, nor our prior investigation with SU5416 and doxorubicin for inflammatory breast cancer (23, 33, 45, 46).
DCE-MRI demonstrated a greater fall in IAUC90 with the combination treatment compared to chemotherapy alone at the end of C1 (P = 0.024), reflecting a greater decrease in tumor blood perfusion by the addition of bevacizumab to docetaxel. No further significant changes in IAUC90 were observed after C1, which suggests that the optimal benefit of antiangiogenic therapy may occur early in the course of disease treatment in areas of large tumor burden.
The model of utilizing inoperable disease to investigate biologic correlates of clinical disease response proved successful, and the results lend support to the results from other studies involving different patient populations. This study additionally demonstrates the safety and relative efficacy of the combination of the angiogenesis inhibitor bevacizumab and docetaxel. Unfortunately, the relatively small size of this study may have been insufficient to detect true differences in biologic markers of angiogenesis, and a larger randomized trial may support a greater biologic role of angiogenesis inhibitors. In addition, further investigation of biologic correlates should continue to provide information on the optimal administration of antiangiogenic therapy and improve patient selection for these specific treatments.
Acknowledgments
Supported in part by NIH grants: K23 CA87725 (BO); P30 CA43703, Avon Supplement to P30 C143703 (BO), U01 CA62502 and NCI Translational Research Initiative; M01 RR00080; Sanofi Aventis.
Footnotes
National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology; http://www.nccn.org
STATEMENT OF TRANSLATIONAL RELEVANCE
The purpose of this study was to determine the added biologic effect from an angiogenesis inhibitor to a taxane that possesses anti-angiogenic properties. Forty nine previously untreated patients with locally advanced breast cancer were randomized to receive either weekly docetaxel or weekly docetaxel with bevacizumab every other week for a total of 16 weeks. Biologic correlatives were obtained prior to treatment, between two cycles of therapy, and at the conclusion of pre-operative treatment. There was a statistically significant reduction in tumor perfusion calculated by DCE-MRI with combination chemotherapy and angiogenic inhibitor compared with single agent chemotherapy. While there was no significant difference in VCAM-1, ICAM or E-selectin levels between the two treatment groups, the clinical outcomes of these patients could be predicted by changes in VCAM-1 and E-selectin. We believe that these important results can be applied to breast cancer patients receiving pre-operative treatment to predict disease outcome.
REFERENCES
- (1).Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–74. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- (2).Ferrara N, Davis-Smyth T. Biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- (3).Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:527–43. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- (4).Ferrara N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999;56:794–814. doi: 10.1046/j.1523-1755.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- (5).Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18:5356–62. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- (6).Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB Journal. 1999;13:9–22. [PubMed] [Google Scholar]
- (7).Veikkola T, Aliltalo K. VEGFs, receptors and angiogenesis. Semin Cancer Biol. 1999;9:211–20. doi: 10.1006/scbi.1998.0091. [DOI] [PubMed] [Google Scholar]
- (8).Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60:203–12. [PubMed] [Google Scholar]
- (9).Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–39. [PMC free article] [PubMed] [Google Scholar]
