Abstract
Genistein (GEN), a soy isoflavone, stimulates growth of estrogen-dependent human tumor cells (MCF-7) in a preclinical mouse model for postmenopausal breast cancer. Antiestrogens and aromatase inhibitors are frontline therapies for estrogen-dependent breast cancer. We have demonstrated that dietary GEN can negate the inhibitory effect of tamoxifen. In this study, we evaluated the interaction of dietary GEN (at 250–1000 p.p.m. in the American Institute of Nutrition 93 growth diet) and an aromatase inhibitor, letrozole (LET), on the growth of tumors in an aromatase-expressing breast cancer xenograft model (MCF-7Ca) in the presence and absence of the substrate androstenedione (AD). Dietary GEN (250 and 500 p.p.m.) or implanted AD stimulated MCF-7Ca tumor growth. Implanted LET inhibited AD-stimulated MCF-7Ca tumor growth. In the presence of AD and LET, dietary GEN (250, 500 and 1000 p.p.m.) reversed the inhibitory effect of LET in a dose-dependent manner. Uterine wet weight, plasma estradiol (E2) levels (enzyme-linked immunosorbent assay) and total plasma GEN and LET levels (liquid chromatography-electrospray/tandem mass spectrometry) were measured. Ki-67 (cellular proliferation), aromatase and pS2 protein expression in tumors were evaluated using immunohistochemical (IHC) analysis. In conclusion, dietary GEN increased the growth of MCF-7Ca tumors implanted in ovariectomized mice and could also negate the inhibitory effect of LET on MCF-7Ca tumor growth. These findings are significant because tumors, which express aromatase and synthesize estrogen, are good candidates for aromatase therapy dietary and GEN can reverse the inhibitory effect of LET on tumor growth and adversely impact breast cancer therapy. Caution is warranted for consumption of dietary GEN by postmenopausal women with estrogen-dependent breast cancer taking LET treatment.
Introduction
Breast cancer is the second leading cause of cancer death in USA women (1) and has been increasing in men (2). Predictions indicate 176 296 new cases and 40 515 deaths from breast cancer in 2007 (1). Most breast cancer cases (∼75%) occur in postmenopausal women, and most (∼70%) are estrogen dependent (3). A recent study showed that trend in estrogen receptor (ER) (+) breast cancer incidence rates from 2002 through 2006 sharply dropped, possibly because many women stopped hormone replacement therapy (4). The stimulatory effect of estrogens on the growth of breast cancers can be blocked by two manipulations: competitive binding interactions at the ER by antiestrogens like tamoxifen and competitive inhibition of estrogen synthesis by aromatase inhibitors. Adjuvant endocrine therapy with tamoxifen has proved to be highly effective and has led to significant improvements in survival for postmenopausal women with early-stage estrogen-dependent breast cancer. However, concerns regarding tamoxifen-associated side effects, including endocrine resistance and endometrial cancer (5), have lead to a reduction in its usage.
Aromatase [cytochrome P450 (CYP) 19], also known as 11β-hydroxysteroid dehydrogenase, is a member of the CYP enzyme superfamily (6) and plays a crucial role in estrogen biosynthesis [i.e. CYP19 converts androstenedione (AD) and testosterone to estrone and estradiol (E2), respectively (7)]. The highest levels of aromatase activity are present in the ovaries of premenopausal women (8) with smaller amounts present in the adipose tissue and skin where the precursor is circulating AD derived from the adrenal cortex (9). In postmenopausal women, ovarian production and circulating levels of estrogen declines and normal stromal and mammary epithelial synthesis of estrogens from C19 steroids by aromatase increases (10). The major estrogen production sites are extragonadal, the adipose and skin fibroblasts. The expression of aromatase in breast cancer tissue has been demonstrated by enzyme activity measurement (11), immunohistochemical (IHC) analysis (12) and quantitative reverse transcription (qRT)–polymerase chain reaction (PCR) (13).
The development of aromatase inhibitors [anastrozole, exemestane and letrozole (LET)] provides an alternative strategy to tamoxifen for endocrine therapy, which appears to have improved outcome compared with tamoxifen alone (14,15). Third-generation aromatase inhibitors, exemestane, anastrozole and LET, have shown equivalent effects or superiority compared with tamoxifen efficacy as an initial treatment method and demonstrated a tolerable side effect profile in comparison with tamoxifen (16). LET (Femara®; Novartis Pharma AG, Basel, Switzerland), an imidazole derivative, is a potent, orally administered non-steroidal inhibitor of aromatase. LET (2.5 mg) administration has been shown to be effective before, after or instead of antiestrogens (17–19).
We have demonstrated that genistein (GEN) stimulates estrogen-dependent tumor growth in mice at levels of dietary exposures that produce total GEN levels in blood that are comparable with those observed in humans (20). GEN at very high concentrations weakly inhibits aromatase (21,22). GEN (10 μM–10 mM) inhibited the phase I enzyme induction (CYP1A1, CYP1A2, CYP1B1, CYP2E1, CYP3A4 and CYP19) (23,24) and increased the phase II enzyme induction (uridine diphosphate-glucuronosyltransferase and quinone reductase) (25,26) in various cells and tumors. GEN also competitively inhibited CYP1B1 (Ki = 1.9 μM). Inhibition of extrahepatic human CYP1A1 and 1B1 by metabolism of isoflavones found in Trifolium pratense (red clover) was observed (27). However, inhibition of aromatase by GEN at low concentrations (<1 μM) was not observed (28).
It is possible that a postmenopausal breast cancer patient being treated with LET and consuming GEN-containing dietary supplements to relieve postmenopausal symptoms may be altering the effectiveness of LET on breast cancer treatment. Since the interaction of LET and GEN has not been investigated, it is critical that this important health issue be evaluated in appropriate preclinical models. Previously, we have demonstrated that dietary GEN negates the inhibitory effect of tamoxifen on the growth of MCF-7 tumors (29). Therefore, the elucidation of the interaction of GEN with an aromatase inhibitor on aromatase-expressing estrogen-dependent breast cancer is a logical extension of our research on the effect of GEN. Here, we report that dietary GEN, at dosages relevant to human exposure, negates the inhibitory effect of LET on the growth of MCF-7Ca tumors.
Materials and methods
Materials
GEN was purchased from Indofine Chemical Company (Somerville, NJ). AD was purchased from Sigma Chemicals (St Louis, MO). Minimal essential medium (without gentamicin and with glutamine) and phenol red-free minimal essential medium were purchased from the Media Facility at the University of Illinois at Urbana-Champaign. Bovine calf serum was purchased from Hyclone (Logan, UT). Penicillin–streptomycin and trypsin–ethylenediaminetetraacetic acid were purchased from Invitrogen (Carlsbad, CA). Laboratory animal diet and dietary components were purchased from Dyets (Bethlehem, PA). Reagents for qRT–PCR were purchased from PE Applied Biosystems (Foster City, CA), Synthegen (Houston, TX) and Invitrogen.
MCF-7Ca cells
MCF-7Ca cells (30) were generated by transfecting the human aromatase gene into estrogen-dependent breast cancer (MCF-7) cells. MCF-7Ca cells were kindly provided by Dr Richard Santen at the University of Virginia. MCF-7Ca cells were maintained in minimal essential medium with 1 mM pyruvate, 2 mM glutamine, 5% heat-inactivated bovine calf serum, 1% penicillin–streptomycin and 600 mg/l neomycin.
