Abstract
Objective
To determine whether the flight attendants who were exposed to secondhand tobacco smoke (SHS) in the aircraft cabin have abnormal pulmonary function.
Methods
We administered questionnaires and performed pulmonary function testing in 61 never-smoking female flight attendants who worked in active air crews before the smoking ban on commercial aircraft (pre-ban).
Results
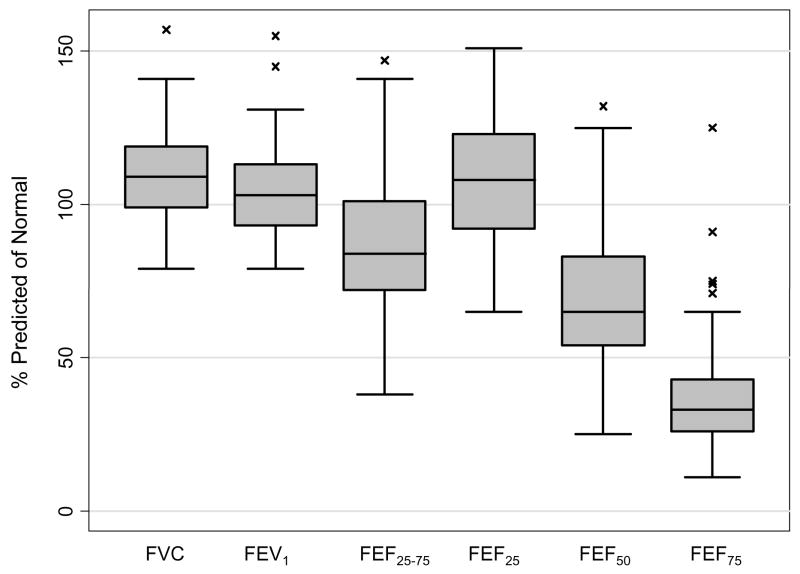
While the pre-ban flight attendants had normal FVC, FEV1, and FEV1/FVC ratio, they had significantly decreased flow at mid- and low-lung volumes, curvilinear flow-volume curves, and evidence of air trapping. Furthermore, the flight attendants had significantly decreased diffusing capacity (77.5±11.2 %predicted normal) with 51% having a diffusing capacity below their 95% normal prediction limit.
Conclusions
This cohort of healthy never-smoking flight attendants who were exposed to SHS in the aircraft cabin showed pulmonary function abnormalities suggestive of airway obstruction and impaired diffusion.
INTRODUCTION
Secondhand tobacco smoke (SHS) consists of the side-stream smoke from the burning end of the cigarette, which contains the highest concentration of particulate matter, and the exhaled mainstream smoke (1, 2). Exposure to SHS is associated with diverse health effects in nonsmokers. These adverse health outcomes include heart disease, lung cancer, asthma flares, chronic obstructive pulmonary disease (COPD), and upper airway problems such as sinusitis (3–10). Occupational exposure to SHS presents a substantial health risk to workers (11, 12). Flight attendants who worked on commercial aircraft before the ban on cigarette smoking (pre-ban flight attendants) were at potentially increased risk due to their previous high exposure to SHS in aircrafts. Flight attendants experienced poor air quality and high levels of SHS in aircraft before the smoking ban regardless of their class or cabin section (13, 14). Furthermore, a pre-ban era chemical analysis of post-flight urine samples from these flight attendants has shown elevated levels of urinary cotinine (a major metabolite of nicotine) indicating the flight attendants had been exposed to substantial levels of tobacco smoke on these aircraft (15).
While SHS exposure has been clearly established as a cause of cardiovascular diseases and lung cancer (4, 9, 10, 16–21), its effect on pulmonary function and development of COPD is less well studied. A few epidemiologic studies have reported associations between occupational or environmental SHS exposure and reduced lung function or the diagnosis of COPD in relatively large cohorts of both smokers and non-smokers based on multivariable regression analyses (3, 7, 22). However, none of these studies has looked exclusively at the long term effects of SHS in a never-smoking cohort. Here, we report abnormal pulmonary function in an otherwise healthy cohort of never-smoking flight attendants who worked on commercial aircraft in the United States before the ban on cigarette smoking in flights. Our hypothesis was that the flight attendants who were previously exposed to SHS within the relatively confined space of aircraft suffer from SHS-related long-term damage to their lungs.
METHODS
Study Population
Between July 2003 and December 2007, we recruited 86 pre-ban female flight attendants as part of a clinical investigation of the health effects of the cabin environment on flight attendants employed before and after the ban on smoking on commercial aircraft. We recruited flight attendants by various means such as announcements at Flight Attendants Medical Research Institute (FAMRI) meetings, notices at union meetings, “word of mouth”, and distribution of business cards. Flight attendants were eligible to participate in the study if they had worked for at least five years on aircraft before the airline ban on cigarette smoking, were never smokers (smoked no more than 100 cigarettes lifetime), and had no previous clinical diagnosis of cardiac or pulmonary diseases that could have adversely affected their pulmonary function. All subjects completed health and SHS exposure questionnaires, had a physical examination, and underwent pulmonary function testing.
