Abstract
Resveratrol is a phytochemical that has been under consideration for use as a prostate cancer chemopreventive agent. However, the efficacy, as well as the mechanisms of action of resveratrol on prostate cancer prevention, remains largely unknown. This study seeks to address these questions and examine the cancer preventive effects of resveratrol using complementary human LNCaP prostate cancer cell culture and xenograft models. In cultured LNCaP cells, we found that resveratrol inhibited cell growth. The growth inhibitory effects of resveratrol appeared to be through modulation of both androgen- and estrogen-mediated events. Global gene expression analysis using microarrays identified androgen-responsive genes as a group of genes universally affected by resveratrol in LNCaP cells in vitro. The effect of resveratrol on expression of these genes appeared to be through inhibition of both androgen- and estrogen-mediated transcription. In a xenograft model, resveratrol delayed LNCaP tumor growth and inhibited expression of a marker for steroid hormone responses. However, exposure to resveratrol also led to increased angiogenesis and inhibition of apoptosis in the xenograft. In summary, resveratrol may act through modulation of steroid hormone-dependent pathways to inhibit prostate cancer cell growth in both culture and xenografts, but exposure in vivo may be of concern.
Introduction
Prostate cancer is the second leading cause of cancer death in American men (1). Although the etiology of prostate cancer remains unknown, elevated levels of steroid hormones such as androgens and estrogens, as well as growth factors such as insulin-like growth factor-1 (IGF-1), are considered to be important risk factors (2–4). These hormones and growth factors have been shown to promote proliferation of prostate cancer cells through the activation of receptor-mediated signaling pathways (2–4). Therapeutic as well as preventive strategies have explored modulation of these pathways as potential approaches to prevent or control prostate cancer (5–7).
Bioactive food components, in particular, are increasingly being evaluated as potential prostate cancer chemopreventive agents because of their presumed safety (8). One such agent is resveratrol (9), a polyphenol (trans-3,5,4′-trihydroxystilbene) categorized as a phytoalexin (10), found principally in the skin of grapes, but also in peanuts and other plant species (11). Red wine, often mentioned as a good source of resveratrol, contains 1–10 mg of resveratrol/l (4–40 μM) (12). Recent studies attributed a variety of health benefits to consumption of foods containing resveratrol, including protection against cancers, cardiovascular disease and aging (13).
Results from rodent carcinogenesis models suggest that resveratrol can inhibit initiation, promotion and progression (14). Moreover, molecular studies show that resveratrol possesses anticancer activities, including acting as an antioxidant (15), possessing anti-inflammatory properties (11,13) and functioning as a weak estrogen (16). In vitro experiments using prostate cancer cell lines provide support for resveratrol to serve as a candidate prostate cancer preventive agent. Resveratrol has been shown to inhibit prostate cancer cell growth in culture (17,18), to inhibit DNA synthesis (19) and to increase apoptosis in LNCaP cells, a human prostate cancer cell line (20). It has been reported that resveratrol may increase expression and serine phosphorylation levels of the tumor suppressor protein p53 (21), thereby affecting activation of p53-dependent signaling pathways, such as inhibition of cell cycle progression and induction of apoptosis. Resveratrol has also been found to decrease expression of prostate-specific antigen (PSA), an androgen-responsive gene (ARG) that is often used as a marker for prostate cancer cell growth (17). Moreover, a recent microarray study revealed that resveratrol may exert global effects on ARG expression in LNCaP cells (22). ARGs such as PSA play important roles in cellular functions, including cell cycle regulation, transcription, cell proliferation and differentiation, as well as metabolism (23,24). We have shown previously that estrogen as well as androgen can regulate ARG expression (25), suggesting that the effects of resveratrol on ARGs may be through modulation of steroid hormone-mediated pathways. Given the roles of androgen and estrogen in prostate cancer development (2,3), modulation of these pathways may contribute to resveratrol’s protective effects against prostate cancer. However, despite the in vitro work suggesting that resveratrol shows promise as a prostate cancer chemopreventive agent, the in vivo effects of resveratrol, as well as the mechanisms underlying those effects on prostate cancer, remain largely unknown.
The present study uses complementary LNCaP cell culture and xenograft models to test the hypothesis that resveratrol is protective against prostate cancer. We also test the hypothesis that resveratrol exerts its effects, in part, through modulation of steroid hormone-dependent pathways. We report here that resveratrol in vitro appeared to affect multiple pathways that impact prostate cancer cell growth, and that the effects of resveratrol are mediated in part by modulation of androgen receptor (AR)- and estrogen receptor-dependent signaling pathways. In vivo, resveratrol initially delayed tumor growth but was also found to decrease tumor apoptosis and increase tumor angiogenesis.
Materials and methods
Chemicals
Resveratrol, 17β-estradiol, dihydrotestosterone and dimethylsulfoxide were from Sigma Chemical Co. (St Louis, MO). The synthetic androgen R1881 was from NEN Life Science Products (Boston, MA).
Cells and cell culture
LNCaP human prostate cancer cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained in Media A [RPMI 1640 medium with phenol red (Invitrogen, Carlsbad, CA), 2 mM L-glutamine (Sigma, St. Louis, MO), 100 U/ml penicillin and 100 μg/ml streptomycin (BioSource International, Camarillo, CA) with 10% fetal bovine serum (FBS) (Invitrogen)]. Cells were incubated in the presence of 5% CO2 in air at 37°C.
Cell growth assay
LNCaP cells (5 × 104 cells per well) were plated in 24-well plates (Costar, Corning Incorporated, Corning, NY); 24 h later, treatments were begun. Cells were treated with 0, 1, 5, 10 or 25 μM resveratrol (dimethylsulfoxide as vehicle) for 0–96 h, and the medium containing resveratrol was replaced every 24 h. Cell growth was analyzed using the sulforhodamine B assay as described previously (25). For experiments using the synthetic androgen R1881 or 17β-estradiol, cells were switched to Media B [RPMI 1640 medium without phenol red (Invitrogen), 2 mM L-glutamine (Sigma), 100 U/ml penicillin and 100 μg/ml streptomycin with 10% charcoal–dextran-treated FBS (CDS; Hyclone, Logan, UT)] 24 h after plating to minimize the effect of serum steroid hormones. The cells were then incubated in Media B for an additional 24 h before the treatments were begun.
Microarray analysis of the effect of resveratrol on global gene expression in LNCaP cells
Microarray gene expression profiling experiments were conducted as described previously (26). Briefly, LNCaP cells (2 × 107 cells per T-175 flask) were exposed to 0, 1, 5 or 25 μM resveratrol for 48 h, total RNA was isolated and microarray analysis was performed as described previously using the Affymetrix (Affymetrix, Santa Clara, CA) platform (26). The Affymetrix U133 chip was used for gene expression analysis. Resveratrol-responsive genes were identified using the MAS 5.0 suite (Affymetrix) following criteria of a 1.5-fold increase and P < 0.01 as a cutoff as described previously (26). Triplicate treatments were performed for each concentration. Microarray data are available upon request and also will be deposited in public database.
Determination of the effects of resveratrol on gene expression in LNCaP cells using reverse transcription–polymerase chain reaction
LNCaP cells were plated in six-well plates (1 × 106 cells per well) in Media A and switched to Media B containing 10% CDS 24 h after plating to minimize the effect of serum hormones. Twenty-four hours later, the medium was replaced with fresh medium containing 1 nM R1881 or 17β-estradiol with or without 25 μM resveratrol. Fresh medium containing the test compounds was changed daily and cells were harvested for total RNA isolation using the Trizol method (Invitrogen) after 48 h as described previously (25,26). Real-time reverse transcription–polymerase chain reaction (RT–PCR) was used to quantify expression of the following genes as described previously (25,26): PSA; beta-2-microglobulin (B2M); selenoprotein P, plasma, 1 (SEPP1); serine threonine kinase 39 (STK39); cyclin-dependent kinase inhibitor 1A/p21WAF1/CIP1 (CDKN1A) and insulin-like growth factor-1 receptor (IGF-1R).
