Abstract
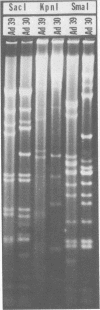
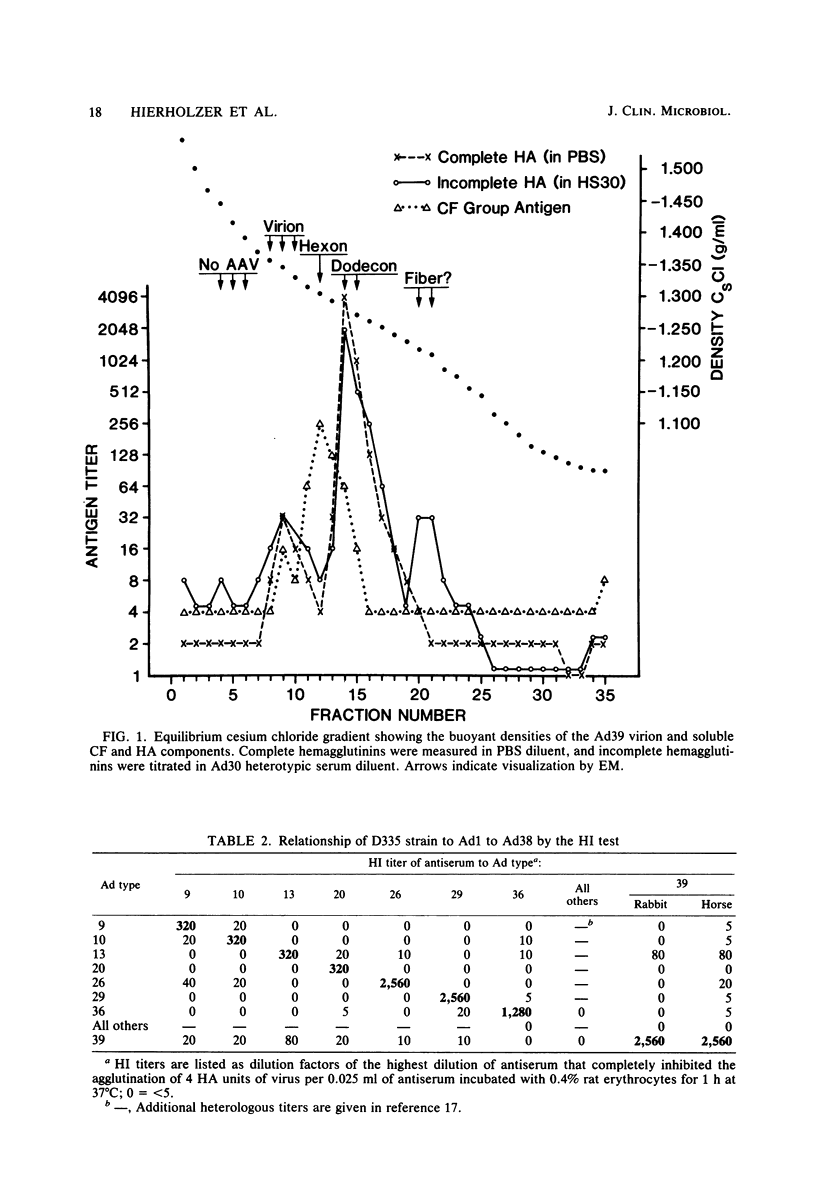
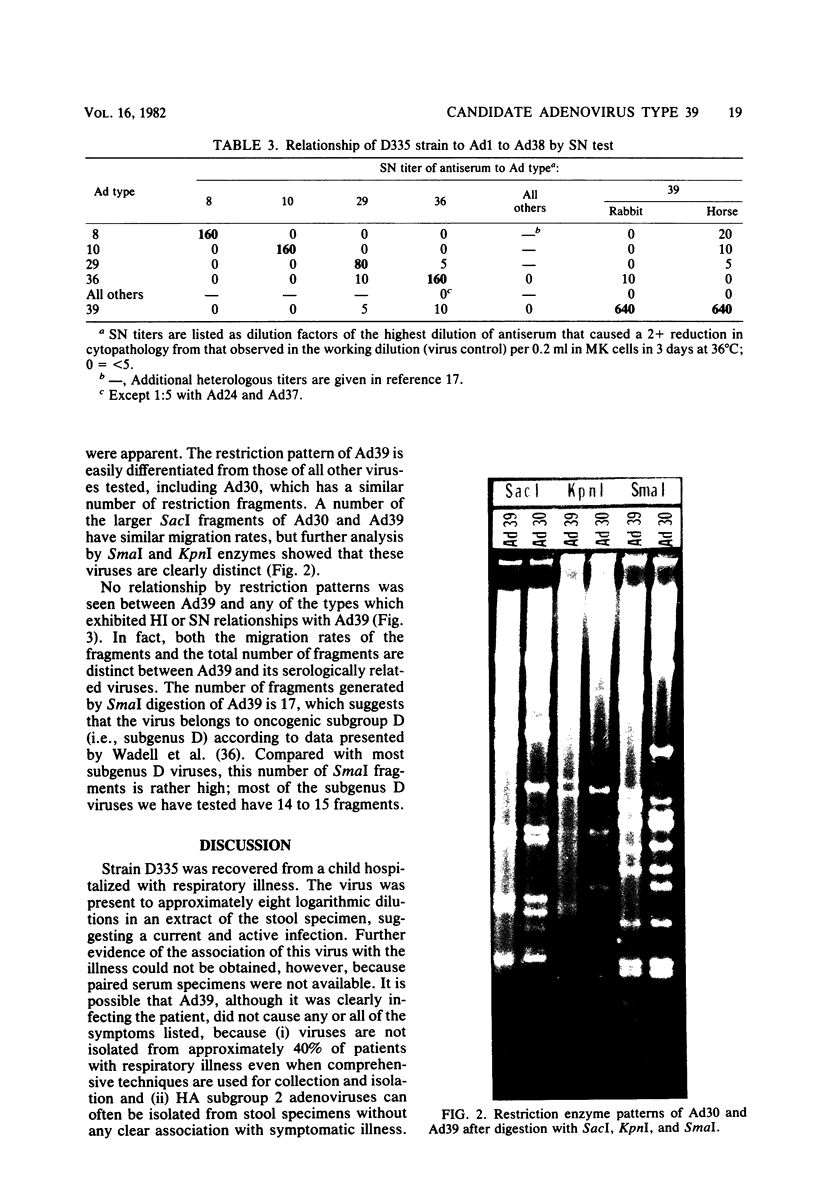
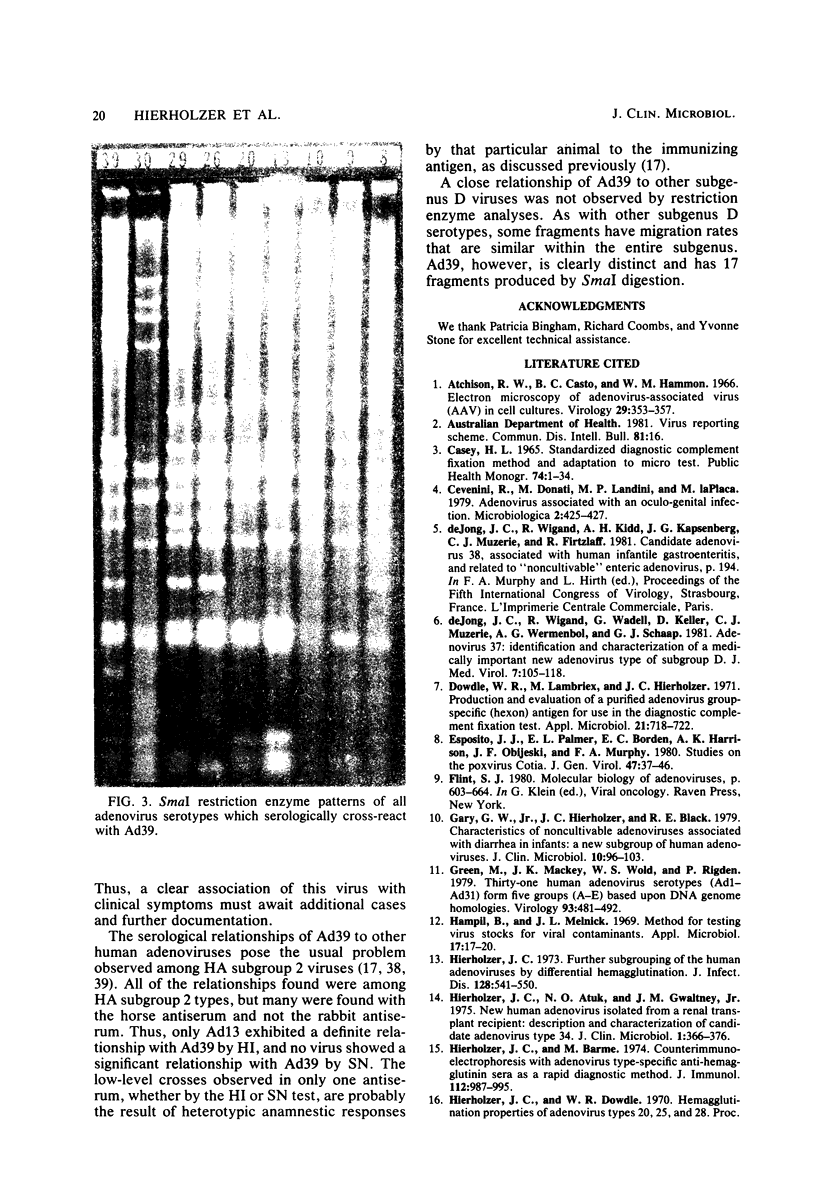
A new human adenovirus was isolated from a stool specimen from a 2-year-old El Salvadorian male hospitalized with severe respiratory illness. The virus, strain D335, is a typical adenovirus by routine classification tests and bears little resemblance to the other 38 adenovirus serotypes by standard hemagglutination inhibition and serum neutralization tests. The only significant cross-reaction observed was between adenovirus types 39 and 13, bilaterally, by the hemagglutination inhibition test. The virus was clearly differentiated from the other human adenoviruses by restriction enzyme analyses with SmaI, SacI, and KpnI enzymes. Strain D335 agglutinates human and rat erythrocytes to moderately high titers and is placed in hemagglutination subgroup 2B on the basis of differential hemagglutination with erythrocytes from 13 animal species. It has a density in cesium chloride of 1.339 g/ml and produces soluble components in human embryonic kidney culture that band at 1.303 g/ml (hexon), 1.283 g/ml (dodecon), and 1.212 g/ml (fiber), the last component being the only incomplete hemagglutinin found. The viral DNA is cleaved into 17 fragments by the SmaI restriction enzyme, indicating that strain D335 is a member of adenovirus subgenus D. Strain D335 is herein described as candidate adenovirus type 39 (Mastadenovirus h 39).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atchison R. W., Casto B. C., Hammon W. M. Electron microscopy of adenovirus-associated virus (AAV) in cell cultures. Virology. 1966 Jun;29(2):353–357. doi: 10.1016/0042-6822(66)90045-6. [DOI] [PubMed] [Google Scholar]
- CASEY H. L. STANDARDIZED DIAGNOSTIC COMPLEMENT FIXATION METHOD AND ADAPTATION TO MICRO TEST. I. LABORATORY BRANCH COMPLEMENT FIXATION METHOD BY LABORATORY BRANCH TASK FORCE. II. ADAPTATION OF LBCF METHOD TO MICRO TECHNIQUE. Public Health Monogr. 1965;74:1–34. [PubMed] [Google Scholar]