From the initial 86 respondents, 21 subjects were excluded who were found to have history of tobacco use. Four other subjects were excluded due to history of illnesses that may have affected their lung function (one subject had received treatment for non-Hodgkin’s lymphoma, two had history of hepatitis C infection, and one had history of Sarcoidosis). The remaining 61 subjects completed the health and SHS exposure questionnaire, and underwent pulmonary function testing. Due to incomplete information, the SHS exposure history (the number of pre-ban years of employment) could only be computed accurately for 49 of these 61 subjects.
Subjects received no monetary compensation for participation in this study other than a meal and parking voucher. This study was approved by the UCSF Institutional Review Board, the Committee on Human Research.
Health and SHS exposure characterization
The SHS questionnaire is a modification of a SHS exposure questionnaire previously used by UCSF FAMRI Center of Excellence investigators (3). The questionnaire was modified to acquire information on airline-related occupational history including the employer airlines, the duration of employment, flight routes (domestic vs. international), and the cabin section. A copy of the SHS exposure questionnaire is available in our online supplement (http://links.lww.com/A1098).
The length of employment before and after the smoking ban was calculated using the dates of flight attendants’ employment and the dates at which specific airlines enforced the smoking ban on their domestic or international routes. The smoking ban was introduced on different dates by successive congressional legislation; first for domestic flights of 2 hours duration or less, then for domestic and North American flights of 6 hours duration or less, then for international flights to Europe, and finally for all international flights. In addition, some airlines voluntarily banned tobacco smoking before the required date by federal legislation.
We defined “pre-ban years” as the number of years of employment before the smoking ban during which a flight attendant was exposed to SHS in the aircraft cabin. For each airline, we used the date of the ban on domestic and North American flights of 6 hours duration or less as those airlines’ domestic cut-off for pre-ban years, and the ban date for all international flights as the cut-off for those specific airlines. If the ban was enforced after the first quarter of the year, that year was included as a pre- ban year. The smoking ban dates for domestic and international fights for airlines included in this study are listed in Table 1 (23, 24).
Table 1.
Smoking ban dates for domestic and international flights of different airlines.
| Ban on Domestic Flights of 6 hour Duration or Less | ||
|---|---|---|
| Date | Airlines | Last year included in pre-ban years |
| Apr-88 | Northwest | 88 |
| Jan-90 | Delta | 89 |
| Feb-90 | All others | 89 |
|
| ||
| Ban on All International Flights | ||
| Date | Airlines | Last year included in pre-ban years |
|
| ||
| Jan-95 | Delta | 94 |
| Jun-96 | US Air | 96 |
| Apr-97 | TWA | 97 |
| Jul-97 | All others | 97 |
Pulmonary function testing
Routine pulmonary function tests were performed on a dry rolling seal spirometer (Warren E. Collins Co., Braintree, MA, USA) in the seated position, including: flow-volume curve; spirometry (25); lung volume by single breath dilution (26, 27), multiple breath helium dilution (28), and plethysmograph (29); airway resistance during panting at functional residual capacity (FRC) (30, 31); and single breath carbon monoxide diffusing capacity (32). Pulmonary function studies were conducted according to the American Thoracic Society (ATS) and European Respiratory Society (ERS) guidelines (33–38). Briefly, we selected a group of healthy, nonsmoking subjects (50 males and 50 females) from the San Francisco Bay area with no personal history of cardiopulmonary disease, who were skin test negative to six local allergens, with normal chest x-ray and physical exam, and who were matched for age-range, race and ethnicity, and environmental characteristics of the reference populations. We used similar instruments and lung function protocols as in the reference populations as recommended by the ATS. Wherever possible, we compared parameters taken from the same reference source. We selected the specific reference equation for each parameter that provided the sum of residuals closest to zero as the most appropriate for our laboratory. We used suitable adjustment factors for African Americans and Asian Americans, as suggested by the ATS (37). The percent predicted values for spirometry and diffusing capacity are based on Crapo’s reference equations (39); the percent predicted values for lung volumes are based on Knudson’s reference equations (40). The largest coefficient of variation for diffusing capacity measurements in our laboratory among our biological standards is 4.1%. Body plethysmography was only performed in 40 subjects.