Western analysis of the effects of resveratrol on PSA expression in LNCaP cells
LNCaP cells were plated on 100 mm Falcon tissue culture plates (2 × 106 cells per plate) in Media A. After 24 h, the medium was changed to Media B with 10% CDS, and the cells were incubated for an additional 24 h. After this incubation, the cells were then treated with either 1 nM R1881 or 1 nM 17β-estradiol in the presence or absence of 25 μM resveratrol. Medium containing the test compounds was replaced every 24 h. Cells were treated with the test compounds for a total of 96 h. Immunodetection of PSA was performed following the protocol described previously (27).
AR binding assays
To assess the affinity of resveratrol for AR, the Androgen Receptor Competitor Assay Kit, Green (Invitrogen), a fluorescence polarization AR binding assay, was used according to the manufacturer’s protocol. Dihydrotestosterone (0–1.5 μM) was used as a positive control. The resveratrol concentration range used was 0–50 μM. Fluorescence polarization was detected using a TECAN ULTRA fluorescence plate reader (Tecan Systems, San Jose, CA) set at 485 nm excitation and 535 nm emission wavelengths. Results were calculated as percent control = [(no compound control fluorescence units (FU) − with compound FU)/(no compound control FU − with 1.5 μM dihydrotestosterone FU)] × 100%. Triplicate assays were performed and results expressed as percent control ± SD.
Effects of resveratrol on LNCaP cancer cell xenografts in athymic nude mice
To determine the prostate cancer protective effects of resveratrol in vivo, a nude mouse xenograft model (28) was used. Five-week-old male athymic nude mice (BALB/cAnNCr-nu/nu, 20–22 g; Charles River, Frederick, MD) were individually housed in filter-top cages at the Beltsville Human Nutrition Research Center animal facility. Animals were randomly assigned to the following diet groups: (i) control (AIN-93M) diet, (ii) AIN-93M supplemented with 50 mg resveratrol/kg diet (RES50) or (iii) AIN-93M supplemented with 100 mg resveratrol/kg diet (RES100). Pellet diets were prepared by Research Diets (New Brunswick, NJ). The concentrations of resveratrol were selected based on published effective doses for cancer prevention and those used in prior animal studies (13,14,29). Twenty-two mice were used per treatment group. After 2 weeks of adaptation on the diets, human LNCaP prostate tumors were established in the animals by a subcutaneous injection of LNCaP cells (2 × 106 cells) resuspended in 50 μl of phosphate-buffered saline (PBS) (pH 7.4) plus 50 μl of Matrigel (BD Biosciences, Bedford, MA). The animals were palpated for tumors, and measurement of tumor volume began on the third week after injection. The cancer preventive efficacy of resveratrol was assessed by twice-weekly measurements of tumor volume, which was calculated using the equation: tumor volume (cm3) = 0.523 × [length (cm) × width2 (cm2)] (30). Mice were fed the diets described above until 7 weeks after the injection of cells, at which time the animals were euthanized. Tumors were removed from the animals, and a sample of tumor tissue was fixed in 10% neutral buffered formalin, embedded in paraffin and cut into 5 μm sections for in situ immunohistochemical (IHC) analysis. Remaining tumor samples were flash frozen under liquid nitrogen and stored at −80°C for resveratrol analysis as described below. Plasma samples were collected and stored at −80°C using the BD Vacutainer® PPT™ Plasma Preparation Tube Procedure as provided by the manufacturer (BD Biosciences, San Jose, CA).
IHC determination of steroid hormone-responsive pathway response, proliferation, apoptosis and angiogenesis
IHC staining protocol for PSA
PSA, a classic ARG responsive to both androgen and estrogen (25), was used to assess the effect of resveratrol on steroid hormone-dependent pathways. Paraffin-embedded sections were deparaffinized into ethanol. Endogenous peroxidase activity was blocked using 0.6% H202 in methanol. Slides were briefly rinsed in PBS prior to application of the serum block (Rabbit Elite ABC Kit, Vector Laboratories, Burlingame, CA, Kit# PK-6101). Slides were incubated with 250 μg/ml of rabbit polyclonal anti-Human PSA (Dako, Carpinteria, CA, Ref. A0562, Lot 00012666, 3.0 g/l) in 0.1% bovine serum albumin–PBS overnight at 4°C and rinsed in PBS; secondary antibody (Rabbit Elite Kit) was then applied as directed. Slides were again rinsed in PBS, ABC reagent (Rabbit Elite Kit) was applied, a final PBS wash was performed and slides were stained with 3,3-diaminobenzidine tetrahydrochloride (Sigma–Aldrich, St. Louis, MO, 10 mg of substrate/tablet). Slides were counterstained with hematoxylin. Human prostate tissue was used as the positive control.
IHC staining protocol for proliferating cell nuclear antigen
Proliferating cell nuclear antigen (PCNA) was used as a proliferation marker (31). Freshly cut paraffin-embedded sections were deparaffinized into ethanol. Endogenous peroxidase activity was blocked using 0.6% H2O2 in methanol. Antigen retrieval was performed by microwaving in deionized water for two 5 min cycles. Slides were cooled for 20 min and rinsed in deionized water. Monoclonal mouse anti-PCNA (Dako, clone PC10, code number M0879, 570 mg/l) was diluted to 0.7 μg/ml. Following a final PBS wash, slides were stained with 3,3-diaminobenzidine tetrahydrochloride (Sigma–Aldrich, 10 mg of substrate/tablet). Slides were counterstained with hematoxylin. Mouse small intestine tissue was used as a positive control.
IHC staining protocol for apoptosis
Paraffin-embedded sections were deparaffinized into PBS. An apoptosis detection kit using the terminal deoxynucleotidyl transferase dUTP nick and labeling assay (32) was used (ApopTag Peroxidase In Situ Apoptosis Detection Kit, Chemicon International, Temecula, CA) according to the manufacturer’s instructions. Mouse testes tissue was used as a positive control.
IHC staining protocol for platelet/endothelial cell adhesion molecule-1
Micro blood vessels were identified using platelet/endothelial cell adhesion molecule-1 (PECAM-1) staining (33). Paraffin-embedded sections were deparaffinized and placed in water. Slides were postfixed in zinc fixative (BD Pharmigen, San Jose, CA) for 10 min followed by a deionized water and PBS rinse. Endogenous peroxidase activity was blocked using 0.6% H2O2 in methanol. Antigen retrieval was performed by microwaving the slide for two 10 min cycles in 1 mM ethylenediaminetetraacetic acid buffer. Slides were cooled for 20 min at room temperature, followed by a 30 min avidin–biotin block (Vector Avidin–Biotin Blocking Kit). Slides were briefly rinsed in PBS prior to application of the serum block (Goat Elite ABC Kit, Vector Laboratories, Kit# PK-6105). Slides were incubated with 1 μg/ml of anti-PECAM-1 antibody (M-20, sc-1506, goat polyclonal IgG, 200 μg/ml, Santa Cruz Biotechnologies, Santa Cruz, CA) in 0.1% bovine serum albumin–PBS for 30 min at room temperature and rinsed in PBS; secondary antibody (Goat Elite Kit) was then applied as directed. Slides were rinsed in PBS, ABC reagent (Goat Elite Kit) was applied, a final PBS wash was performed and Vector Nova Red (Vector Nova Red Substrate Kit, for Peroxidase, sk-4800) was applied. Slides were counterstained with hematoxylin. Mouse kidney and heart tissues were used as positive controls.
IHC staining protocol for vascular endothelial growth factor
Paraffin-embedded sections were deparaffinized into ethanol. Endogenous peroxidase activity was blocked using 0.6% H2O2 in methanol. Antigen retrieval was performed by microwaving for two 10 min cycles in citrate buffer. Slides were cooled for 20 min at room temperature, followed by a 20 min serum block (Goat Elite ABC Kit, Vector Laboratories, Kit# PK-6105). Anti-mouse vascular endothelial growth factor (VEGF) antibody, P20 from Santa Cruz Biotechnologies (sc1836, lot A1405, goat polyclonal IgG, 200 μg/ml), was used. Slides were incubated with 0.2 μg/ml of P20 in 0.1% bovine serum albumin–PBS for 60 min at room temperature. Slides were rinsed in PBS (0.05% Tween); secondary antibody (Goat Elite Kit) was then applied as directed. Slides were again rinsed in PBS (0.05% Tween), ABC reagent (Goat Elite Kit) was applied, a final PBS wash was performed and slides were stained with 3,3-diaminobenzidine tetrahydrochloride (Sigma–Aldrich, 10 mg of substrate/tablet). Slides were counterstained with hematoxylin. Mouse kidney tissue was used as a positive control. Five samples from each diet group were evaluated by a certified pathologist to obtain qualitative measurement of VEGF expression.