Athymic nude mice
Female Balb/c (nude) mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were ovariectomized at 21 days of age by the vendor. After their arrival, animals were acclimated for 1 week. During the study, animals were single caged and were maintained under the standard light–dark cycle (12 h light and 12 h dark).
AD and LET implants
Silastic implants containing 5 mg of AD were prepared with 10 mg of cholesterol (0.2 cm inner diameter × 0.1 cm wall, 1.5 cm length). For mice in the MCF-7Ca group, entire 15 mg implants were used. Similarly, silastic implants containing 1 mg of LET and 2 mg of cholesterol (0.1 cm inner diameter × 0.06 cm wall, 1.5 cm length) were prepared. Silastic tubes were purchased from SF Medical (Hudson, MA) and silicon adhesive was purchased from Dow Corning (Midland, MI). Both ends of the implants were sealed with silicon adhesive. Implants were sterilized with 70% ethanol and conditioned with phosphate-buffered saline before implantation and were implanted subcutaneously on the backs of the mice. The dosages of AD and LET were selected based on our dose range-finding data (data not shown).
Analysis of tumor growth
Three days after implantation of the AD pellets, MCF-7Ca cells were harvested using 500 μl trypsin–ethylenediaminetetraacetic acid (0.5% trypsin, 5.3 mM ethylenediaminetetraacetic acid•4Na) (Gibco BRL, Grand Island, NY) per 100 mm culture plate. Cells were adjusted to 1 × 105 cells per 40 μl of Matrigel® (Collaborative Biomedical Products, Bedford, MA) and injected at 40 μl per site into two sites on the backs of the athymic mice. Mice were divided into 10 treatment groups: MCF-7Ca, 250 p.p.m. GEN, 500 p.p.m. GEN, 1000 p.p.m. GEN, AD (5 mg), LET (1 mg), AD + LET, AD + LET + 250 p.p.m. GEN, AD + LET + 500 p.p.m. GEN and AD + LET + 1000 p.p.m. GEN. During the study, tumor growth and body weight were monitored weekly and food intake was measured throughout the study.
Diets formulation
American Institute of Nutrition 93 growth diet semipurified diet (Dyets) was selected as a base diet for control mice as it has been established as meeting all the nutritional requirements of mice (31). Mice in the MCF-7Ca, AD, LET and AD + LET groups were on isoflavone-free American Institute of Nutrition 93 growth diet, and mice in the GEN treatment groups were fed with American Institute of Nutrition 93 growth diet containing GEN (250, 500 or 1000 p.p.m.). Soy oil was substituted with corn oil as a fat source to eliminate any additional components of soy being added to the diets.
Serum E2 levels
Blood samples were collected by cardiac puncture at the time of killing and centrifuged at 500g for 20 min at 4°C. Plasma samples were stored at −20°C until analyzed. E2 levels in plasma were measured using E2 enzyme-linked immunosorbent assay kit according to the company’s protocol (Alpha Diagnostic International, San Antonio, TX). Controls included non-specific binding, total counts and a plasma blank plus a known amount of E2 (for recovery). Plasma samples (50 μl) were transferred into an E2 antibody-coated plate in duplicate. After 1 h incubation at 24°C, the plate was read at 450 nm using a plate reader (μQuant, Bio-Tek, LabTech International Ltd. East Sussex, UK). The limit of detection was 10 pg/ml and the intra-assay coefficient of variation was <3%.
Analysis of plasma concentrations of total GEN and LET
Plasma samples (10 μl aliquots) from mice fed either an isoflavone-free diet or a GEN-fortified diet with and without LET implantation were analyzed for total GEN and LET using LC/MS/MS as previously validated for isoflavones with modifications (32). The method detection limit for total GEN was ∼0.005 μM, accuracy was in the range of 88–96% and precision was in the range of 3–8% (32). The method detection limit for LET was ∼0.001 μM, accuracy was in the range of 90–108% and precision was in the range of 2–9%.
RNA preparation and analysis of changes in gene expression using qRT–PCR
Expression of pS2 [a breast cancer-specific marker (33) that linked to ER activation and regulated by aromatase inhibitor treatment] and aromatase messenger RNAs (mRNAs) in tumors was analyzed using qRT–PCR. Tumors with areas similar to the mean tumor surface area of the each treatment group (six to seven tumors per group) were used for mRNA analysis. RNA from frozen tumors (≤200 mg) was prepared as described in Ju et al. (29). Complementary DNA was generated using 10 ng of RNA and TaqMan Reverse Transcription Reagents (PE Applied Biosystems). The pS2 and aromatase primers and fluorescence 6-carboxyfluorescein (6-FAM)-labeled probes were designed using Primer and Probe Design Express (PE Applied Biosystems)—pS2 forward: 5′-TCCCCTGGTGCTTCTATCCTAA-3′, pS2 reverse: 5′-CGTCAGGATGCAGGCAGAT-3′ and pS2 probe: 6-FAM-5′-ACCATCGACGTCCCTCCAGAAGAGG-3′-tetramethyl-6-carboxyrhodamine (TAMRA) and aromatase forward: 5′-TGACCAATGAATCGGGCTATGTG-3′, aromatase reverse: 5′-GATCCTCAAGAAGAGCGTGTTAGA-3′ and aromatase probe: 6-FAM-5′-ACCCTTCTGCGTCGTGTCATGCTGGA-3′-TAMRA. Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers and a fluorescent (6-FAM–TAMRA)-labeled probe (User Bulletin #2, PE Applied Biosystems) were used as a control. PCR and analysis of PCR products were performed using an ABI PRISM 7700 Sequence Detector (PE Applied Biosystems). Data were analyzed using a comparative threshold cycle method (User Bulletin, PE Applied Biosystems). Amplicons were run as triplicates in separate tubes to permit quantification of target genes normalized to a control, GAPDH.
IHC analysis
Expression of Ki-67 (a universal marker of cellular proliferation), pS2 and aromatase proteins in tumors was evaluated using IHC analysis (34). The expression of the human Ki-67 protein is associated with cell proliferation (35). Anti-human Ki-67 antibody (PharMingen, San Diego, CA) (1:3000 dilution in 1% bovine serum albumin/phosphate-buffered saline), anti-human pS2 antibody or anti-human aromatase antibody (Biomeda, Foster City, CA) (1:200 dilution) was used. Slides were analyzed using a light microscope. Both positive- and background-stained cells were counted in a given area of tissue. A total of 50 fields from five tumors per treatment group were evaluated. The data were then presented as a percentage of cells proliferating in a given area of tumor.
Statistics
Data from tumor surface area at final week, IHC analysis, qRT–PCR, plasma analysis (E2 and GEN), uterine wet weight and feed intake were analyzed using one-way or repeated measures analysis of variance according to the characteristics of the data set using the SAS program (SAS, Cary, NC). If the overall treatment F ratio was significant (P < 0.05), the differences between treatment means were tested with Fisher’s least significant difference test.