- Dowdle W. R., Lambriex M., Hierholzer J. C. Production and evaluation of a purified adenovirus group-specific (hexon) antigen for use in the diagnostic complement fixation test. Appl Microbiol. 1971 Apr;21(4):718–722. doi: 10.1128/am.21.4.718-722.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito J. J., Palmer E. L., Borden E. C., Harrison A. K., Obijeski J. F., Murphy F. A. Studies on the poxvirus Cotia. J Gen Virol. 1980 Mar;47(1):37–46. doi: 10.1099/0022-1317-47-1-37. [DOI] [PubMed] [Google Scholar]
- Gary G. W., Jr, Hierholzer J. C., Black R. E. Characteristics of noncultivable adenoviruses associated with diarrhea in infants: a new subgroup of human adenoviruses. J Clin Microbiol. 1979 Jul;10(1):96–103. doi: 10.1128/jcm.10.1.96-103.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Mackey J. K., Wold W. S., Rigden P. Thirty-one human adenovirus serotypes (Ad1-Ad31) form five groups (A-E) based upon DNA genome homologies. Virology. 1979 Mar;93(2):481–492. doi: 10.1016/0042-6822(79)90251-4. [DOI] [PubMed] [Google Scholar]
- Hampil B., Melnick J. L. Method for testing virus stocks for viral contaminants. Appl Microbiol. 1969 Jan;17(1):17–20. doi: 10.1128/am.17.1.17-20.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Atuk N. O., Gwaltney J. M., Jr New human adenovirus isolated from a renal transplant recipient: description and characterization of candiate adenovirus type 34. J Clin Microbiol. 1975 Apr;1(4):366–376. doi: 10.1128/jcm.1.4.366-376.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Barme M. Counterimmunoelectrophoresis with adenovirus type-specific anti-hemagglutinin sera as a rapid diagnostic method. J Immunol. 1974 Mar;112(3):987–995. [PubMed] [Google Scholar]