Quantitation of IHC results using image analysis
All IHC slides, other than VEGF, were quantitated using the following protocol. The image was acquired using a Nikon DXM1200F Digital Camera (Nikon Eclipse 80i Microscope, Nikon ACT-1 v2.70 software) and analyzed using Image-Pro Plus v5.0 with custom-designed macros. Parameters for image analysis were as follows: (i) All images were taken at a constant exposure associated with a particular group of stains. (ii) Images were acquired pseudorandomly with no images overlapping the same areas. (iii) All macros were constructed from a random set of images from the associated stain to compensate for variability within the group. (iv) All images were acquired at ×20 (×200 total magnification). (v) Ten areas were randomly taken for each slide, and data are presented as an average. Special notes per stain are as follows. For PSA analysis, no post-capture modification was done to the images in order to preserve the color intensity of each sample. A 3-tier intensity scale was arbitrarily constructed to sort the varying degree of positive cells into groups. Data supplied included raw area in millimeter square and percent composition of each group of cells that make up the particular image and were expressed as PSA expression indices. For PCNA analysis, all images were calibrated to a master (single image) color profile to maximize accuracy. Total cells were counted as well as total tissue area (excluding white space). Data were presented as cells per area of tissue in millimeter square and expressed as proliferation indices. For apoptosis analysis, all images were calibrated to a master (single image) color profile to maximize accuracy. Apoptotic cells were analyzed through contrast enhancement algorithms. Data were presented as raw apoptotic cell counts and expressed as apoptosis indices. For PECAM-1 analysis, all images were calibrated to a master (single image) color profile to maximize accuracy. Vessels were analyzed through contrast enhancement algorithms. Non-specific/background signal was filtered out via morphological algorithms. Data were presented as raw vessel counts and expressed as angiogenesis indices.
Determination of resveratrol content in mouse plasma and tumor samples
Plasma and tumor resveratrol concentrations were determined using liquid chromatography–electrospray ionization–mass spectrometry following the protocol described below.
Liquid chromatography–electrospray ionization–mass spectrometry system and conditions
The liquid chromatography–electrospray ionization–mass spectrometry system used was a Shimadzu LCMS-2010 EV liquid chromatograph mass spectrometer system (Kyoto, Japan) connected to the LC portion consisting of two LC-10AD pumps, a SIL-10AD VP auto injector, a SPD-10A VP ultraviolet detector and a SCL-10A VP system controller. Data qualitation and quantitation were accomplished using Shimadzu LCMS Solutions Version 3 software (Kyoto, Japan). The analytical column used was a Phenomenex Luna C18 (2) (150 × 4.6 mm intradermally, 5 μm particle size). The mobile phase consisted of (i) acetonitrile and (ii) 0.5% aqueous acetic acid (vol/vol), filtered and degassed under reduced pressure prior to use. Separation was achieved using gradient elutions of 18–31% A at 0–10 min and 31–48% A at 10–20 min at a flow rate of 1.0 ml/min at ambient temperature (25 ± 1°C). This was followed by a 5 min equilibration period with initial conditions prior to injection of the next sample. Ultraviolet detection was set at 320 nm.
The mass spectrometer conditions consisted of a curved desolvation line temperature of 200°C and a block temperature of 200°C. The curved desolvation line, interface and detector voltages were −20.0 V, 4.5 kV and 1.2 kV, respectively. Vacuum was maintained by an Edwards® E2M30 rotary vacuum pump (Edwards, UK). Liquid nitrogen (Washington State University Central Stores, Pullman, WA) was used as a source of nebulizer gas (1.5 l/min). Resveratrol and chlorogenic acid internal standard (IS) were qualitated in selected ion monitoring negative mode. The monitored single plot transitions were resveratrol at m/z 227 and chlorogenic acid at m/z 353.
Stock and working standard solution
Methanolic stock solutions of resveratrol (100 μg/ml) and the IS, chlorogenic acid (100 μg/ml), were prepared. These solutions were protected from light and stored at −20°C between uses, for no longer than 3 months. Calibration standards in serum were prepared daily from the stock solution of resveratrol by sequential dilution with blank rat serum, yielding a series of concentrations, namely, 0.05, 0.1, 0.5, 1.0, 5.0, 10.0, 50.0 and 100.0 g/ml.
Sample preparation
Plasma samples (0.1 ml) were aliquoted and 25 μl of chlorogenic acid (IS) was added to each sample. The proteins present were precipitated using 1 ml of ice-cold high-performance liquid chromatography (HPLC)-grade acetonitrile, vortexed for 1 min (Vortex Genie-2, VWR Scientific, West Chester, PA) and centrifuged at 5 000 r.p.m. for 5 min (Beckman Microfuge centrifuge, Beckman Coulter, Fullerton, CA). The supernatants were evaporated to dryness under compressed nitrogen gas. The residue was reconstituted with 100 μl of mobile phase, vortexed for 1 min and centrifuged at 5000 r.p.m. for 5 min; the supernatant was transferred to HPLC vials, and 25 μl of it was injected into the LC/MS system. Weighed tumors were rapidly frozen in liquid nitrogen and pulverized to a fine powder with a mortar and pestle under liquid nitrogen. To each sample, 25 μl of chlorogenic acid (IS) and 1 ml ice-cold HPLC-grade acetonitrile were added. Samples were vortexed for 1 min and centrifuged at 5000 r.p.m. for 5 min. The supernatants were evaporated to dryness under compressed nitrogen gas. The residue was reconstituted with 100 μl of mobile phase, vortexed for 1 min and centrifuged at 5000 r.p.m. for 5 min; the supernatant was transferred to HPLC vials, and 25 μl of it was injected into the LC/MS system.
Statistical methods
For in vitro experiments, StatView (SAS Institute, Cary, NC) software was used for statistical analysis. Multiple group data were analyzed using analysis of variance followed by post hoc analysis with Fisher’s PLSD test. The unpaired Student’s t-test was used to compare experiments with two groups. Values were considered significant at P < 0.05. The animal tumor data for each treatment group were analyzed using SAS® Proc MIXED (SAS Institute). Data measured on animals that did not survive the entire experiment were not included in statistical analyses. A random coefficients model (34) was fit to the data to model the linear relationships between tumor size and time after injection for each treatment group and to make comparisons among rates of tumor growth and average tumor size at specific times. Correlation among the weekly measured tumor sizes in each animal was modeled using a Toeplitz covariance structure with heterogeneous variances to accurately represent the increasing variation in tumor size relative to time (35). Only prespecified comparisons were conducted, using no more than the total of five degrees of freedom available for comparing treatment group means without risking inflation of the Type I error (P = 0.05).