Results
Effect of dietary GEN on growth of MCF-7Ca tumors
Tumors in the MCF-7Ca, 250 p.p.m. GEN, 500 p.p.m. GEN and 1000 p.p.m. GEN groups were monitored until 19 weeks at which point mice in these groups were terminated. The average tumor sizes of the 250 and 500 p.p.m. GEN groups were significantly larger than other MCF-7Ca and 1000 p.p.m. GEN groups (P < 0.05). No statistically significant difference was observed between the 250 and 500 GEN groups (P = 0.505) or between the MCF-7Ca and 1000 GEN groups (P = 0.495).
Interaction of dietary GEN with AD and LET on growth of MCF-7Ca tumors
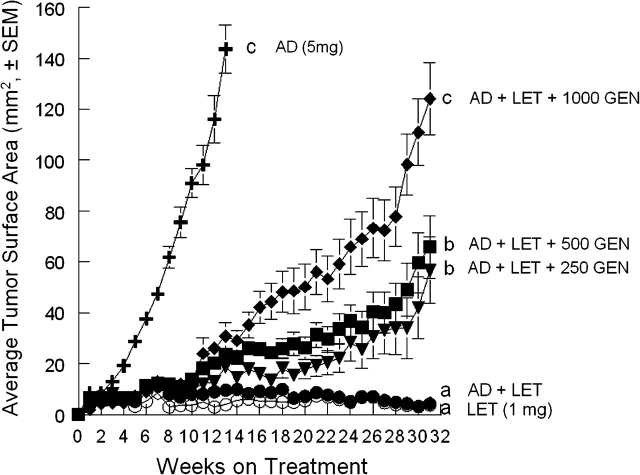
Mean tumor surface area in the AD-positive control group reached 143.6 ± 9.5 mm2 13 weeks after the AD implantation, at which point the mice in this group were terminated because of a high tumor burden. Tumors in the LET, AD + LET, AD + LET + 250 GEN, AD + LET + 500 GEN and AD + LET + 1000 GEN groups were monitored for 31 weeks. LET blocked MCF-7Ca tumor growth in the LET group relative to the MCF-7Ca controls, and LET also blocked AD-stimulated MCF-7Ca tumor growth in the AD + LET group (Figure 2). There were statistical differences in tumor size between the AD + LET group and AD + LET + GEN groups (P < 0.05). There were no statistical differences between the LET and the LET + AD groups (P = 0.729) or between the LET + AD + 250 GEN and the LET + AD + 500 GEN (P = 0.065). Among the GEN groups, the tumor size in the AD + LET + 1000 GEN was significantly larger than the AD + LET + 250 GEN and AD + LET + 500 GEN (P < 0.05).
Fig. 2.
Effect of dietary GEN in the presence of AD and LET on the growth of MCF-7Ca cells implanted in ovariectomized athymic mice. Animals were randomly assigned into five treatment groups: LET (12 mice; n = 15 tumors), AD (12 mice; n = 24 tumors), AD + LET (12 mice; n = 17 tumors), AD + LET + 250 GEN (15 mice; n = 20 tumors), AD + LET + 500 GEN (15 mice; n = 24 tumors) and AD + LET + 1000 GEN (14 mice; n = 25 tumors). Data are expressed as average cross-sectional area (mm2 ± SEM) for all tumors in each treatment group. Mean tumor surface area in the AD-positive control group reached 143.6 ± 9.5 mm2 13 weeks after the AD implantation. Average tumor sizes at 31 weeks were 3.7 ± 0.9 mm2 for the LET, 4.2 ± 1.3 mm2 for the AD + LET, 56.7 ± 13.1 mm2 for the AD + LET + 250 GEN, 65.9 ± 12.3 mm2 for the AD + LET + 500 GEN and 124.1 ± 14.0 mm2 for the AD + LET + 1000 GEN groups, respectively. Bars with different letters are significantly different, P < 0.05.
Plasma E2 levels
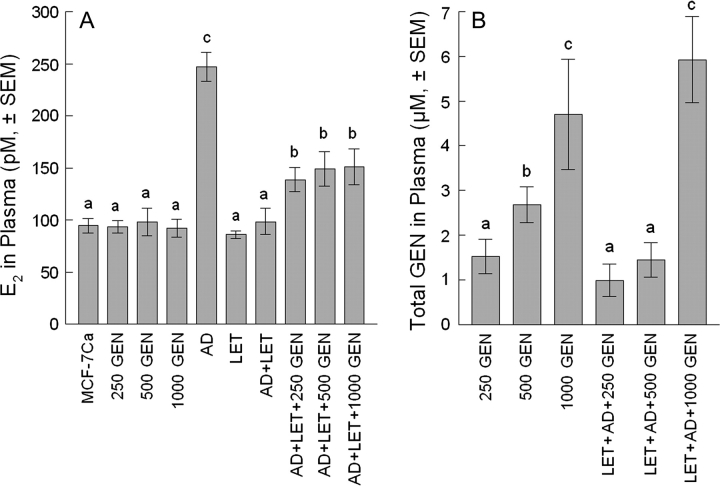
The E2 levels in the AD + LET + 250 GEN, AD + LET + 500 GEN and AD + LET+ 1000 GEN groups were significantly lower than those in the AD groups (P < 0.05). No significant difference among GEN groups or among AD + LET + GEN groups and between the MCF-7Ca group and the GEN groups were detected (Figure 3A).
Fig. 3.
Plasma E2 and GEN levels. Plasma E2 (A). Data are expressed as average E2 concentration (pM ± SEM). E2 levels in plasma were 247.1 ± 14.0 pM for the AD group, 94.7 ± 6.9 pM for the MCF-7Ca group, 93.7.0 ± 6.1 pM for the 250 GEN, 98.4 ± 13.3 pM for the 500 GEN, 92.3 ± 8.4 pM for the 1000 GEN, 86.2 ± 3.6 pM for the LET group, 98.5 ± 12.6 pM for the AD + LET group, 138.8 ± 11.4 pM for the AD + LET + 250 GEN, 149.1 ± 16.4 pM for the AD + LET + 500 GEN and 151.2 ± 17.1 pM for the AD + LET + 1000 GEN, respectively. E2 levels in plasma were 30.4 ± 1.7 pM for the 250 GEN, 30.6 ± 1.9 pM for the 500 GEN and 32.4 ± 1.8 pM for the 1000 GEN, respectively (A). (B) Total GEN (aglycone + conjugated forms). Less than 5% of total GEN was present as free GEN. Plasma concentration of total GEN was 1.5 ± 0.4, 2.7 ± 0.4 and 4.7 ± 1.2 μM for the 250 GEN, 500 GEN and 1000 GEN groups, respectively. Plasma concentration of total GEN was 1.0 ± 0.4, 1.5 ± 0.4 and 5.9 ± 1.0 μM for the AD + LET + 250 GEN, AD + LET + 500 GEN and AD + LET + 1000 GEN groups, respectively. Bars with different letter are significantly different, P < 0.05.
Plasma total GEN concentrations
While no GEN was detected in plasma from mice in the MCF-7Ca, AD, LET and LET + AD groups, the concentration of total GEN in plasma from GEN-fed animals increased regularly as the dietary dose increased (250–1000 p.p.m.), with levels that ranged from 0.99 to 5.92 μM (Figure 3B). There was a statistical difference among the GEN groups (P < 0.05). There was a statistical difference between the AD + LET + 250 GEN and AD + LET + 1000 GEN groups and between the AD + LET + 500 GEN and AD + LET + 1000 GEN groups (P < 0.05) (Figure 3B).