- (10).Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- (11).Melder RJ, Koenig GC, Witwer BP, Safabakhsh N, Munn LL, Jain RK. During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nature Med. 1996;2:992–7. doi: 10.1038/nm0996-992. [DOI] [PubMed] [Google Scholar]
- (12).Byrne GJ, Ghellal A, Iddon J, et al. Serum soluble vascular cell adhesion molecule-1: role as a surrogate marker of angiogenesis. J Natl Cancer Inst. 2000;92:1329–36. doi: 10.1093/jnci/92.16.1329. [DOI] [PubMed] [Google Scholar]
- (13).Koch AE, Halloran MM, Haskell CJ, Shah MR, Polverini PJ. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature. 1995;376:517–519. doi: 10.1038/376517a0. [DOI] [PubMed] [Google Scholar]
- (14).O’Hanlon DM, Fitzsimons H, Lynch J, Tormey S, Malone C, Given HF. Soluble adhesion molecules (E-selectin, ICAM-1 and VCAM-1) in breast carcinoma. Eur J Cancer. 2002;38:2252–7. doi: 10.1016/s0959-8049(02)00218-6. [DOI] [PubMed] [Google Scholar]
- (15).Klein B, Levin I, Kfir B, Mishaeli M, Shapira J, Klein T. The significance of soluble interleukin-2, soluble interleukin-2 receptors, soluble ICAM-1 and beta 2-microglobulin in breast cancer patients. Tumour Biol. 1995;16:290–296. [PubMed] [Google Scholar]
- (16).Moss MA, Zimmer S, Anderson KW. Role of metastatic potential in the adhesion of human breast cancer cells to endothelial monolayers. Anticancer Res. 2000;20:1425–1433. [PubMed] [Google Scholar]
- (17).Banks RE, Gearing AJH, Hemingway IK, Norfolk DR, Perren TJ, Selby PJ. Circulating intercellular adhesion molecule-1 (ICAM-1), E-selectin, and vascular cell adhesion molecule-1 (VCAM-1) in human malignancies. Br J Cancer. 1993;68:122–124. doi: 10.1038/bjc.1993.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Fox SB, Turner GDH, Gatter KC, Harris AL. The increased expression of adhesion molecules ICAM-3, E- and P-selectins on breast cancer endothelium. J Pathol. 1995;177:369–376. doi: 10.1002/path.1711770407. [DOI] [PubMed] [Google Scholar]
- (19).Madhavan M, Srinivas P, Abraham E, Ahmed I, Vijayalekshmi NR, Balaram P. Down regulation of endothelial adhesion molecules in node positive breast cancer: possible failure of host defence mechanism. Path Oncol Res. 2002;8:125–128. doi: 10.1007/BF03033721. [DOI] [PubMed] [Google Scholar]
- (20).Cobleigh MA, Langmuir VK, Sledge GW, et al. A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol. 2003;30(5 Suppl 16):117–24. doi: 10.1053/j.seminoncol.2003.08.013. [DOI] [PubMed] [Google Scholar]
- (21).Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–9. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- (22).Wedam SB, Low JA, Yang SX, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24:769–77. doi: 10.1200/JCO.2005.03.4645. [DOI] [PubMed] [Google Scholar]
- (23).Ramaswamy B, Elias AD, Kelbick NT, et al. Phase II trial of bevacizumab in combination with weekly docetaxel in metastatic breast cancer patients. Clin Cancer Res. 2006;12:3124–9. doi: 10.1158/1078-0432.CCR-05-2603. [DOI] [PubMed] [Google Scholar]
- (24).Miller KD, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Eng J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- (25).Schimming R, Hunter NR, Mason KA. Inhibition of tumor neo-angiogenesis and induction of apoptosis as properties of docetaxel. Mund-, Kiefer- und Gesichtschirurgie. 1999;3:210–2. doi: 10.1007/s100060050132. [DOI] [PubMed] [Google Scholar]
- (26).Zoli W, Flamigni A, Frassineti GL, et al. In vitro activity of taxol and taxotere in comparison with doxorubicin and cisplatin on primary cell cultures of human breast cancers. Breast Cancer Res Treat. 1995;34:63–9. doi: 10.1007/BF00666492. [DOI] [PubMed] [Google Scholar]
- (27).Sweeney CJ, Miller KD, Sissons SE, et al. The Antiangiogenic Property Of Docetaxel Is Synergistic With A Recombinant Humanized Monoclonal Antibody Against Vascular Endothelial Growth Factor Or 2-Methoxyestradiol But Antagonized By Endothelial Growth Factors. Cancer Res. 2001;61:3369–3372. [PubMed] [Google Scholar]