Data analysis
Distributions of subjects’ characteristics (i.e., age, lung function) were computed for the 61 subjects, and distributions of different SHS exposure variables were computed in the subset of 49 subjects with complete pre-ban airline employment history. Differences in characteristics between the two groups with and without SHS exposure history were examined. Measures of lung function, based on percent predicted of normal, were calculated and examined with box-whisker plots. Subjects’ predicted diffusing capacity was calculated and plotted relative to subjects’ observed diffusing capacity. The Wilcoxon signed-rank test was used as a non-parametric comparison of the paired variables (41). The potential contribution of non-airline related SHS exposure to subjects’ diffusing capacity was investigated by comparing the mean predicted values of subjects with airline SHS exposure only and those who reported additional non-airline SHS exposure (i.e., exposure during childhood and/or adulthood), in a subset of 46 subjects. One-way ANOVA was used to test for differences in mean predicted diffusing capacity between the different groups. Lastly, diffusing capacity, adjusted for age, height, hemoglobin, and total years of employment, was examined relative to pre-ban years of employment. All analyses were conducted in STATA (version 10.0) and SAS (version 9.1.3).
RESULTS
Subjects characteristics
Characteristics of the flight attendants are shown in Table 2. Subjects were all healthy women between the ages of 47 and 79 with no complaint of cardiac or pulmonary symptoms. All subjects were relatively fit as indicated by their body mass index (BMI). Total years of airline employment varied in length between 7 and 50. Among subjects whose pre-ban years of service were known (49 of 61 subjects), the estimated years of pre-ban employment were between 3 and 45 representing a range of 15% to 100% of the total length of their active duty employment. The characteristics of the 12 flight attendants with unknown pre-ban years of employment were not significantly different than those of the rest of the cohort (data not shown). Details of subjects’ pre-ban employment and other measures of SHS exposure are given below (Table 3).
Table 2.
Characteristics of pre-ban flight attendants (N=61). Data is shown in median [interquartile range] {range}.
| Subject Characteristics* | Median [IQR] | {Range} |
|---|---|---|
| Age (years) | 59 [56, 61] | {47, 79} |
| Height (cm) | 165 [160, 169] | {152, 181} |
| BMI (kg/m2) | 23.8 [21.3, 26.7] | {17.2, 41.2} |
| Hemoglobin level (g/dl) | 13.6 [13.0, 14.1] | {11.2, 16.5} |
| Total length of employment (years)** | 34 [22, 36] | {7, 50} |
Subjects were all female.
Detailed information of SHS exposure history (pre-ban years of employment) was obtained for 49 subjects (see Table 3).
Table 3.
SHS exposure history of the flight attendants (N=49).
| SHS Exposure History | |
|---|---|
| Airline occupation | |
| Total length of employment (years) | 34 [22, 37] {7, 50} |
| Pre-ban length of employment (years) | 24 [14, 29] {3, 45} |
| Pre-ban years as a fraction of total years (%) | 79.4 [63.2, 88.4] {15.0, 100.0} |
| Non-airline occupation § | |
| SHS exposure (N (%)) | 15 (33) * |
| Length of employment (years) | 0 [0, 1.5] {0, 15} * |
| Home exposure § | |
| Childhood (up to age 18) (N (%)) | 30 (61) |
| Adulthood (after age 18) (N (%)) | 21 (43) |
Length of employment data is shown in median [interquartile range] {range}; non-airline occupational and home exposures data are reported as number (percentage) of flight attendants.
Data available for 46 flight attendants only.
Subjects with childhood or adulthood home exposure or subjects with non-airline occupational exposure are not mutually exclusive.
Secondhand tobacco smoke exposure
Detailed history of exposure was available from 49 of the participating 61 flight attendants. These data are presented in Table 3. Total length of employment for these 49 subjects was comparable to the entire sample. The distribution of pre-ban years as a proportion of total flight years of employment indicated that for most subjects their employment overlapped with pre-ban years such that the flight attendants with longer length of employment had disproportionately higher SHS exposure. Most subjects reported additional SHS exposure apart from exposure during their experience as flight attendants; however, it is expected that their non-airline related SHS exposure was relatively insignificant compared to the intensity of their exposure while aboard aircraft (Table 3).
Spirometry and lung volumes
Pulmonary function measured by spirometry was, overall, within the normal range: FVC 3.48 ± 0.49L (109.1 ± 15.0 % predicted of age, height, and sex-adjusted normal value), FEV1 2.62 ± 0.38L (104.2 ± 15.2 % predicted normal) with an FEV1 to FVC ratio of 0.76 ± 0.05 indicating no overt evidence of airway obstruction by these tests (only eight out of 61 subjects had mildy decreased ratio below 0.70). In addition, airway resistance by plethysmography was also normal: 2.03 ± 0.74 cmH2O/LPS (N=40 for airway resistance). However, the flow-volume curves were curvilinear and the maximal airflow at mid- and low-lung volume was on average significantly decreased suggestive of an obstructive ventilatory defect: FEF25–75% 2.22 ± 0.66 L/s (88.7 ± 25.0 % predicted normal), FEF50% 3.20 ± 0.97 L/s (70.6 ± 21.9 % predicted normal) and FEF75% 0.81 ± 0.35 L/s (38.1 ± 19.1 % predicted normal) (Wilcoxon signed-rank test p<0.05; N=61) (Figure 1). Moreover, there was also associated air trapping, reflected by the increased difference (0.49 ± 0.25 L; Wilcoxon signed-rank test p<0.001; N=40) between total lung capacity measured by plethysmography (TLC) (5.23 ± 0.57 L; 103.2 ± 10.3 % predicted normal) and by single breath dilution (VA) (4.73 ± 0.58 L; 92.7 ± 10.3 % predicted normal).