Plasma LET concentrations
The concentrations of LET were determined in plasma samples from all mice. LET concentrations were not detectable (<0.001 μM) in non-implanted mice (data not shown). Concentrations of LET in implanted mice were ∼0.01 μM and did not vary significantly between groups (data not shown).
Relative pS2 and aromatase mRNA expressions in tumors
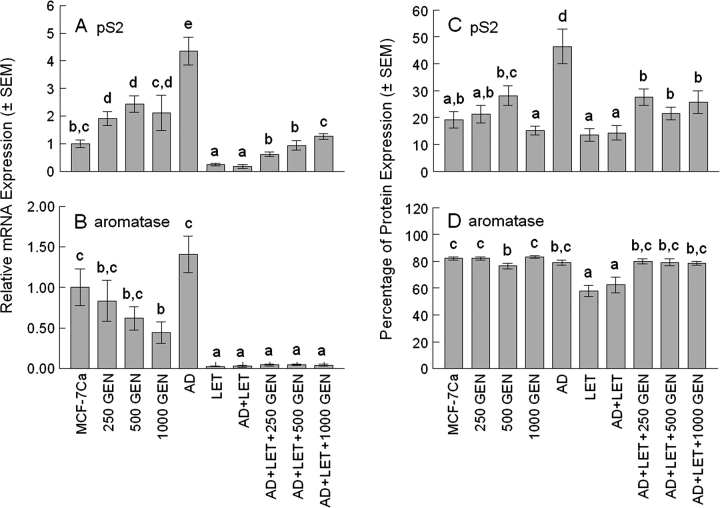
Total RNA from tumors was evaluated for expression of gene markers pS2 and aromatase using qRT–PCR (Figure 4A andB).
Fig. 4.
Expression of mRNA and protein in tumors. Relative pS2 (A) and aromatase (B) mRNA expression in tumors. Six to seven tumors (≤200 mg each) from each treatment group were used for qRT–PCR analysis. Average relative pS2 mRNA expression in tumors was 1.9 ± 0.25× for 250 GEN, 2.4 ± 0.30× for 500 GEN, 2.1 ± 0.63× for 1000 GEN, 4.4 ± 0.49× for AD, 0.3 ± 0.04× for LET, 0.2 ± 0.07× for AD + LET, 0.6 ± 0.08× for AD + LET + 250 GEN, 0.9 ± 0.16× for AD + LET + 500 GEN and 1.3 ± 0.10× for AD + LET + 1000 GEN relative to MCF-7Ca. In the presence of AD and LET, dietary 250 GEN, 500 GEN and 1000 GEN treatment increased pS2 mRNA expression in tumors by 3.3×, 5.1× and 6.8× over the AD + LET group (P < 0.05) (A). Average relative aromatase mRNA expression in tumors was 0.83 ± 0.25× for 250 GEN, 0.62 ± 0.14× for 500 GEN, 0.44 ± 0.13× for 1000 GEN, 1.41 ± 0.22× for AD, 0.02 ± 0.00× for LET, 0.03 ± 0.01× for AD + LET, 0.04 ± 0.01× for AD + LET + 250 GEN, 0.04 ± 0.01× for AD + LET + 500 GEN and 0.04 ± 0.01× for AD + LET + 1000 GEN relative to MCF-7Ca. In the presence of AD and LET, dietary GEN treatment did not alter aromatase mRNA expression levels compared with the AD + LET group (B). Percentage of pS2 (C) and aromatase (D) protein expression in tumors. Six to seven tumors from each treatment group were used for IHC analysis. Average percentage of pS2 expression in tumors was 19.1 ± 3.0% for MCF-7Ca, 21.2 ± 3.3% for 250 GEN, 28.2 ± 3.7% for 500 GEN, 15.2 ± 1.6% for 1000 GEN, 46.4 ± 6.4% for AD, 13.5 ± 2.3% for LET, 14.3 ± 2.7% for AD + LET, 27.5 ± 3.0% for AD + LET + 250 GEN, 21.5 ± 2.3% for AD + LET + 500 GEN and 25.7 ± 4.3% for AD + LET + 1000 GEN, respectively. In the presence of AD and LET, dietary GEN treatment increased pS2 mRNA expression in tumors by 1.9×, 1.5× and 1.7× over the AD + LET group (P < 0.05) (C). Average percentage of aromatase expression in tumors was 82.1 ± 1.0% for MCF-7Ca, 82.1 ± 1.1% for 250 GEN, 76.6 ± 2.2% for 500 GEN, 83.2 ± 1.0% for 1000 GEN, 79.1 ± 1.8% for AD, 57.8 ± 4.1% for LET, 62.5 ± 5.8% for AD + LET, 80.0 ± 2.0% for AD + LET + 250 GEN, 79.1 ± 2.5% for AD + LET + 500 GEN and 78.6 ± 1.4% for AD + LET + 1000 GEN, respectively. In the presence of AD and LET, dietary GEN treatment increased aromatase expression in tumors by 1.28×, 1.27× and 1.26× for the AD + LET + 250 GEN, AD + LET + 500 GEN and AD + LET + 1000 GEN, respectively, over the AD + LET group (P < 0.05) (D). Bars with different letters are significantly different, P < 0.05.
pS2.
We observed a significant increase in pS2 expression from AD treatment (4.4× over the MCF-7Ca) and that 250 GEN, 500 GEN and 1000 GEN treatments increased pS2 mRNA expression in tumors by 1.9×, 2.4× and 2.1× over the MCF-7Ca, respectively (Figure 4A). No statistical difference was observed among the 250 GEN, 500 GEN and 1000 GEN groups. There was no significant difference between the AD + LET + 250 GEN and AD + LET + 500 GEN groups.
Aromatase.
GEN (1000 p.p.m.) reduced aromatase mRNA levels in tumors by 55.7% compared with MCF-7Ca tumors (P < 0.05). AD treatment did not significantly increase mRNA expression in the AD group over the MCF-7Ca (P = 0.1894). LET treatment reduced aromatase mRNA level in the LET (by 95.7% compared with MCF-Ca tumors) and LET + AD (by 97.3% compared with MCF-7Ca tumors) (P < 0.05). No significant difference was observed among the AD + LET + GEN groups.
IHC analysis
pS2.
AD treatment significantly increased pS2 expression in tumors (by 2.4× over the MCF-7Ca) and LET treatment reduced AD-stimulated pS2 expression (by 69.1%) compared with the AD group. No statistical difference was observed between the MCF-7Ca and GEN groups. There was no significant difference among the AD + LET + GEN groups.
Aromatase.
Unlike aromatase mRNA expression, AD treatment did not increase aromatase protein expression in tumors. LET treatment reduced aromatase expression by 24.8% for the LET group and by 18.7% for the AD + LET group. No statistical difference was observed between the MCF-7Ca and GEN groups. There was no significant difference among the AD + LET + GEN groups.
Cellular proliferation (Ki-67).