Results
Effects of resveratrol on LNCaP cell growth in vitro
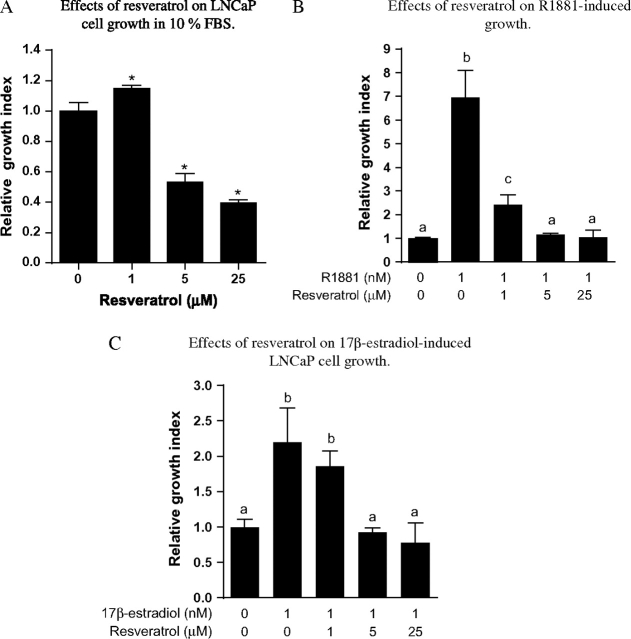
As shown in Figure 1A, in vitro exposure of androgen-responsive LNCaP human prostate cancer cells to medium containing 10% FBS with various concentrations of resveratrol (0–25 μM), relative to medium without resveratrol, resulted in concentration-dependent growth inhibition. The inhibitory effects of resveratrol occurred at concentrations as low as 5 μM (Figure 1A). As both androgen and estrogen can contribute to cell growth in LNCaP cells cultured in 10% FBS (25), we also examined the interactive effects of resveratrol, androgen and estrogen on growth to elucidate the underlying mechanisms. LNCaP cells were cultured in 10% CDS with 1 nM synthetic androgen R1881 or 17β-estradiol in the presence of 0, 1, 5 or 25 μM resveratrol for 96 h. As shown in Figure 1B and C, resveratrol inhibited cell growth induced by R1881 or 17β-estradiol in a concentration-dependent manner. The effects of resveratrol occurred at concentrations as low as 1 μM for androgen (Figure 1B) and as low as 5 μM for 17β-estradiol (Figure 1C).
Fig. 1.
Effects of resveratrol on LNCaP cell growth in culture. (A) Concentration-dependent effects of resveratrol on LNCaP cells cultured in 10% FBS. LNCaP cells were plated in 24-well plates; 24 h after plating, cells were treated with 0, 1, 5 or 25 μM resveratrol for 96 h and cell growth determined as described in Materials and Methods. Results are expressed as mean ± SD (n = 4). Bars with *are significantly different from vehicle control (0) at P < 0.05. (B) Effects of resveratrol on R1881-induced cell growth. LNCaP cells were plated in 24-well plates; 24 h after plating, the medium was switched to Media B, which contains 10% CDS, for an additional 24 h. Cells were then treated with and without R1881 (1 nM) in the presence of 0, 1, 5 or 25 μM resveratrol for 96 h and cell growth determined as described in Materials and Methods. Results are expressed as percent inhibition (mean ± SD, n = 4). Bars with different letters are significantly different from each other at P < 0.05. (C) Effects of resveratrol on 17β-estradiol-induced growth. LNCaP cells were seeded in 24-well plates; 24 h after plating, medium was switched to Media B, which contains 10% CDS, for an additional 24 h. Cells were then treated with and without 17β-estradiol (1 nM) in the presence of 0, 1, 5 and 25 μM resveratrol for 96 h, and cell growth was determined as described in Materials and Methods. Results are expressed as percent inhibition (mean ± SD, n = 4). Bars with different letters are significantly different from each other at P < 0.05.
Microarray and RT–PCR analysis of the effects of resveratrol on gene expression in LNCaP cells in vitro
To further elucidate the molecular mechanisms underlying the growth inhibitory effects of resveratrol, we performed microarray analysis to study the effects of resveratrol on global gene expression in LNCaP cells. As shown in Figure 2A, ARGs appeared to be globally affected by treatment with resveratrol. Expression of classic ARGs such as kallikrein 3 [commonly known as PSA (23,25)] was downregulated, whereas androgen-downregulated genes such as BCHE (23,25) were upregulated by resveratrol. In addition, several messenger RNAs (mRNAs) coding for genes involved in the AKT-mediated pathway (36,37) were also affected by exposure to resveratrol. These included IGF-1R, PIK3R3, FRAP/mTOR and FOX3A (Figure 2A). Of these genes, IGF-1R, PIK3R3 and FRAP/mTOR are known to be ARGs (23,38). Selected ARGs were subjected to RT–PCR confirmation, and consistent with the microarray results, expression of PSA and STK39 was upregulated in response to resveratrol, whereas no changes were observed for B2M and SEPP1 (Figure 2B). Additionally, IGF-1R, a receptor for IGF-1, was found to be downregulated by resveratrol in microarray analysis (Figure 2A), which was confirmed by RT–PCR (Figure 2C). Our microarray results also indicated that resveratrol induced expression of CDKN1A. CDKN1A is a cyclin inhibitor regulated by p53, a tumor suppressor protein involved in cell cycle arrest and apoptosis (39). Using RT–PCR, we found that in LNCaP cells exposed to resveratrol for 48 h, CDKN1A mRNA increased significantly after 24 h exposure to 25 μM resveratrol, but not at lower resveratrol concentrations (Figure 2D).
Fig. 2.
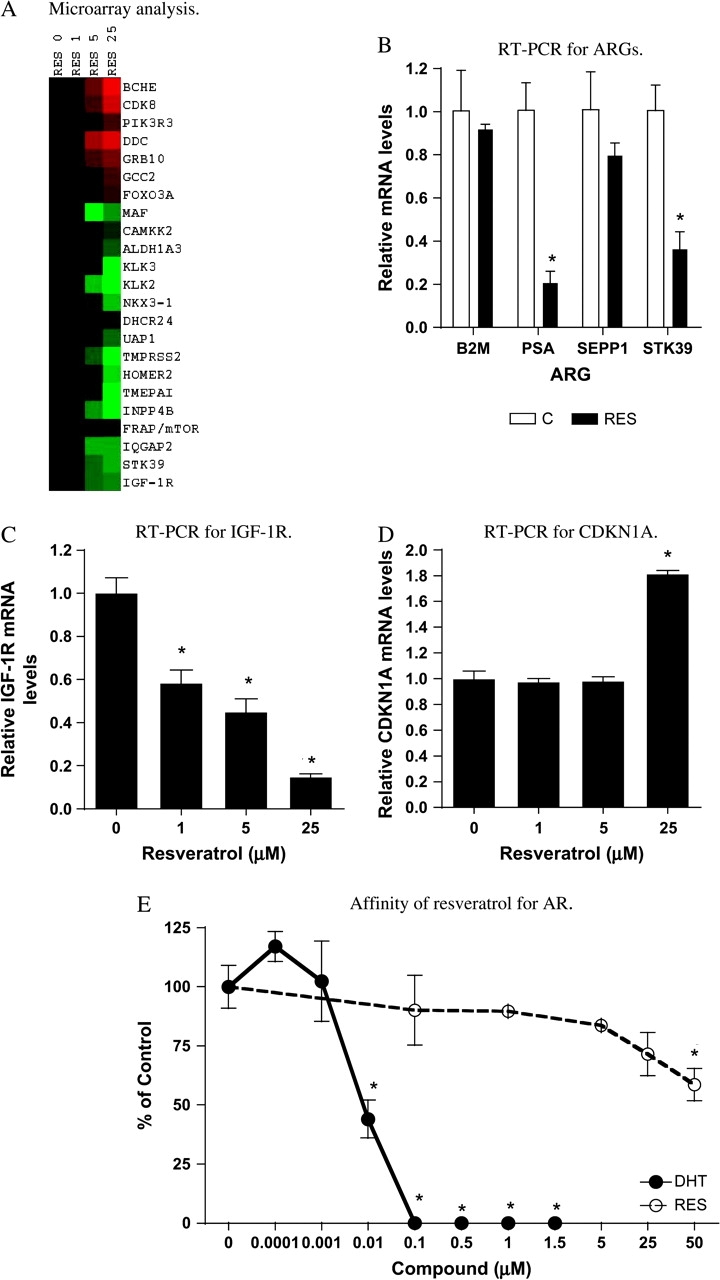
Effects of resveratrol on ARG expression and affinity of resveratrol for AR. (A) Microarray analysis of ARG gene expression. LNCaP cells cultured in 10% FBS were treated with 0, 1, 5 or 25 μM resveratrol for 48 h, and total RNA isolation and microarray analysis for ARG expression were performed as described in Materials and Methods. Triplicate treatments and analyses were conducted for each concentration. Results are expressed as a heat map. (Red color: upregulated gene and green color: downregulated gene.) Intensity of the color is proportional to the magnitude of effects on a log 2 scale, −3 to +3. (B) RT–PCR analysis of effects of resveratrol on ARG expression. LNCaP cells cultured in 10% FBS were treated with or without resveratrol (25 μM) for 48 h, total RNA was isolated and mRNA levels of selected ARGs (B2M, PSA, SEPP1 and STK39) were determined as described in Materials and Methods. Results are expressed as mean ± SD (n = 3). Bar with * is significantly different from vehicle control at P < 0.05. (C) RT–PCR analysis of effects of resveratrol on IGF-1R expression. LNCaP cells cultured in 10% FBS were treated with 0, 1, 5 or 25 μM resveratrol for 48 h, and total RNA was isolated and mRNA levels of IGF-1R determined as described in Materials and Methods. Results are expressed as mean ± SD (n = 3). Bars with *are significantly different from vehicle control (0) at P < 0.05. (D) RT–PCR analysis of effects of resveratrol on CDKN1A expression. LNCaP cells cultured in 10% FBS were treated with 0, 1, 5 or 25 μM resveratrol for 48 h, and total RNA was isolated and mRNA levels of CDKN1A determined as described in Materials and Methods. Results are expressed as mean ± SD (n = 3). Bar with *is significantly different from vehicle control (0) at P < 0.05. (E) Affinity of resveratrol for AR. AR binding assays were performed as described in Materials and Methods. Dihydrotestosterone (DHT) was used as positive control. Results are expressed as percent control ± SD (n = 3). Points with *are significantly different from vehicle control (0) at P < 0.05.