Figure 1.
Box plot distribution of spirometry of the flight attendants as percent predicted values for their sex, age, and height (N=61). The box represents the interquartile range; the horizontal line inside the box represents the median; and the vertical lines (whiskers) represent the minimum and maximum non-outlier values. Outlier values are shown by a cross (x).
Diffusing capacity
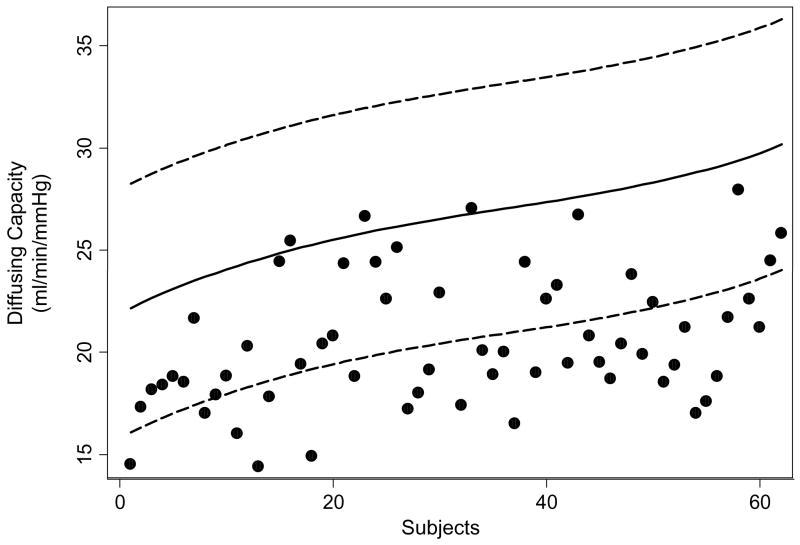
The single breath carbon monoxide diffusing capacity was abnormally decreased as shown in Table 4. Thirty one of the 61 subjects (51%) had diffusing capacity adjusted for hemoglobin below the 95% prediction limit of their normal values for their sex, age, and height, based on Crapo’s reference equations (39) (Figure 2). Subjects’ diffusing capacity means did not differ between those subjects who reported additional non-airline related SHS exposure and those who reported exposure only on board of aircraft during their employment as flight attendants (F-test: p<0.50) (Table 5).
Table 4.
Single breath carbon monoxide diffusing capacity at rest (N=61)
| Diffusing Capacity (ml/min/mmHg) | Actual value | %Predicted value | p-value* |
|---|---|---|---|
| Unadjusted | 20.1 ± 3.1 | 75.9 ± 10.4 | <0.001 |
| Adjusted for hemoglobin value | 20.5 ± 3.3 | 77.5 ± 11.2 | <0.001 |
| Adjusted for hemoglobin and alveolar volume (Dco/VA) | 4.3 ± 0.5 | 84.8 ± 15.3 | <0.001 |
Wilcoxon signed-rank test for comparison of actual diffusing capacity values with the predicted values.
Figure 2.
Diffusing capacity (adjusted for hemoglobin) of flight attendants compared to 95% prediction limits of their predicted values (N=61). Subjects are presented in increasing order of their predicted diffusing capacity. Black solid line: predicted diffusing capacity; dash lines: upper and lower 95% prediction limits; black dots: diffusing capacity of flight attendants.
Table 5.
Single breath carbon monoxide diffusing capacity at rest (adjusted for hemoglobin) of flight attendants categorized by their non-airline SHS exposure (N= 46)
| Non-airline SHS Exposure | N (%) | %Predicted Diffusing Capacity * |
|---|---|---|
| None | 6 (13) | 75.8 ± 10.1 |
| Childhood only | 11 (24) | 75.7 ± 11.5 |
| Adulthood only | 12 (26) | 77.6 ± 11.2 |
| Childhood & Adulthood | 17 (37) | 81.8 ± 11.7 |
|
| ||
| Total | 46 (100) | 78.5 ± 11.3 |
Number of flight attendants in each category is reported as number (percentage) and diffusing capacity is reported as mean ± SD. Data was available for 46 flight attendants only. Significance based on one-way ANOVA for differences in the means of diffusing capacity between categories was p<0.50.