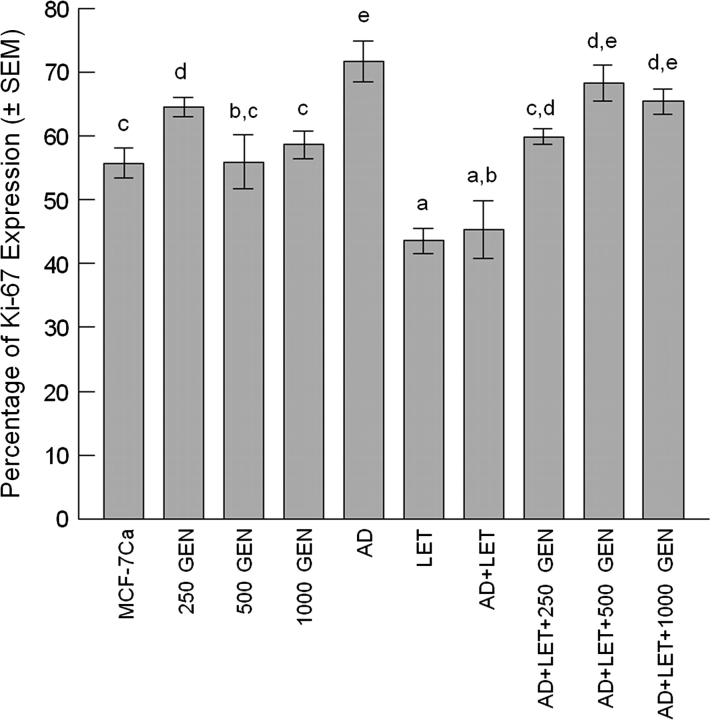
No statistical difference was observed between the MCF-7Ca and GEN groups. AD treatment increased Ki-67 expression in tumors by 1.3× over the MCF-7Ca tumors (P < 0.05) (Figure 5). LET treatment reduced Ki-67 expression by 21.9% for the LET group compared with the MCF-7Ca group (P < 0.05) and by 36.8% for the AD + LET group compared with the AD group (P < 0.05). There was no significant difference among the AD + LET + GEN groups.
Fig. 5.
Percentage of Ki-67 expression in tumors. Six to seven tumors from each treatment group were used for IHC analysis. Average percentage of Ki-67 expression in tumors was 55.8 ± 2.3% for MCF-7Ca, 64.6 ± 1.5% for 250 GEN, 56.0 ± 4.2% for 500 GEN, 58.7 ± 2.2% for 1000 GEN, 71.8 ± 3.2% for AD, 43.6 ± 2.0% for LET, 45.3 ± 4.5% for AD + LET, 60.0 ± 1.2% for AD + LET + 250 GEN, 68.3 ± 2.8% for AD + LET + 500 GEN and 65.4 ± 2.0% for AD + LET + 1000 GEN, respectively. In the presence of AD and LET, dietary GEN treatment increased Ki-67 expression in tumors by 1.3×, 1.5× and 1.4× for the AD + LET + 250 GEN, AD + LET + 500 GEN and AD + LET + 1000 GEN, respectively, over the AD + LET group (P < 0.05). Bars with different letters are significantly different, P < 0.05.
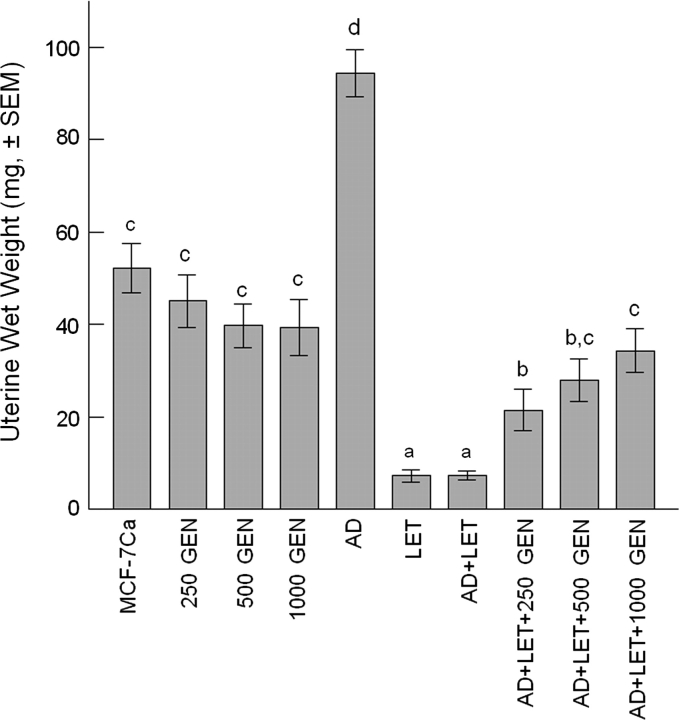
Uterine wet weight
Uterotrophic effect is directly affected by an ER-mediated mechanism, especially by ERα agonists. We observed that the AD implantation resulted in an increase in uterine wet weight by 1.8× over the MCF-7Ca group (P < 0.05). Dietary GEN treatment (250, 500 and 1000 p.p.m.) did not change uterine wet weight in ovariectomized MCF-7Ca-implanted mice. There was no statistical difference between the MCF-7Ca and GEN groups (250, 500 and 1000 GEN). LET reduced uterine weight by 86.1% compared with the MCF-7Ca and by 92.2% compared with the AD (P < 0.05). There was a significant difference between the AD + LET + 250 GEN and AD + LET + 1000 GEN (P < 0.05), but not between the AD + LET + 250 GEN and the AD + LET + 500 GEN (P = 0.1805), or between the AD + LET + 500 GEN and the AD + LET + 1000 GEN (P = 0.3278) groups.
Discussion
In July 2002, the Women’s Health Initiative announced that the Prempro (estrogen plus progestin) trial had been stopped because there were more adverse—than beneficial—effects (36). Notable was the reported increase in risk for breast cancer. Because of the potential risks, physicians greatly reduced prescriptions for hormone replacement therapy. The reduced rate of hormone replacement therapy that began in 2003 has resulted in a significant reduction of breast cancer diagnosis (37). Because of these findings, warning against the use of equine estrogens, it is possible that women might consider the hypothesized potential beneficial estrogenic effects of soy- and isoflavone-containing products. The consumption of GEN-containing dietary supplements has recently gained acceptance as an alternative to conventional hormone replacement therapies. This acceptance has occurred despite lack of consistent evidence for any beneficial effects of GEN (38). Approximately 30% of Americans are using at least one complementary and alternative medicine (CAM) per year, typically for chronic rather than acute medical conditions (39). Consumption of dietary supplements and herbal supplements are considered as CAM utilization. Recent review on CAM in early breast cancer showed that 45% of breast cancer patients used CAM (40). Isoflavone-containing dietary supplements are among the most widely consumed by older women; however, the effects of isoflavone supplements on postmenopausal symptoms in human trials are at best inconsistent in producing beneficial effects (41,42). The interaction of estrogenic isoflavone supplements with prescription drugs for breast cancer is a critical health issue that has not been adequately investigated.