- Hierholzer J. C., Dowdle W. R. Hemagglutination properties of adenovirus types 20, 25, and 28. Proc Soc Exp Biol Med. 1970 Jun;134(2):482–488. doi: 10.3181/00379727-134-34817. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C. Further subgrouping of the human adenoviruses by differential hemagglutination. J Infect Dis. 1973 Oct;128(4):541–550. doi: 10.1093/infdis/128.4.541. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C., Gamble W. C., Dowdle W. R. Reference equine antisera to 33 human adenovirus types: homologous and heterologous titers. J Clin Microbiol. 1975 Jan;1(1):65–74. doi: 10.1128/jcm.1.1.65-74.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Gamble W. C., Quist K. D., Chappell W. A. Comparison of immunization methods for producing reference adenovirus antisera in horses. Appl Microbiol. 1972 Sep;24(3):398–404. doi: 10.1128/am.24.3.398-404.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Suggs M. T., Hall E. C. Standardized viral hemagglutination and hemagglutination-inhibition tests. II. Description and statistical evaluation. Appl Microbiol. 1969 Nov;18(5):824–833. doi: 10.1128/am.18.5.824-833.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Suggs M. T. Standardized viral hemagglutination and hemagglutination-inhibition tests. I. Standardization of erythrocyte suspensions. Appl Microbiol. 1969 Nov;18(5):816–823. doi: 10.1128/am.18.5.816-823.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggan M. D., Blacklow N. R., Rowe W. P. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Mayor H. D. Hemagglutinin of type 4 adeno-associated satellite virus. J Immunol. 1968 Jan;100(1):61–68. [PubMed] [Google Scholar]
- Jacobsson P. A., Johansson M. E., Wadell G. Identification of an enteric adenovirus by immunoelectroosmophoresis (IEOP) technique. J Med Virol. 1979;3(4):307–312. doi: 10.1002/jmv.1890030409. [DOI] [PubMed] [Google Scholar]
- Johansson M. E., Uhnoo I., Kidd A. H., Madeley C. R., Wadell G. Direct identification of enteric adenovirus, a candidate new serotype, associated with infantile gastroenteritis. J Clin Microbiol. 1980 Jul;12(1):95–100. doi: 10.1128/jcm.12.1.95-100.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M. E., Wadell G., Jacobsson P. A., Svensson L. Preparation of specific antisera against adenoviruses by affinity bead immunization (ABI). J Immunol Methods. 1979;26(2):141–149. doi: 10.1016/0022-1759(79)90077-2. [DOI] [PubMed] [Google Scholar]