Adulthood exposure included domestic and occupational sources of exposure.
DISCUSSION
In this study, we found that our 61 never-smoking female flight attendants who worked on commercial aircraft before the ban on cigarette smoking in flights had on average significantly decreased diffusing capacity, with 51% of them having diffusing capacity below the lower limit of the 95% prediction interval for their sex, age, and height. In addition, these same flight attendants had decreased maximal airflow at mid- and low-lung volumes as well as pulmonary function evidence of air trapping suggestive of airflow obstruction. Although these pulmonary function abnormalities are consistent with the presence of a mild degree of COPD, our cohort on average had a normal FEV1 to FVC ratio (only 8 out of 61 subjects had ratios less than 0.70) and thus does not meet the GOLD criteria for mild COPD (42). Despite this, the pulmonary function of these pre-ban flight attendants, particularly their diffusing capacity, is abnormal.
Furthermore, we developed a questionnaire-based estimate of air cabin-related occupational SHS exposure for our cohort of flight attendants by determining the number of pre-smoking ban years they had worked on domestic and international flights. We found that on average our cohort of flight attendants had served 74.6 ± 20.7 % of their active duty time in the pre-smoking ban era, which reflects that they had experienced considerable occupational SHS exposure. We then examined but did not find any association between the years of pre-ban employment, our surrogate of SHS exposure, and adjusted diffusing capacity (data not shown). However, our relatively small sample size along with other confounding factors, such as healthy worker effect (i.e. those employed longer had higher diffusing capacity) and possible exposure misclassification, limit our ability to draw any conclusion from this lack of association.
Occupational exposure to tobacco smoke has been an important source of SHS exposure in adults (15, 43–47) and presents a substantial health risk to workers (11, 12). Indeed, abnormal lung function has been reported in men exposed to SHS at their workplace (48, 49). Masi estimated that a never-smoking young woman who worked in an SHS-contaminated office would have her diffusing capacity reduced by three units below the value observed if she worked in a smoke-free office environment (46). Recently, Rizzi et al reported for the first time that current exposure to SHS in a cohort of healthy male adolescents was associated with decreased diffusing capacity; this lung function impairment was independent of exposure to maternal smoking during pregnancy, but it was dependent on the amount of exposure to SHS (50).
Air cabin-related SHS exposure in particular presented a substantial occupational health risk for the flight attendants because their SHS exposure occurred within the relatively confined space of commercial aircraft, which resulted in high intensity SHS exposures. Studies based on urine and serum concentrations of cotinine, a biomarker of exposure to tobacco smoke, have shown that, during the pre-ban era, the flight attendants experienced 6 to 7 times the SHS exposure compared to airlines ground-based workers, and 14 times that of the average person (51). In fact, the urinary cotinine levels in these flight attendants approached the levels that are observed in active cigarette smokers (52). This is important especially as the environmental condition of the aircraft cabin, including extremely low humidity (mean humidity of 5%) (13) and presence of air pollutants such as ozone (53), may have a compounding influence on the effects of SHS on lungs (54).
Recently, Ebbert et al reported an association between sinusitis, middle ear infection, and asthma symptoms and the hours of time spent in a smoky cabin among never-smoking pre-ban flight attendants (55). Our study extends the Ebbert et al study by showing that the never-smoking pre-ban flight attendants who have a significant history of occupational SHS exposure have abnormal pulmonary function suggestive of long-term damage to their lungs as seen in COPD.
Our study is limited by several factors. First, the small sample size of the study limits its statistical power. Second, recruitment in our study was not based on a random selection of flight attendants who flew prior to the ban on smoking on commercial aircraft, and thus the study population may not be a representative sample of the larger population of pre-ban flight attendants. We offered a comprehensive cardiopulmonary exam to all of flight attendants as an integral part of the study, but did not offer any other form of financial reimbursement. Therefore, subjects who joined this study may have done so for reasons related to underlying health concerns (although asymptomatic based on their answers to our questionnaire), employment status (e.g., retired), or other reasons (e.g., ability to travel to the clinic).
The sixty-one subjects selected for the current study had no smoking history, no pulmonary disease, and no underlying health problems that could have affected their lung function. Subjects’ ages indicate that they were older on average (mean 59.2 years, range 47–79) than flight attendants who flew prior to the airline smoking ban, based on data available from larger studies (56), although we did not find this to account for the decrease in their diffusing capacity levels. While the study population may not reflect a wider population of pre-ban women flight attendants, it does constitute a group of relatively healthy older flight attendants from the pre-ban era known to have no underlying respiratory disease and for whom objective measures of lung function could be obtained.