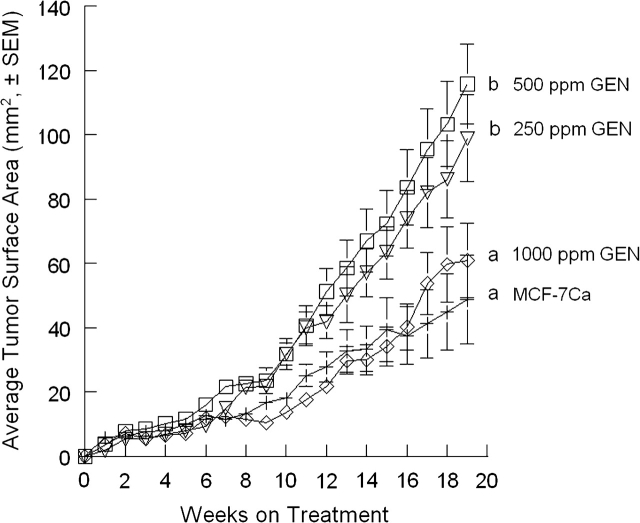
Aromatase is locally produced in breast tumors (43,44) and level of aromatase is significantly increased in breast cancer patients (50- to 100-fold above normal) (45,46). Aromatase inhibitors are effective either as initial therapy for estrogen-dependent breast cancer or after a course of tamoxifen. In this study, we evaluated the interaction of dietary GEN with LET using a MCF-7Ca xenografted ovariectomized athymic mouse model. We utilized MCF-7Ca cells because of their higher expression and activity of aromatase, 10-fold compared with the parental MCF-7 cells (47). Increased aromatase activity, protein and mRNA have been observed in breast tumors (48), so this model could be more relevant to postmenopausal advanced breast cancer patients. Tumors from implanted MCF-7Ca cells, which express aromatase, can grow without exogenous estrogen or estrogen precursor supplementation (Figure 1).
Fig. 1.
Effect of dietary GEN on the growth of MCF-7Ca cells implanted in ovariectomized athymic mice. Animals were randomly assigned into five treatment groups: MCF-7Ca (12 mice; n = 15 tumors), 250 GEN (15 mice; n = 30 tumors), 500 GEN (15 mice; n = 28 tumors) and 1000 GEN (14 mice; n = 22 tumors). Data are expressed as average cross-sectional area (mm2 ± SEM) for all tumors in each treatment group. Average tumor sizes at week 19 were 48.8 ± 13.9 mm2, 98.6 ± 13.5 mm2, 115.8 ± 12.3 mm2 and 60.9 ± 11.6 mm2, respectively. Bars with different letters are significantly different, P < 0.05.
Dietary GEN (250–1000 p.p.m.) generated total GEN levels (free plus conjugated forms) in plasma that ranged 1.0–5.9 μM (Figure 3B). There was a correlation between dietary GEN concentration and serum GEN level (Figure 3B). The concentration of GEN circulating in plasma of women consuming soy products has been reported in the range of 0.74–6.0 μM (20). However, GEN-induced tumor growth was not dose dependent (Figure 1). GEN at 250 and 500 p.p.m. stimulated the growth of MCF-7Ca tumors implanted in ovariectomized mice, whereas the higher dose, 1000 p.p.m. GEN, did not differ from the MCF-7Ca control group (Figure 1), suggesting that dietary GEN may have multiple activities—a stimulatory effect at lower concentrations and an inhibitory effect at higher concentrations. In a recent study, multiple activities of a dietary supplement (Avlimil, a herbal mixture) were observed—a stimulatory effect at a low dietary dose and no effect at a high dietary dose on ER (+) human breast tumor growth (49). It is important to note that only minor changes in dose can dramatically alter biological responses. AD can be converted to E2 by aromatase and used to stimulate the growth of MCF-7Ca cells in vitro (50) and in nude mice (51). We observed that AD implantation highly stimulated MCF-7Ca tumors (Figure 1). LET implantation effectively blocked the basal level of growth of aromatase-expressing tumors (MCF-7Ca) in the LET group and blocked AD-stimulated MCF-7Ca tumor growth in the AD + LET group (Figure 2). The plasma concentration of LET in implanted mice was in the range of 0.01 μM, a concentration ∼50-fold lower than the apparent steady state level observed in women receiving a 2.5 mg/kg/day dose of LET (52).
Dietary GEN reversed the inhibitory effect of LET in a dose-dependent manner (Figure 2). AD supplementation increased circulating E2 levels and LET reduced AD-induced E2 levels (Figure 3A). The E2 plasma levels were in the range of the circulating E2 levels observed in postmenopausal women (20–200 pM) (53,54). In our previous studies (29), we observed that dietary GEN can decrease serum E2 levels following E2 implantation. In this study, we observed an increase in serum E2 by GEN in the presence of AD (Figure 3A). Brooks et al. (22) reported that GEN (10 μM) inhibited aromatase in MCF-7 cells resulting in lower production of estrone (by 70%) (28). We observed that dietary GEN (250–1000 p.p.m.) did not alter the expression of aromatase mRNA in the presence of AD, whereas GEN (only at 1000 p.p.m.) reduced expression of aromatase mRNA in the absence of AD (Figure 4B). However, data obtained from IHC analysis were not consistent with mRNA expression. GEN (only at 500 p.p.m.) reduced aromatase protein expression in the absence of AD and GEN (250 and 1000 p.p.m.) did not affect aromatase protein expression in tumors (Figure 4D), whereas dietary GEN treatment resulted in a concentration-dependent increase in pS2 gene transcription (Figure 4C), suggesting that GEN may directly affect pS2 transcription in MCF-7Ca tumors. Several promoters are required for aromatase expression in breast cancer (55). Discrepancy between aromatase transcription and translation suggest that GEN may involve a complex system on aromatase transcription. There is a correlation between aromatase activity and aromatase mRNA in breast tumors (44). Aromatase gene transcription is regulated in a tissue-specific manner, using different promoter regions (56,57). Aromatase gene transcription was not further elevated by AD treatment, which was expected since AD is a substrate for the aromatase enzyme and not an inducer of aromatase gene transcription. pS2 gene transcription was increased by AD (Figure 4A). Transcriptional activation of the pS2 gene is a primary response to estrogens in the MCF-7 cell line, and pS2 gene expression is an adequate way to assess estrogenicity in humans (58). As dietary GEN increased pS2 gene transcription in the absence of AD, MCF-7Ca tumor growth was stimulated (Figure 4A).
The implants produced plasma concentrations of LET that effectively blocked E2 synthesis from AD (Figure 3A), but dietary GEN reversed LET-inhibited MCF-7Ca tumor growth. In the presence of AD and LET, dietary GEN increased tumor growth (Figure 2), cellular proliferation in tumors (Figure 5), plasma E2 levels (Figure 3A) (and possibly local E2 levels), expression of pS2 mRNA (Figure 4A) and protein (Figure 4C) and uterine wet weight (Figure 6). The correlation between changes in tumor growth and circulating concentrations of GEN suggest that ER agonist activity may be involved in GEN-stimulated MCF-7Ca cell growth. Furthermore, the uterotrophic effect observed further suggests that dietary GEN-stimulated MCF-7Ca tumor growth may involve an ERα-mediated mechanism.
Fig. 6.
Uterine wet weight in ovariectomized athymic mice. Average uterine wet weight was 52.1 ± 5.3 mg for the MCF-7Ca (ovariectomized), 45.1 ± 5.8 mg for the 250 GEN, 39.7 ± 4.7 mg for the 500 GEN, 39.3 ± 6.2 mg for the 1000 GEN, 94.3 ± 5.1 mg for the AD, 7.3 ± 1.4 mg for the LET, 7.3 ± 0.9 mg for the AD + LET, 21.5 ± 4.6 mg for the AD + LET + 250 GEN, 28.0 ± 4.7 mg for the AD + LET + 500 GEN and 34.3 ± 4.7 mg for the AD + LET + 1000 GEN groups, respectively. In the presence of AD and LET, dietary GEN increased uterine weight by 2.9× (250 GEN), 3.8× (500 GEN) and 4.6× (1000 GEN) (P < 0.05). Data are expressed as average uterine wet weights in milligrams for each treatment group. Bars with different letters are significantly different, P < 0.05.