- Keller E. W., Rubin R. H., Black P. H., Hirsch M. S., Hierholzer J. C. Isolation of adenovirus type 34 from a renal transplant recipient with interstitial pneumonia. Transplantation. 1977 Feb;23(2):188–191. doi: 10.1097/00007890-197702000-00020. [DOI] [PubMed] [Google Scholar]
- Kidd A. H., Madeley C. R. In vitro growth of some fastidious adenoviruses from stool specimens. J Clin Pathol. 1981 Feb;34(2):213–216. doi: 10.1136/jcp.34.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerla H. C., Hierholzer J. C. Physicochemical and serological characteristics of respiratory virus fluorescein-isothiocyanate conjugates for fluorescent-antibody diagnosis. J Clin Microbiol. 1975 May;1(5):451–461. doi: 10.1128/jcm.1.5.451-461.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerowitz R. L., Stalder H., Oxman M. N., Levin M. J., Moore M., Leith J. D., Gantz N. M., Hierholzer J. C., Hierholzer J. C. Fatal disseminated adenovirus infection in a renal transplant recipient. Am J Med. 1975 Oct;59(4):591–598. doi: 10.1016/0002-9343(75)90267-3. [DOI] [PubMed] [Google Scholar]
- Norrby E., Bartha A., Boulanger P., Dreizin R. S., Ginsberg H. S., Kalter S. S., Kawamura H., Rowe W. P., Russell W. C., Schlesinger W. Adenoviridae. Intervirology. 1976;7(3):117–125. doi: 10.1159/000149945. [DOI] [PubMed] [Google Scholar]
- Schaap G. J., de Jong J. C., van Bijsterveld O. P., Beekhuis W. H. A new intermediate adenovirus type causing conjunctivitis. Arch Ophthalmol. 1979 Dec;97(12):2336–2338. doi: 10.1001/archopht.1979.01020020552010. [DOI] [PubMed] [Google Scholar]