Because resveratrol has been shown to be estrogenic but appears to have low affinity for estrogen receptors (16), we also examined the affinity of resveratrol for ARs in LNCaP cells. As shown in Figure 2E, in a competition assay, resveratrol appeared to have little affinity for ARs, as indicated by its inability to displace fluorescent ligand from ARs.
Since expression of selected ARG mRNAs, as with cell growth, is subject to regulation by androgen and 17β-estradiol (25), the effects of resveratrol on both androgen- and estrogen-mediated changes in ARGs were further examined using RT–PCR. As shown in Figure 3A, treatment of LNCaP cells with resveratrol effectively inhibited the R1881-induced increase in several known ARGs: B2M, PSA, SEPP1, STK39 and IGF-1R (23,25). Resveratrol also inhibited 17β-estradiol induction of PSA, STK39 and IGF-1R (Figure 3B). Expression of B2M and SEPP1 was not 17β-estradiol inducible. Consistent with changes at the message level, the effect of resveratrol on the expression of the classic ARG, PSA, was also reflected at the protein expression level. As shown in Figure 3C by western analysis, 25 μM resveratrol effectively inhibited the induction of PSA by 1 nM R1881 or 17β-estradiol.
Fig. 3.
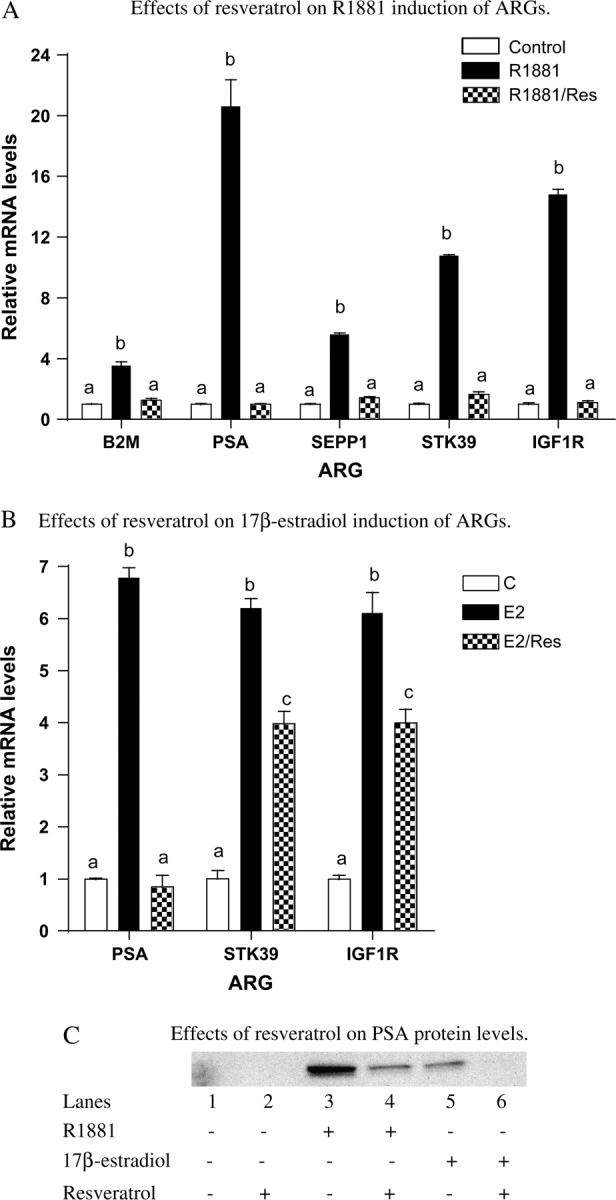
Effects of resveratrol on R1881- and 17β-estradiol-induced ARG mRNA and protein levels. (A) Effects of resveratrol on the R1881-induced increase in ARG mRNA levels. LNCaP cells were plated in six-well plates; 24 h after plating, the medium was switched to Media B, which contains 10% CDS, for an additional 24 h. Cells were then treated with or without R1881 (1 nM) in the presence or absence of resveratrol (25 μM) for 48 h, total RNA was isolated and mRNA levels of selected ARGs (B2M, PSA, SEPP1 and STK39) were determined as described in Materials and Methods. Results are expressed as mean ± SD (n = 3). Bars with different letters are significantly different from each other at P < 0.05. (B) Effects of resveratrol on the 17β-estradiol-induced increase in ARG mRNA levels. LNCaP cells were plated in six-well plates; 24 h after plating, the medium was switched to Media B, which contains 10% CDS, for an additional 24 h. Cells were then treated with or without 17β-estradiol (1 nM) in the presence or absence of resveratrol (25 μM) for 48 h, total RNA was isolated and mRNA levels of selected ARGs (PSA and STK39) were determined as described in Materials and Methods. Results are expressed as mean ± SD (n = 3). Bars with different letters are significantly different from each other at P < 0.05. (C) Effects of resveratrol on PSA protein levels. LNCaP cells were plated in 100 mm plates; 24 h after plating, the medium was switched to Media B, which contains 10% CDS, for an additional 24 h. Cells were then treated with or without R1881 or 17β-estradiol (1 nM) in the presence or absence of resveratrol (25 μM) for 48 h, protein was isolated and immunodetection of PSA was performed as described in Materials and Methods.