Third, our study could not fully control for factors, other than aircraft SHS (e.g. SHS in childhood, adulthood, or other cabin effects such as exposure to ozone, low atmospheric pressures, or low humidity), that may have contributed to the decrease in subjects’ diffusing capacity levels. We did inquire whether participants were ever exposed to SHS in childhood or in their adult years when not aboard aircraft. However, for these exposures, we did not develop a SHS exposure surrogate similar to the one that we used for quantification of the flight attendants airline exposure. As discussed above, studies have shown that the concentration of aircraft SHS exposure was particularly high and considerably more than most other sources of environmental or occupational SHS exposure (51). In addition, while we were able to calculate the duration of aircraft SHS exposure with relative accuracy by determination of flight attendants’ dates of employment, we expected the calculation of non-airline related SHS exposure would be more subject to recall bias. In our analysis, however, we did investigate the potential impact of any non-airline SHS exposure that the flight attendants may have received. Our results indicated that the diffusing capacity was not significantly different between the subjects who reported any additional non-airline SHS exposure and those who reported no additional exposure. It is possible that subjects’ non-airline SHS exposure in these instances was negligible compared to the exposure they received aboard aircraft. Other studies have quantified the differences in SHS exposure from aircraft and other sources (51), though we were not able to verify these differences with our data.
Given these limitations, our study represents an initial step toward improved understanding of the health effects of aircraft SHS exposure in otherwise healthy older flight attendants based on objective measures of pulmonary function.
In conclusion, we found that in our cohort of 61 never-smoking pre-ban flight attendants, single breath carbon monoxide diffusing capacity and maximal airflow at mid- and low-lung volumes were significantly decreased suggestive of long-term damage to the lungs of these flight attendants. The most likely factor contributing to the pulmonary function abnormalities in these never-smoking pre-ban flight attendants is their occupational exposure to SHS in commercial aircraft. Further studies of a larger number of pre-ban as well as post-smoking ban flight attendants and better assessment of their cabin-related exposure to SHS and other pollutants may help identify the causes of these abnormalities.
Supplementary Material
Acknowledgments
Supported by (1) the Flight Attendants Medical Research Institute (FAMRI) and (2) the National Institutes of Health (K23 HL083099).
We would like to thank Susan Beaulien for assistance with subject recruitment, Frank Seitzinger, Jolina Saliendra, and Oliver Beech for their help with performance of pulmonary function testing, and Drs. Ira Tager, Mark Eisner, Katharine Hammond, and Stan Glantz from UCSF FAMRI Center of Excellence for their guidance and input into data collection and analyses. We also would like to appreciate the contribution of the flight attendants who took time out of their busy schedules to participate as research subjects in this study.
References
- 1.First M. Constitutents of sidestream and mainstream tobacco and markers to quantify exposure to them. Indoor Air and Human Health. 1985:195–203. [Google Scholar]
- 2.Spengler J. Long term measurements of respirable sulfates and particles inside and outside homes. Atmos Environ. 1981;15:23–30. [Google Scholar]
- 3.Eisner MD, Wang Y, Haight TJ, Balmes J, Hammond SK, Tager IB. Secondhand smoke exposure, pulmonary function, and cardiovascular mortality. Ann Epidemiol. 2007;17:364–373. doi: 10.1016/j.annepidem.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Kawachi I, Colditz GA, Speizer FE, et al. A prospective study of passive smoking and coronary heart disease. Circulation. 1997;95:2374–2379. doi: 10.1161/01.cir.95.10.2374. [DOI] [PubMed] [Google Scholar]