- Stalder H., Hierholzer J. C., Oxman M. N. New human adenovirus (candidate adenovirus type 35) causing fatal disseminated infection in a renal transplant recipient. J Clin Microbiol. 1977 Sep;6(3):257–265. doi: 10.1128/jcm.6.3.257-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D. A., Schaeffer M., Fox J. P., Brandt C. D., Romano M. Standardization and certification of reference antigens and antisera for 30 human adenovirus serotypes. Am J Epidemiol. 1967 Nov;86(3):617–633. doi: 10.1093/oxfordjournals.aje.a120771. [DOI] [PubMed] [Google Scholar]
- Tullo A. B., Higgins P. G. An outbreak of adenovirus keratoconjunctivitis in bristol. Br J Ophthalmol. 1979 Sep;63(9):621–626. doi: 10.1136/bjo.63.9.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullo A. B., Higgins P. G. Epidemic adenovirus keratoconjunctivitis. Lancet. 1978 Feb 25;1(8061):442–443. doi: 10.1016/s0140-6736(78)91229-1. [DOI] [PubMed] [Google Scholar]
- Wadell G., Hammarskjöld M. L., Winberg G., Varsanyi T. M., Sundell G. Genetic variability of adenoviruses. Ann N Y Acad Sci. 1980;354:16–42. doi: 10.1111/j.1749-6632.1980.tb27955.x. [DOI] [PubMed] [Google Scholar]
- Wadell G., Sundell G., de Jong J. C. Characterization of candidate adenovirus 37 by SDS-polyacrylamide gel electrophoresis of virion polypeptides and DNA restriction site mapping. J Med Virol. 1981;7(2):119–125. doi: 10.1002/jmv.1890070205. [DOI] [PubMed] [Google Scholar]
- Wigand R., Fliedner D. Serologically intermediate adenovirus strains: a regular feature of group II adenoviruses. Arch Gesamte Virusforsch. 1968;24(3):245–256. doi: 10.1007/BF01241296. [DOI] [PubMed] [Google Scholar]
- Wigand R., Gelderblom H., Wadell G. New human adenovirus (candidate adenovirus 36), a novel member of subgroup D. Arch Virol. 1980;64(3):225–233. doi: 10.1007/BF01322702. [DOI] [PubMed] [Google Scholar]
- Wigand R. Serologische Beziehungen der Adenoviren der Gruppe II. Arch Gesamte Virusforsch. 1968;23(1):40–47. [PubMed] [Google Scholar]
- de Jong J. C., Wigand R., Wadell G., Keller D., Muzerie C. J., Wermenbol A. G., Schaap G. J. Adenovirus 37: identification and characterization of a medically important new adenovirus type of subgroup D. J Med Virol. 1981;7(2):105–118. doi: 10.1002/jmv.1890070204. [DOI] [PubMed] [Google Scholar]