Effects of resveratrol on a LNCaP prostate cancer cell xenograft model
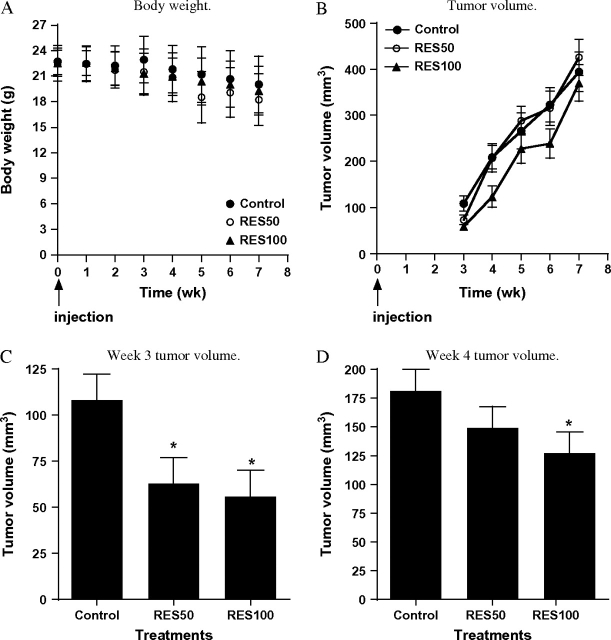
Having established the in vitro effects of resveratrol, we sought to extend the findings in vivo using a LNCaP cell tumor xenograft model (28,30). Resveratrol in the diet did not significantly affect body weight of the animals (Figure 4A). As shown in Figure 4B, tumor volume increased in all diet groups (Figure 4B). However, as shown in Figure 4C and D, there was a significant delay in tumor growth in animals consuming the RES50 diet (week 3 after injection of LNCaP cells) or RES100 diet (weeks 3 and 4). By the seventh week, there were no differences in tumor volume. The rate of tumor growth was not statistically different across weeks 3–7 for control versus RES50 versus RES100. By IHC analysis, expression of PSA was significantly less in tumors from mice fed resveratrol, and this effect was dose dependent (Figure 5A). The expression of PSA within the tumors appeared to be heterogeneous, with some cells containing more intense PSA staining than others. The IHC analysis showed no differences between the treatments in overall levels of PCNA, a marker for proliferation (Figure 5B). Interestingly, we observed significantly lower levels of apoptosis (as assessed by ApopTag) in tumors from the RES50- and RES100-fed animals compared with control (Figure 5C). Moreover, we also observed an increase in microvessel formation in RES100-fed animals as assessed by PECAM-1 staining (Figure 5D), suggesting an increase in angiogenesis in this group. However, we did not observe qualitative differences in VEGF staining (data not shown) between the diet groups.
Fig. 4.
Effects of resveratrol on LNCaP cell-derived tumor growth in athymic nude mice. LNCaP cell tumor xenografts were established in athymic nude mice (n = 22 per diet group) as described in Materials and Methods. Tumor volumes were measured twice a week and calculated as described in Materials and Methods. The linear coefficients and standard errors from the random effects model are shown in the graph. (A) Body weights of control, RES50 and RES100 groups during the study. (B) Temporal effects on tumor growth in animals fed control (filled circles), RES50 (open circles) or RES100 (filled triangles) diets. (C) Comparison of tumor volumes between animals fed control, RES50 or RES100 diet in week 3. Week 3 results from Panel B were regraphed, and *indicates significantly different from control at P < 0.05. (D) Comparison of tumor volumes between animals fed control, RES50 or RES100 diets in week 4. Week 4 results from Panel B were regraphed, and *indicates significantly different from control at P < 0.05.
Fig. 5.
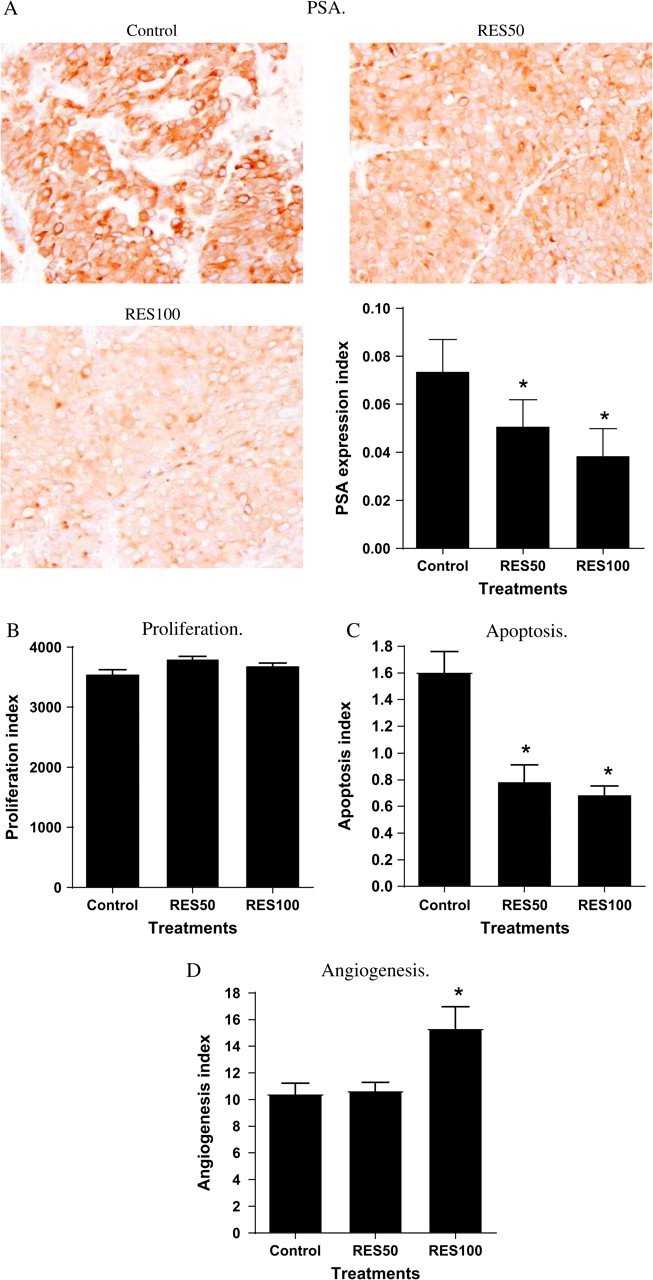
IHC analysis of markers for the steroid hormone-responsive pathway, proliferation, apoptosis and angiogenesis. IHC analyses for the steroid hormone-responsive gene PSA, the proliferation marker PCNA, apoptosis and the angiogenesis marker PECAM-1 were performed on paraffin-embedded tumor samples as described in Materials and Methods. (A) PSA. PSA expression index = average PSA-positive cells/mm2 (B) PCNA. Proliferation index = average PCNA-positive cells/mm2 (C) ApopTag. Apoptosis index = average apoptotic cells per field (D) PECAM-1. Angiogenesis index = average vessel counts per field *indicates significantly different from control at P < 0.05.
Resveratrol concentrations in plasma and tumor samples were determined. We found plasma resveratrol concentrations for animals fed with resveratrol averaged 0.78 ± 0.0326 μM (mean ± SE, n = 12) and 1.30 ± 0.336 μM (mean ± SE, n = 12) for the RES50 and RES100 groups, respectively, with concentrations ranging from 0.7 μM to as high as 5.25 μM was observed. Resveratrol was also detectable in tumors, and the average concentrations were 0.33 ± 0.051 μM (mean ± SE, n = 12) and 0.33 ± 0.045 μM (mean ± SE, n = 12) for the RES50 and RES100 groups, respectively, with concentrations as high as 0.7 μM in some tumor samples. We did not detect modified forms of resveratrol metabolites using the described analytical method.
Discussion
In the present study, we examined the chemopreventive effects and mechanisms of action of resveratrol on the androgen-responsive human prostate cancer cell LNCaP in cell culture and in a xenograft model. Our results suggest that resveratrol exerts differential effects in these in vitro and in vivo models.
Resveratrol exerted growth inhibitory effects in our cell culture model (Figure 1). This is consistent with observations for resveratrol in similar in vitro models (17–22) and appeared to be due in part to modulation of androgen- and estrogen-responsive pathways, as we found that resveratrol inhibited both synthetic androgen R1881-induced and 17β-estradiol-induced LNCaP cell growth (Figure 1B and C). The actions of resveratrol on these two pathways were further supported by our microarray and RT–PCR analyses of gene expression. Our microarray results indicated that resveratrol exerts a global effect on ARG mRNA expression (Figure 2A and B), confirming findings by Jones et al. (22). As in the cell growth experiment, resveratrol’s action on ARG mRNAs appeared to involve modulation of androgen- and estrogen-responsive pathways. Resveratrol inhibited both androgen and estrogen induction of ARG mRNA in LNCaP cells (Figure 3A and B). The effect of resveratrol on ARG mRNAs correlated with expression at the protein level, as we showed resveratrol inhibited the R1881- and 17β-estradiol-induced increases in PSA protein levels (Figure 3C), similar to those reported by Hsieh et al. (40). These molecular results are consistent with recent reports of in vitro effects of resveratrol (41–43) and provide further support for modulation of steroid hormone-dependent events as potential mechanisms that contribute to the overall growth inhibitory effects of resveratrol on LNCaP cells. Resveratrol is known to have weak estrogenic activity (16), but in our experiments resveratrol appeared to act mainly as an antiestrogen, as resveratrol inhibited both 17β-estradiol-induced growth and increases in ARG mRNA levels. Interestingly, our binding study result (Figure 2E), as well as that reported by others (16), suggests that resveratrol may not exerts its actions on androgen- and estrogen-mediated effects through direct competition of binding of steroid hormones to their receptors. Additional research is needed to elucidate the precise mechanisms of action.