In summary, dietary GEN, which produced circulating concentrations consistent with human exposures, did not act as an aromatase inhibitor; rather, dietary intake of GEN negated the inhibitory effect of an aromatase inhibitor, LET, by stimulating the growth of aromatase-expressing estrogen-dependent breast tumors by an ER agonist-mediated mechanism. Results from this study raise concerns about the consumption of GEN-containing products by postmenopausal women with advanced breast cancer who may be treated with LET. The potential to reverse the beneficial effects of E2 suppression achieved by LET in women with breast cancer should be carefully considered.
Funding
National Cancer Institute (CA77355) to W.G.H.; National Institute on Aging (P01 AG024387) to W.G.H., D.D. and Y.H.J.; National Institute for Complementary and Alternative Medicine (P01 AG024387); Office of Dietary Supplements; Women’s Health Initiative (P01 AG024387).
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AD
androstenedione
- CAM
complementary and alternative medicine
- CYP
cytochrome P450
- E2
estradiol
- ER
estrogen receptor
- 6-FAM
6-carboxyfluorescein
- GEN
genistein
- IHC
immunohistochemical
- LET
letrozole
- mRNA
messenger RNA
- PCR
polymerase chain reaction
- qRT
quantitative reverse transcription
References
- 1.American Cancer Society. Cancer Statistics 2008. 2008. http://www.cancer.org/downloads/STT/Cancer_Statistics_2008.ppt#2. [Google Scholar]
- 2.Bagchi S. Men with breast cancer have high risk of second cancer. Lancet Oncol. 2007;8:198. doi: 10.1016/s1470-2045(07)70067-0. [DOI] [PubMed] [Google Scholar]
- 3.Harlan LC, et al. Adjuvant therapy for breast cancer: practice patterns of community physicians. J. Clin. Oncol. 2002;20:1809–1817. doi: 10.1200/JCO.2002.07.052. [DOI] [PubMed] [Google Scholar]
- 4.Ravdin PM, et al. The decrease in breast-cancer incidence in 2003 in the United States. N. Engl J. Med. 2007;356:1670–1674. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 5.Howell A, et al. Defining the roles of aromatase inhibitors in the adjuvant treatment of early-stage breast cancer. Clin. Breast Cancer. 2005;6:302–309. doi: 10.3816/CBC.2005.n.032. [DOI] [PubMed] [Google Scholar]
- 6.Labrie F, et al. Intracrinology: role of the family of 17 beta-hydroxysteroid dehydrogenases in human physiology and disease. J. Mol. Endocrinol. 2000;25:1–16. doi: 10.1677/jme.0.0250001. [DOI] [PubMed] [Google Scholar]
- 7.Zhu BT, et al. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Simpson ER. Biology of aromatase in the mammary gland. J. Mammary Gland Biol. Neoplasia. 2000;5:251–258. doi: 10.1023/a:1009590626450. [DOI] [PubMed] [Google Scholar]
- 9.Parker CR, Jr, et al. Effects of aging on adrenal function in the human: responsiveness and sensitivity of adrenal androgens and cortisol to adrenocorticotropin in premenopausal and postmenopausal women. J. Clin. Endocrinol. Metab. 2000;85:48–54. doi: 10.1210/jcem.85.1.6265. [DOI] [PubMed] [Google Scholar]
- 10.Bulun SE, et al. Aromatase in aging women. Semin. Reprod. Endocrinol. 1999;17:349–358. doi: 10.1055/s-2007-1016244. [DOI] [PubMed] [Google Scholar]
- 11.James VH, et al. Aromatase activity in normal breast and breast tumor tissues: in vivo and in vitro studies. Steroids. 1987;50:269–279. doi: 10.1016/0039-128x(83)90077-6. [DOI] [PubMed] [Google Scholar]
- 12.Lu Q, et al. Expression of aromatase protein and messenger ribonucleic acid in tumor epithelial cells and evidence of functional significance of locally produced estrogen in human breast cancers. Endocrinology. 1996;137:3061–3068. doi: 10.1210/endo.137.7.8770932. [DOI] [PubMed] [Google Scholar]
- 13.Bhatnagar AS, et al. Intracellular aromatase and its relevance to the pharmacological efficacy of aromatase inhibitors. J. Steroid Biochem. Mol. Biol. 2001;76:199–202. doi: 10.1016/s0960-0760(01)00050-4. [DOI] [PubMed] [Google Scholar]
- 14.Coombes RC, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N. Engl J. Med. 2004;350:1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 15.Howell A, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 16.Herold CI, et al. Aromatase inhibitors for breast cancer: proven efficacy across the spectrum of disease. Clin. Breast Cancer. 2008;8:50–64. doi: 10.3816/CBC.2008.n.003. [DOI] [PubMed] [Google Scholar]
- 17.Mouridsen H, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J. Clin. Oncol. 2001;19:2596–2606. doi: 10.1200/JCO.2001.19.10.2596. [DOI] [PubMed] [Google Scholar]
- 18.Bertelli G, et al. Maintenance hormone therapy with letrozole after first-line chemotherapy for advanced breast cancer. Oncology. 2005;68:364–370. doi: 10.1159/000086976. [DOI] [PubMed] [Google Scholar]
- 19.Koeberle D, et al. Letrozole as adjuvant endocrine therapy in postmenopausal women with breast cancer. Expert Rev. Anticancer Ther. 2006;6:5–10. doi: 10.1586/14737140.6.1.5. [DOI] [PubMed] [Google Scholar]
- 20.Ju YH, et al. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J. Nutr. 2001;131:2957–2962. doi: 10.1093/jn/131.11.2957. [DOI] [PubMed] [Google Scholar]
- 21.Pelissero C, et al. Effects of flavonoids on aromatase activity, an in vitro study. J. Steroid Biochem. Mol. Biol. 1996;57:215–223. doi: 10.1016/0960-0760(95)00261-8. [DOI] [PubMed] [Google Scholar]
- 22.Brooks JD, et al. Mammalian lignans and genistein decrease the activities of aromatase and 17[beta]-hydroxysteroid dehydrogenase in MCF-7 cells. J. Steroid Biochem. Mol. Biol. 2005;94:461–467. doi: 10.1016/j.jsbmb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, et al. Potential beneficial metabolic interactions between tamoxifen and isoflavones via cytochrome P450-mediated pathways in female rat liver microsomes. 2004;21:2095. doi: 10.1023/b:pham.0000048202.92930.61. [DOI] [PubMed] [Google Scholar]
- 24.Foster BC, et al. In vitro inhibition of human cytochrome P450-mediated metabolism of marker substrates by natural products. 2003;10:334. doi: 10.1078/094471103322004839. [DOI] [PubMed] [Google Scholar]
- 25.Sun XY, et al. Increased UDP-glucuronosyltransferase activity and decreased prostate specific antigen production by biochanin A in prostate cancer cells. 1998;58:2379. [PubMed] [Google Scholar]
- 26.Yannai S, et al. Characterization of flavonoids as monofunctional or bifunctional inducers of quinone reductase in murine hepatoma cell lines. 1998;36:623. doi: 10.1016/s0278-6915(98)00022-2. [DOI] [PubMed] [Google Scholar]