- (28).Dowlati A, Robertson K, Radivoyevitch T, et al. Novel phase I dose de-escalation design trial to determine the biological modulatory dose of the anti-angiogenic agent SU5416. Clin Cancer Res. 2005;11:7938–44. doi: 10.1158/1078-0432.CCR-04-2538. [DOI] [PubMed] [Google Scholar]
- (29).Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- (30).Costa SD, von Minckwitz G, Raab G. The role of docetaxel (Taxotere) in neoadjuvant chemotherapy of breast cancer. Semin Oncol. 1999;26:24–31. [PubMed] [Google Scholar]
- (31).Wood LS, Ziats NP. Vascular targeting - case study: 55-year-old man with stage IV anaplastic thyroid cancer. Horiz Cancer Ther. 2002;3:24–5. [Google Scholar]
- (32).Dale BM, Jesberger JA, Lewin, et al. Determining and optimizing the precision of quantitative measurements of perfusion. JMRI. 2003;18:575–584. doi: 10.1002/jmri.10399. [DOI] [PubMed] [Google Scholar]
- (33).Overmoyer B, Fu P, Hoppel C, et al. Inflammatory breast cancer as a model disease to study tumor angiogenesis: results of a phase IB trial of combination SU5416 and doxorubicin. Clin Cancer Res. 2007;13:5862–8. doi: 10.1158/1078-0432.CCR-07-0688. [DOI] [PubMed] [Google Scholar]
- (34).Brix G, Semmler W, Port R, Schad LR, Layer G, Lorenz WJ. Pharmacokinetic Parameters in CNS Gd-DTPA Enhanced MR Imaging. J Comput.Assist.Tomogr. 1991;15(4):621–628. doi: 10.1097/00004728-199107000-00018. [DOI] [PubMed] [Google Scholar]
- (35).Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- (36).Cox DR. Regression Models and Life Tables (with Discussion) Journal of the Royal Statistical Society. 1972;B 34:187–220. [Google Scholar]
- (37).Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–836. [Google Scholar]
- (38).Mazouni C, Peintinger F, Wan-Kau S, et al. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol. 2007;25:2650–2655. doi: 10.1200/JCO.2006.08.2271. [DOI] [PubMed] [Google Scholar]
- (39).El-Maraghi RH, Eisenhauer EA. Review of phase II trial designs used in studies of molecular targeted agents: Outcomes and predictors of success in phase III. J Clin Oncol. 2008;26:1346–1354. doi: 10.1200/JCO.2007.13.5913. [DOI] [PubMed] [Google Scholar]
- (40).Bewick M, Conlon M, Lee H, et al. Evaluation of sICAM-1, sVCAM-1, and sE-Selectin levels in patients with metastatic breast cancer receiving high-dose chemotherapy. Stem Cells Dev. 2004;13:281–94. doi: 10.1089/154732804323099217. [DOI] [PubMed] [Google Scholar]
- (41).Zhang GJ, Adachi I. Serum levels of soluble intercellular adhesion molecule-1 and E-selectin in metastatic breast carcinoma: correlations with clinicopathological features and prognosis. Int J Oncol. 1999;14:71–77. [PubMed] [Google Scholar]
- (42).Mooney CJ, Nagaiah G, Fu Pingfu, et al. A phase II trial of fosbretabulin in advanced anaplastic thyroid carcinoma and correlation of baseline sICAM with outcome. Manuscript in process. doi: 10.1089/thy.2008.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Dowlati A, Gray R, Sandler A, Schiller JH, Johnson DH. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab - an Eastern Cooperative Oncology Group study. Clin Cancer Res. 2008;14:1407–1412. doi: 10.1158/1078-0432.CCR-07-1154. [DOI] [PubMed] [Google Scholar]
- (44).Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–34. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Salven P, Perhoniemi V, Tykka H, Maenpaa H, Joensuu H. Serum VEGF levels in women with a benign breast tumor or breast cancer. Breast Cancer Res and Treat. 1999;53:161–166. doi: 10.1023/a:1006178517505. [DOI] [PubMed] [Google Scholar]
- (46).Tung-Ping R, Fan ST, Wong J. Clinical Implications of circulating angiogenic factors in cancer patients. J Clin Oncol. 2001;19:1207–1225. doi: 10.1200/JCO.2001.19.4.1207. [DOI] [PubMed] [Google Scholar]