Data presented here also show a novel effect of resveratrol on expression of steroid hormone-regulated genes in the LNCaP model. Resveratrol selectively reduced PSA, STK39 and IGF-1R (Figure 2B and C) mRNAs in cells cultured in 10% FBS, but not expression of two other ARGs, B2M and SEPP1 (Figure 2B). We reason that the presence of estrogen-like activity in cell culture medium may explain these observations. Consistent with this interpretation, we found a lack of effects on expression of B2M and SEPP1 in the presence of externally added 17β-estradiol but not in the presence of the synthetic androgen R1881 in cells cultured in medium that is free of phenol red, a weak estrogen, and contains 10% CDS. Thus, culture conditions may greatly affect the interpretation of resveratrol’s biological activities.
In addition to the steroid hormone-responsive pathways, modulation of p53- and IGF-1-dependent events by resveratrol was also observed, as we found by microarray and confirmed by RT–PCR that resveratrol modulated both CDKN1A and IGF-1R mRNA levels. However, activation of the p53 pathway appeared to require a higher concentration (25 μM), whereas regulation of the steroid hormone and IGF-1 pathways appeared to have a lower threshold (1–5 μM). Thus, resveratrol may exert effects on multiple pathways in a concentration-dependent fashion. Given that, we measured circulating levels of resveratrol no higher than ∼5 μM in the plasma of our animals, it is probably that modulation of steroid hormone–IGF-1-mediated events may be the more physiologically relevant mechanisms.
As illustrated in Figure 2A, we observed that exposure to resveratrol can lead to downregulation of IGF-1R and FRAP1–mTOR mRNAs but to upregulation of PIK3R3 mRNA. All three genes have been reported to be upstream regulators of AKT activity (36–38). Activation of IGF-1R and FRAP/mTOR upregulates AKT activity (36,37), whereas PIK3R3 downregulates AKT activity (37). Modulation of IGF-1R, PIK3R3 and FRAP1–mTOR by resveratrol as reported here is consistent with recent reports of inhibitory effects of resveratrol on AKT-mediated pathways (41–43). These results and our findings on modulation of androgen- and estrogen-mediated expression by resveratrol (Figure 3A and B) lend further support for a recent report on modulation of androgen- and estrogen-mediated activation of AKT by resveratrol (41). More importantly, we provide evidence that additional upstream molecular targets of AKT pathways such as IGF-1R, FRAP/mTOR and PIK3R3 are also modulated by resveratrol through androgen–estrogen-related events.
Our IHC results on expression of PSA, an ARG–estrogen-responsive gene, in xenograft tumors confirm the cell culture results and support our hypothesis that modulation of androgen- and estrogen-mediated pathways may contribute to resveratrol’s prostate cancer preventive effect in vivo. Tumors from animals fed RES50 and RES100 diets had lower numbers of cells expressing PSA, corresponding to the observed delay in tumor growth in animals consuming these two diets. The effects on tumor growth and PSA protein expression appeared to be dose dependent (Figure 4A and Figure 5A). However, we also observed that not all cells within a tumor expressed similar levels of PSA. One possible explanation is that tumor cells in the xenograft may differ from the parent LNCaP cells, as LNCaP cells are known to undergo changes with different passages (44). Interestingly, our animal experiment results are only partially correlated with the cell culture results. The lag in growth of tumor volume for animals consuming resveratrol appeared to ‘catch up’ to control animals by the seventh week (Figure 4A). Hence, modulation of the steroid hormone-dependent pathways may afford only partial protection against tumor development. Our IHC data on apoptosis and angiogenesis appear to suggest that resveratrol may not be entirely beneficial against tumor development. Tumors from animals fed resveratrol appeared to have lower apoptosis frequencies than animals on control diet. Moreover, tumors from the animals fed the higher dose of resveratrol (100 mg resveratrol/kg diet) also had significantly higher blood vessel counts, suggesting increased angiogenesis in the RES100 group compared with the control diet group. This effect of resveratrol on angiogenesis appeared not to be related to production of VEGF by tumor cells, as we did not observe qualitative differences in VEGF levels using IHC analysis.
The amount of resveratrol ingested by the animals per day (3–6 mg/day) in the present experiment is equivalent to consumption of 500–1000 ml of red wine per day and is readily achievable in humans (45). By body surface area calculations (46), our experimental animals consumed a dose of 12–24 mg/m2 of resveratrol and appeared to be at the lower end of pharmacological doses used in a recent human trial (47). However, our dose was ∼10× lower than that used in a recent study with TRAMP mice (48). Interestingly, the plasma levels in our animals (1.3 ± 0.336 μM, RES100) were higher than those in Harper’s study [52 ± 18 nM, (48)]. Hence, additional work would be necessary to extrapolate our results, as well as those from other animal models, to human populations.
In summary, we report here that resveratrol differentially affects in vivo and in vitro models of prostate cancer. In vitro, resveratrol appeared to exert growth inhibitory effects on cultured LNCaP cells through multiple pathways, including steroid hormone-dependent pathways. In vivo, resveratrol delayed the initial development of xenograft LNCaP cell tumors, consistent with an effect on steroid hormone-mediated events. However, exposure to resveratrol appeared to lead to promotion of angiogenesis and inhibition of apoptosis in LNCaP cell-derived tumors, as assessed by IHC markers.
Funding
USA appropriated funds to United States Department of Agriculture project number 1235-51530-052-00 to T.T.Y.W., T.-C. W and B.V.; National Cancer Institute to T.S.H., Y.S.K. and H.S.; National Institute of Environmental Health Sciences to S.D.H. and S.N.P. (P30ES007784).