- 27.Roberts DW, et al. Inhibition of extrahepatic human cytochromes P450 1A1 and 1B1 by metabolism of isoflavones found in Trifolium pratense (red clover) J. Agric. Food Chem. 2004;52:6623–6632. doi: 10.1021/jf049418x. [DOI] [PubMed] [Google Scholar]
- 28.Almstrup K, et al. Dual effects of phytoestrogens result in u-shaped dose-response curves. Environ. Health Perspect. 2002;110:743–748. doi: 10.1289/ehp.02110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ju YH, et al. Dietary genistein negates the inhibitory effect of tamoxifen on growth of estrogen-dependent human breast cancer (MCF-7) cells implanted in athymic mice. Cancer Res. 2002;62:2474–2477. [PubMed] [Google Scholar]
- 30.Chen S. Aromatase and breast cancer. Front. Biosci. 1998;3:d922–d933. doi: 10.2741/a333. [DOI] [PubMed] [Google Scholar]
- 31.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997;127:838S–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 32.Chang HC, et al. Mass spectrometric determination of Genistein tissue distribution in diet-exposed Sprague-Dawley rats. J. Nutr. 2000;130:1963–1970. doi: 10.1093/jn/130.8.1963. [DOI] [PubMed] [Google Scholar]
- 33.Jakowlew SB, et al. Sequence of the pS2 mRNA induced by estrogen in the human breast cancer cell line MCF-7. Nucleic Acids Res. 1984;12:2861–2878. doi: 10.1093/nar/12.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ju YH, et al. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis. 2006;27:856–863. doi: 10.1093/carcin/bgi320. [DOI] [PubMed] [Google Scholar]
- 35.Scholzen T, et al. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 36.Blake JM, et al. The SOGC statement on the WHI report on estrogen and progestin use in postmenopausal women. J. Obstet. Gynaecol. Can. 2002;24:783–790. doi: 10.1016/s1701-2163(16)30471-6. 793–802. [DOI] [PubMed] [Google Scholar]
- 37.Glass AG, et al. Breast cancer incidence, 1980-2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J. Natl Cancer Inst. 2007;99:1152–1161. doi: 10.1093/jnci/djm059. [DOI] [PubMed] [Google Scholar]
- 38.Sacks FM, et al. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113:1034–1044. doi: 10.1161/CIRCULATIONAHA.106.171052. [DOI] [PubMed] [Google Scholar]
- 39.Eisenberg DM, et al. Unconventional medicine in the United States—prevalence, costs, and patterns of use. N. Engl J. Med. 1993;328:246–252. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- 40.Gerber B, et al. Complementary and alternative therapeutic approaches in patients with early breast cancer: a systematic review. Breast Cancer Res. Treat. 2006;95:199–209. doi: 10.1007/s10549-005-9005-y. [DOI] [PubMed] [Google Scholar]
- 41.Phipps WR, et al. Isoflavones and postmenopausal women: a critical review. Treat. Endocrinol. 2002;1:293–311. doi: 10.2165/00024677-200201050-00003. [DOI] [PubMed] [Google Scholar]
- 42.Kaari C, et al. Randomized clinical trial comparing conjugated equine estrogens and isoflavones in postmenopausal women: a pilot study. Maturitas. 2006;53:49–58. doi: 10.1016/j.maturitas.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Simpson ER, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 44.Miller WR, et al. Regulation of aromatase activity within the breast. J. Steroid. Biochem. Mol. Biol. 1997;61:193–202. [PubMed] [Google Scholar]
- 45.Dorgan JF, et al. Re: plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J. Natl Cancer Inst. 1999;91:380–381. doi: 10.1093/jnci/91.4.380. [DOI] [PubMed] [Google Scholar]
- 46.Purohit A, et al. Aromatase activity and interleukin-6 production by normal and malignant breast tissues. J. Clin. Endocrinol. Metab. 1995;80:3052–3058. doi: 10.1210/jcem.80.10.7559896. [DOI] [PubMed] [Google Scholar]
- 47.Zhou DJ, et al. Stable expression of human aromatase complementary DNA in mammalian cells: a useful system for aromatase inhibitor screening. Cancer Res. 1990;50:6949–6954. [PubMed] [Google Scholar]
- 48.Zhou C, et al. Aromatase gene expression and its exon I usage in human breast tumors. Detection of aromatase messenger RNA by reverse transcription-polymerase chain reaction. J. Steroid Biochem. Mol. Biol. 1996;59:163–171. doi: 10.1016/s0960-0760(96)00100-8. [DOI] [PubMed] [Google Scholar]
- 49.Ju YH, et al. A dietary supplement for female sexual dysfunction, Avlimil, stimulates the growth of estrogen-dependent breast tumors (MCF-7) implanted in ovariectomized athymic nude mice. Food Chem. Toxicol. 2008;46:310–320. doi: 10.1016/j.fct.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Santner SJ, et al. Effect of androstenedione on growth of untransfected and aromatase-transfected MCF-7 cells in culture. J. Steroid Biochem. Mol. Biol. 1993;44:611–616. doi: 10.1016/0960-0760(93)90267-z. [DOI] [PubMed] [Google Scholar]
- 51.Yue W, et al. A new nude mouse model for postmenopausal breast cancer using MCF-7 cells transfected with the human aromatase gene. Cancer Res. 1994;54:5092–5095. [PubMed] [Google Scholar]
- 52.Awada A, et al. The oral mTOR inhibitor RAD001 (everolimus) in combination with letrozole in patients with advanced breast cancer: results of a phase I study with pharmacokinetics. Eur. J. Cancer. 2008;44:84–91. doi: 10.1016/j.ejca.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Lonning PE, et al. Plasma estrogen suppression with aromatase inhibitors evaluated by a novel, sensitive assay for estrone sulphate. J. Steroid Biochem. Mol. Biol. 1997;61:255–260. [PubMed] [Google Scholar]
- 54.Yue W, et al. Aromatase within the breast. Endocr. Relat. Cancer. 1999;6:157–164. doi: 10.1677/erc.0.0060157. [DOI] [PubMed] [Google Scholar]
- 55.Bulun SE, et al. Organization of the human aromatase p450 (CYP19) gene. Semin. Reprod. Med. 2004;22:5–9. doi: 10.1055/s-2004-823022. [DOI] [PubMed] [Google Scholar]
- 56.Purohit A, et al. Regulation of estrogen synthesis in postmenopausal women. Steroids. 2002;67:979–983. doi: 10.1016/s0039-128x(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Y, et al. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology. 1996;137:5739–5742. doi: 10.1210/endo.137.12.8940410. [DOI] [PubMed] [Google Scholar]
- 58.Soto AM, et al. Developing a marker of exposure to xenoestrogen mixtures in human serum. Environ. Health Perspect. 1997;105(suppl. 3):647–654. doi: 10.1289/ehp.97105s3647. [DOI] [PMC free article] [PubMed] [Google Scholar]