- 5.Lam TH, Ho LM, Hedley AJ, et al. Secondhand smoke and respiratory ill health in current smokers. Tob Control. 2005;14:307–314. doi: 10.1136/tc.2005.011775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor AE, Johnson DC, Kazemi H. Environmental tobacco smoke and cardiovascular disease. A position paper from the Council on Cardiopulmonary and Critical Care, American Heart Association. Circulation. 1992;86:699–702. doi: 10.1161/01.cir.86.2.699. [DOI] [PubMed] [Google Scholar]
- 7.Eisner MD, Balmes J, Katz PP, Trupin L, Yelin EH, Blanc PD. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health. 2005;4:7. doi: 10.1186/1476-069X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisner MD, Klein J, Hammond SK, Koren G, Lactao G, Iribarren C. Directly measured second hand smoke exposure and asthma health outcomes. Thorax. 2005;60:814–821. doi: 10.1136/thx.2004.037283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitsavos C, Panagiotakos DB, Chrysohoou C, et al. Association between exposure to environmental tobacco smoke and the development of acute coronary syndromes: the CARDIO2000 case-control study. Tob Control. 2002;11:220–225. doi: 10.1136/tc.11.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitsavos C, Panagiotakos DB, Chrysohoou C, et al. Association between passive cigarette smoking and the risk of developing acute coronary syndromes: the CARDIO2000 study. Heart Vessels. 2002;16:127–130. doi: 10.1007/s003800200008. [DOI] [PubMed] [Google Scholar]
- 11.Hammond SK, Sorensen G, Youngstrom R, Ockene JK. Occupational exposure to environmental tobacco smoke. Jama. 1995;274:956–960. [PubMed] [Google Scholar]
- 12.Hammond SK. Exposure of U.S. workers to environmental tobacco smoke. Environ Health Perspect. 1999;107 (Suppl 2):329–340. doi: 10.1289/ehp.99107s2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindgren T, Norback D, Andersson K, Dammstrom BG. Cabin environment and perception of cabin air quality among commercial aircrew. Aviation, space, and environmental medicine. 2000;71:774–782. [PubMed] [Google Scholar]
- 14.Neilsen K, Glantz SA. A tobacco industry study of airline cabin air quality: dropping inconvenient findings. Tob Control. 2004;13 (Suppl 1):i20–29. doi: 10.1136/tc.2003.004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samet J, Marbury M, Spengler J. Health effects and sources of indoor air pollution. Part I. Am Rev Respir Dis. 1987;136:1486–1508. doi: 10.1164/ajrccm/136.6.1486. [DOI] [PubMed] [Google Scholar]
- 16.Glantz SA, Parmley WW. Passive smoking and heart disease. Epidemiology, physiology, and biochemistry. Circulation. 1991;83:1–12. doi: 10.1161/01.cir.83.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Glantz SA, Parmley WW. Passive smoking and heart disease. Mechanisms and risk. JAMA. 1995;273:1047–1053. [PubMed] [Google Scholar]
- 18.Taylor R, Najafi F, Dobson A. Meta-analysis of studies of passive smoking and lung cancer: effects of study type and continent. Int J Epidemiol. 2007;36:1048–1059. doi: 10.1093/ije/dym158. [DOI] [PubMed] [Google Scholar]
- 19.Takagi H, Sekino S, Kato T, Matsuno Y, Umemoto T. Revisiting evidence on lung cancer and passive smoking: adjustment for publication bias by means of “trim and fill” algorithm. Lung Cancer. 2006;51:245–246. doi: 10.1016/j.lungcan.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Zhong L, Goldberg MS, Parent ME, Hanley JA. Exposure to environmental tobacco smoke and the risk of lung cancer: a meta-analysis. Lung Cancer. 2000;27:3–18. doi: 10.1016/s0169-5002(99)00093-8. [DOI] [PubMed] [Google Scholar]
- 21.Panagiotakos DB, Chrysohoou C, Pitsavos C, et al. The association between secondhand smoke and the risk of developing acute coronary syndromes, among non-smokers, under the presence of several cardiovascular risk factors: The CARDIO2000 case-control study. BMC Public Health. 2002;2:9. doi: 10.1186/1471-2458-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisner MD. Environmental tobacco smoke exposure and pulmonary function among adults in NHANES III: impact on the general population and adults with current asthma. Environ Health Perspect. 2002;110:765–770. doi: 10.1289/ehp.02110765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopipero P, Bero LA. Tobacco interests or the public interest: 20 years of industry strategies to undermine airline smoking restrictions. Tob Control. 2006;15:323–332. doi: 10.1136/tc.2006.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobacco.org: Tobacco News and Information. Airline News at http://www.tobacco.org/Resources/travel.html.