Acknowledgments
T.S.H. is a National Cancer Institute cancer prevention fellow. The authors would like to thank Dr Diana Haines, Maureen Kennedy and Scott Lawrence of the Pathology/Histotechnology Laboratory, SAIC-Frederick, for their assistance in IHC analysis. The authors would also like to thank Drs Chang-hee Kim of SAIC-Frederick and Gadisetti V.R.Chandramouli of the National Cancer Institute for their assistance in microarray analysis.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AR
androgen receptor
- ARG
androgen-responsive gene
- B2M
beta-2-microglobulin
- CDKN1A
cyclin-dependent kinase inhibitor 1A/p21WAF1/CIP1
- CDS
charcoal–dextran-treated FBS
- FBS
fetal bovine serum
- FU
fluorescence units
- HPLC
high-performance liquid chromatography
- IGF-1
insulin-like growth factor-1
- IGF-1R
insulin-like growth factor-1 receptor
- IHC
immunohistochemical
- IS
Internal standard
- mRNA
messenger RNA
- PBS
phosphate-buffered saline
- PCNA
proliferating cell nuclear antigen
- PECAM-1
platelet/endothelial cell adhesion molecule-1
- PSA
prostate-specific antigen
- RT–PCR
reverse transcription–polymerase chain reaction
- SEPP1
selenoprotein P, plasma, 1
- STK39
serine threonine kinase 39
- VEGF
vascular endothelial growth factor
References
- 1.Jemal A, et al. Cancer statistics, 2007. CA Cancer J. Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Taplin ME, et al. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J. Cell. Biochem. 2004;91:483–490. doi: 10.1002/jcb.10653. [DOI] [PubMed] [Google Scholar]
- 3.Ho SM. Estrogens and anti-estrogens: key mediators of prostate carcinogenesis and new therapeutic candidates. J. Cell. Biochem. 2004;91:491–503. doi: 10.1002/jcb.10759. [DOI] [PubMed] [Google Scholar]
- 4.Renehan AG, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman R. Chemoprevention of prostate cancer: current status and future directions. Cancer Metastasis Rev. 2002;21:297–309. doi: 10.1023/a:1021267128567. [DOI] [PubMed] [Google Scholar]
- 6.Moschos SJ, et al. The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncology. 2002;63:317–332. doi: 10.1159/000066230. [DOI] [PubMed] [Google Scholar]
- 7.So AI, et al. Androgens and prostate cancer. World J. Urol. 2003;21:325–337. doi: 10.1007/s00345-003-0373-9. [DOI] [PubMed] [Google Scholar]
- 8.Kris-Etherton PM, et al. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002;113:71S–88S. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 9.Stewart JR, et al. Resveratrol: a candidate nutritional substance for prostate cancer prevention. J. Nutr. 2003;133:2440S–2443S. doi: 10.1093/jn/133.7.2440S. [DOI] [PubMed] [Google Scholar]
- 10.Hain R, et al. Expression of a stilbene synthase gene in Nicotiana tabacum results in synthesis of the phytoalexin resveratrol. Plant Mol. Biol. 1990;15:325–335. doi: 10.1007/BF00036918. [DOI] [PubMed] [Google Scholar]
- 11.Bhat KPL, et al. Biological effects of resveratrol. Antioxid. Redox. Signal. 2001;3:1041–1064. doi: 10.1089/152308601317203567. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg DM, et al. Method to assay the concentrations of phenolic constituents of biological interest in wines. Anal. Chem. 1996;68:1688–1694. doi: 10.1021/ac951083i. [DOI] [PubMed] [Google Scholar]
- 13.Baur JA, et al. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 14.Jang M, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead TP, et al. Effect of red wine ingestion on the antioxidant capacity of serum. Clin. Chem. 1995;41:32–35. [PubMed] [Google Scholar]
- 16.Gehm BD, et al. Estrogenic effects of resveratrol in breast cancer cells expressing mutant and wild-type estrogen receptors: role of AF-1 and AF-2. J. Steroid Biochem. Mol. Biol. 2004;88:223–234. doi: 10.1016/j.jsbmb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh TC, et al. Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines. Exp. Cell Res. 1999;249:109–115. doi: 10.1006/excr.1999.4471. [DOI] [PubMed] [Google Scholar]
- 18.Kim YA, et al. Antiproliferative effect of resveratrol in human prostate carcinoma cells. J. Med. Food. 2003;6:273–280. doi: 10.1089/109662003772519813. [DOI] [PubMed] [Google Scholar]
- 19.Kuwajerwala N, et al. Resveratrol induces prostate cancer cell entry into s phase and inhibits DNA synthesis. Cancer Res. 2002;62:2488–2492. [PubMed] [Google Scholar]
- 20.Lin HY, et al. Resveratrol induced serine phosphorylation of p53 causes apoptosis in a mutant p53 prostate cancer cell line. J. Urol. 2002;168:748–755. [PubMed] [Google Scholar]
- 21.Narayanan BA, et al. Differential expression of genes induced by resveratrol in LNCaP cells: P53-mediated molecular targets. Int. J. Cancer. 2003;104:204–212. doi: 10.1002/ijc.10932. [DOI] [PubMed] [Google Scholar]
- 22.Jones SB, et al. Resveratrol-induced gene expression profiles in human prostate cancer cells. Cancer Epidemiol. Biomarkers Prev. 2005;14:596–604. doi: 10.1158/1055-9965.EPI-04-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson PS, et al. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc. Natl Acad. Sci. USA. 2002;99:11890–11895. doi: 10.1073/pnas.182376299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang H, et al. The role of the androgen receptor in prostate cancer. Crit. Rev. Eukaryot. Gene Expr. 2002;12:193–207. doi: 10.1615/critreveukaryotgeneexpr.v12.i3.30. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi Y, et al. 17Beta-Estradiol differentially regulates androgen-responsive genes through estrogen receptor-beta- and extracellular-signal regulated kinase-dependent pathways in LNCaP human prostate cancer cells. Mol. Carcinog. 2007;46:117–129. doi: 10.1002/mc.20254. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi Y, et al. Molecular signatures of soy-derived phytochemicals in androgen-responsive prostate cancer cells: a comparison study using DNA microarray. Mol. Carcinog. 2006;45:943–956. doi: 10.1002/mc.20247. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi Y, et al. Genistein affects androgen-responsive genes through both androgen- and estrogen-induced signaling pathways. Mol. Carcinog. 2006;45:18–25. doi: 10.1002/mc.20153. [DOI] [PubMed] [Google Scholar]
- 28.Kelland LR. Of mice and men: values and liabilities of the athymic nude mouse model in anticancer drug development. Eur. J. Cancer. 2004;40:827–836. doi: 10.1016/j.ejca.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 29.Marier JF, et al. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J. Pharmacol. Exp. Ther. 2002;302:369–373. doi: 10.1124/jpet.102.033340. [DOI] [PubMed] [Google Scholar]
- 30.Zhou JR, et al. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J. Nutr. 1999;129:1628–1635. doi: 10.1093/jn/129.9.1628. [DOI] [PubMed] [Google Scholar]
- 31.Cher ML, et al. Cellular proliferation in prostatic adenocarcinoma as assessed by bromodeoxyuridine uptake and Ki-67 and PCNA expression. Prostate. 1995;26:87–93. doi: 10.1002/pros.2990260205. [DOI] [PubMed] [Google Scholar]
- 32.Hunter AL, et al. Detection of apoptosis in cardiovascular diseases. Methods Mol. Med. 2005;112:277–289. doi: 10.1385/1-59259-879-x:277. [DOI] [PubMed] [Google Scholar]
- 33.Cao G, et al. Involvement of human PECAM-1 in angiogenesis and in vitro endothelial cell migration. Am. J. Physiol. Cell Physiol. 2002;282:C1181–C1190. doi: 10.1152/ajpcell.00524.2001. [DOI] [PubMed] [Google Scholar]
- 34.Littell RC, et al. SAS® for Mixed Models. 2nd edn. 814p. Cary, NC: 2006. [Google Scholar]
- 35.SAS Institute Inc. SAS® v9.1. Cary, NC: SAS Institute, Inc.; 2002–2007. [Google Scholar]
- 36.Majumder PK, et al. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24:7465–7474. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- 37.Shaw RJ, et al. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y, et al. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66:7783–7792. doi: 10.1158/0008-5472.CAN-05-4472. [DOI] [PubMed] [Google Scholar]
- 39.El-Deiry WS, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh TC, et al. Grape-derived chemopreventive agent resveratrol decreases prostate-specific antigen (PSA) expression in LNCaP cells by an androgen receptor (AR)-independent mechanism. Anticancer Res. 2000;20:225–228. [PubMed] [Google Scholar]
- 41.Benitez DA, et al. Non-genomic action of resveratrol on androgen and oestrogen receptors in prostate cancer: modulation of the phosphoinositide 3-kinase pathway. Br. J. Cancer. 2007;96:1595–1604. doi: 10.1038/sj.bjc.6603755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pozo-Guisado E, et al. Resveratrol modulates the phosphoinositide 3-kinase pathway through an estrogen receptor alpha-dependent mechanism: relevance in cell proliferation. Int. J. Cancer. 2004;109:167–173. doi: 10.1002/ijc.11720. [DOI] [PubMed] [Google Scholar]
- 43.Aziz MH, et al. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3′-kinase/Akt pathway and Bcl-2 family proteins. Mol. Cancer Ther. 2006;5:1335–1341. doi: 10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- 44.Karan D, et al. Decreased androgen-responsive growth of human prostate cancer is associated with increased genetic alterations. Clin. Cancer Res. 2001;7:3472–3480. [PubMed] [Google Scholar]
- 45.Aggarwal BB, et al. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–2840. [PubMed] [Google Scholar]
- 46.Reagan-Shaw S, et al. Dose translation from animal to human studies revisited. FASEB J. 2007;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 47.Boocock DJ, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 48.Harper CE, et al. Resveratrol suppresses prostate cancer progression in transgenic mice. Carcinogenesis. 2007;28:1946–1953. doi: 10.1093/carcin/bgm144. [DOI] [PubMed] [Google Scholar]