- 25.Comroe JH., Jr . Methods in Medical Research. Chicago, Illinois: Year Book Publishers, Inc; 1950. Pulmonary Function Tests; p. 188. [Google Scholar]
- 26.Mitchell MM, Renzetti AD., Jr Evaluation of a single-breath method of measuring total lung capacity. Am Rev Respir Dis. 1968;97:571–580. doi: 10.1164/arrd.1968.97.4.571. [DOI] [PubMed] [Google Scholar]
- 27.Burns CB, Scheinhorn DJ. Evaluation of single-breath helium dilution total lung capacity in obstructive lung disease. Am Rev Respir Dis. 1984;130:580–583. doi: 10.1164/arrd.1984.130.4.580. [DOI] [PubMed] [Google Scholar]
- 28.Schaanning CG, Gulsvik A. Accuracy and precision of helium dilution technique and body plethysmography in measuring lung volumes. Scand J Clin Lab Invest. 1973;32:271–277. doi: 10.3109/00365517309082471. [DOI] [PubMed] [Google Scholar]
- 29.Dubois AB, Botelho SY, Bedell GN, Marshall R, Comroe JH., Jr A rapid plethysmographic method for measuring thoracic gas volume: a comparison with a nitrogen washout method for measuring functional residual capacity in normal subjects. J Clin Invest. 1956;35:322–326. doi: 10.1172/JCI103281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois AB, Botelho SY, Comroe JH., Jr A new method for measuring airway resistance in man using a body plethysmograph: values in normal subjects and in patients with respiratory disease. J Clin Invest. 1956;35:327–335. doi: 10.1172/JCI103282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briscoe WA, Dubois AB. The relationship between airway resistance, airway conductance and lung volume in subjects of different age and body size. J Clin Invest. 1958;37:1279–1285. doi: 10.1172/JCI103715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blakemore WS, Forster RE, Morton JW, Ogilvie CM. A standardized breath holding technique for the clinical measurement of the diffusing capacity of the lung for carbon monoxide. J Clin Invest. 1957;36:1–17. doi: 10.1172/JCI103402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 34.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 35.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 36.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 37.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 38.Standardization of Spirometry, 1994 Update. American Thoracic Society. American journal of respiratory and critical care medicine. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 39.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 40.Knudson RJ, Slatin RC, Lebowitz MD, Burrows B. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. The American review of respiratory disease. 1976;113:587–600. doi: 10.1164/arrd.1976.113.5.587. [DOI] [PubMed] [Google Scholar]
- 41.Rice J. Mathematical Statistics and Data Analysis. 2. Duxbury Press; 1995. [Google Scholar]
- 42.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. American journal of respiratory and critical care medicine. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 43.Samet J, Marbury M, Spengler J. Health effects and sources of indoor air pollution. Part II. Am Rev Respir Dis. 1988;137:221–242. doi: 10.1164/ajrccm/137.1.221. [DOI] [PubMed] [Google Scholar]
- 44.Masjedi M, Kazemi H, Johnson D. Effects of passivev smoking on the pulmonary function of adults. Thorax. 1990;45:27–31. doi: 10.1136/thx.45.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuki H. Personal exposure to NO2 and its health effect wiht urinary hydroxproline to creatine ratio as a biochemical indicator. Indoor Air and Human Health. 1984;2:243–248. [Google Scholar]
- 46.Masi M. Environmental exposure to tobacco smoke and lung function in young adults. Am Rev Respir Dis. 1988;138:296–299. doi: 10.1164/ajrccm/138.2.296. [DOI] [PubMed] [Google Scholar]
- 47.Lebowitz M. Longitudinal study of pulmonary functioni devlopment in childhood, adolescence, and early adulthood. Am Rev Respir Dis. 1987;136:69–75. doi: 10.1164/ajrccm/136.1.69. [DOI] [PubMed] [Google Scholar]
- 48.Kentner M, Triebig G, Weltle D. The influence of passive smoking on pulmonary function--a study of 1,351 office workers. Preventive medicine. 1984;13:656–669. doi: 10.1016/s0091-7435(84)80015-8. [DOI] [PubMed] [Google Scholar]
- 49.Masjedi MR, Kazemi H, Johnson DC. Effects of passive smoking on the pulmonary function of adults. Thorax. 1990;45:27–31. doi: 10.1136/thx.45.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rizzi M, Sergi M, Andreoli A, Pecis M, Bruschi C, Fanfulla F. Environmental tobacco smoke may induce early lung damage in healthy male adolescents. Chest. 2004;125:1387–1393. doi: 10.1378/chest.125.4.1387. [DOI] [PubMed] [Google Scholar]
- 51.Repace J. Flying the smoky skies: secondhand smoke exposure of flight attendants. Tob Control. 2004;13 (Suppl 1):i8–19. doi: 10.1136/tc.2003.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riboli E, Haley NJ, Tredaniel J, Saracci R, Preston-Martin S, Trichopoulos D. Misclassification of smoking status among women in relation to exposure to environmental tobacco smoke. Eur Respir J. 1995;8:285–290. doi: 10.1183/09031936.95.08020285. [DOI] [PubMed] [Google Scholar]
- 53.Rayman RB. Cabin air quality: an overview. Aviation, space, and environmental medicine. 2002;73:211–215. [PubMed] [Google Scholar]
- 54.Yu M, Pinkerton KE, Witschi H. Short-term exposure to aged and diluted sidestream cigarette smoke enhances ozone-induced lung injury in B6C3F1 mice. Toxicol Sci. 2002;65:99–106. doi: 10.1093/toxsci/65.1.99. [DOI] [PubMed] [Google Scholar]
- 55.Ebbert JO, Croghan IT, Schroeder DR, Murawski J, Hurt RD. Association between respiratory tract diseases and secondhand smoke exposure among never smoking flight attendants: a cross-sectional survey. Environ Health. 2007;6:28. doi: 10.1186/1476-069X-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagda NL, Koontz MD. Review of studies on flight attendant health and comfort in airliner cabins. Aviation, space, and environmental medicine. 2003;74:101–109. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.