Abstract
During vertebrate embryogenesis, hematopoietic stem cells (HSC) arise in the aorta-gonads-mesonephros (AGM) region. A zebrafish chemical genetic screen identified compounds that regulate blood flow as modulators of HSC formation. silent heart (sih) embryos that lack a heartbeat and blood circulation exhibited severely reduced HSCs. Blood flow modifiers exerted their effects after the onset of heartbeat; however, nitric oxide (NO) donors affected HSC induction even when treatment occurred prior to the initiation of circulation, and rescued HSCs in sih mutants. NO synthase (Nos) inhibitors and morpholino-knockdown of nos1 (nnos/enos) blocked HSC development. Embryonic transplantation assays demonstrated a cell-autonomous requirement for nos1. Nos3 (eNos) was expressed in HSCs in the murine AGM. Intrauterine Nos inhibition or Nos3 deficiency in mice resulted in the absence of hematopoietic clusters and reduced transplantable progenitors and HSCs. This work links blood flow to AGM hematopoiesis, and identifies NO as a conserved downstream regulator of HSC development.
Introduction
Definitive HSCs that are capable of self-renewal and production of all mature blood lineages arise during embryogenesis. Both the timing of HSC induction and the gene programs regulating this process are well conserved across vertebrate species (Orkin and Zon, 2008). Additionally, factors that affect HSC specification during embryogenesis often similarly function in HSC maintenance and/or recovery after marrow injury. The identification of factors that regulate HSC induction during embryogenesis is of significant therapeutic interest.
Murine transplantation studies revealed that adult-type long-term repopulating (LTR) HSCs arise in the AGM region between e10.5 and e11.5 (Dzierzak and Medvinsky, 2008). Transplantable HSCs localize to the ventral wall of the dorsal aorta and express phenotypic markers of mesenchymal, endothelial or hematopoietic cell types. Runx1, commonly affected in childhood and adult leukemia (Downing et al., 1993; Golub et al., 1995), is required for the formation of functional HSCs (North et al., 1999; North et al., 2002; Wang et al., 1996); its expression is highly conserved across vertebrate species (Orkin and Zon, 2008). Based on the functional conservation of AGM hematopoiesis from fish to man, an evolutionary advantage or necessity for the production of stem cells within the aorta must exist. To identify genes that regulate HSC formation, we conducted a chemical genetic screen for regulators of runx1/cmyb+ cells in the zebrafish AGM at 36 hours post fertilization (hpf). We have previously identified PGE2 as a potent regulator of both HSC induction and marrow repopulation across vertebrate species (North et al., 2007). The wnt pathway similarly regulates stem cell production during embryogenesis, and genetically interacts with PGE2 (Goessling et al., 2008a). The identification of novel regulators of this process will aid in connecting the complex network of signaling pathways that control both HSC development during embryogenesis and marrow regulation in the adult.
Nitric Oxide (NO) plays a key role in the regulation of vascular tone, angiogenesis, and endothelial migration (Davies, 1995; Lucitti et al., 2007). As HSCs are derived from hemogenic endothelial cells within the dorsal aorta, NO produced locally in endothelial cells could link blood flow and HSC formation. NO has been detected in blood cells such and extends replating ability in hematopoietic culture, presumably by maintaining HSCs in a quiescent state in vitro (Krasnov et al., 2008). While NO function in the adult hematopoietic stroma is thought to have a positive effect on hematopoiesis, data from knock-out mice imply that NO production is detrimental to hematopoietic repopulation and recovery after injury (Michurina et al., 2004); this effect, however, may be due to NO-related superoxide complexes induced by irradiation (Epperly et al., 2007). The role of NO in HSC induction in the vertebrate embryo is currently uncharacterized.
Here, we show that a diverse group of compounds that regulate blood flow affect the production of runx1/cmyb+ HSCs. In general, compounds that increased blood flow enhanced HSC number, whereas chemicals that decreased blood flow diminished HSCs. Blood flow modifying agents primarily exerted their effects after the onset of the heartbeat; compounds that increase NO production, could modify HSC formation when exposure occurred prior to the initiation of circulation. silent heart (sih) embryos that lack a heartbeat and fail to establish blood circulation had impaired HSC formation. Expression of nos1 (nnos/enos) was found to be significantly downregulated in sih-/- embryos, and NO donors, such as S-nitroso-N-acetyl-penicillamine (SNAP), could rescue HSC production in sih mutants. Inhibition of NO production by N-nitro-L-arginine methyl ester (L-NAME) blocked the inductive effect of several blood flow modulators on HSCs, suggesting that NO serves as the connection between blood flow and HSC formation. In the mouse, NO synthase 3 (Nos3; eNos) is expressed in AGM endothelium and hematopoietic clusters, and marks LTR-HSCs. Intrauterine Nos inhibition by L-NAME blocked hematopoietic cluster formation within the AGM and reduced transplantable CFU-S12 progenitors or HSCs; similar results, although not as severe, were found for the Nos3-/- knockout mice. Our work provides a direct link between the initiation of circulation and the onset of hematopoiesis within the AGM, and identifies NO signaling as a conserved regulator of HSC development.
Results
Modulators of blood flow regulate HSC formation
A chemical genetic screen was conducted to identify regulators of AGM HSC formation (North et al., 2007). Of the chemicals found to regulate runx1 and cmyb co-expression by in situ hybridization at 36hpf, several were known modulators of heartbeat and blood flow. These compounds were categorized into distinct classes based on their hemodynamic mechanism of action (Sup.Fig.1A). Well-established agonists and antagonists of each category were secondarily screened for effects on HSCs (Fig.1A-L). The adrenergic signaling pathways affect both cardiac and vascular physiology. Exposure to the α1-adrenergic blocker doxasozin (10μM) enhanced HSCs (58 increased (inc)/86 scored), while the α-agonist ergotamine (10μM) decreased HSC number (Fig.1B,H, 42 decreased (dec)/82). Similarly, the β1-adrenergic blocker metoprolol increased (49 inc/77) and the β1-agonist epinephrine decreased runx1/cmyb staining (Fig.1C,I, 40 dec/70). Changes in electrolyte balance potently regulate cardiac and vascular reactivity. The Ca2+-channel blocker nifedipine enhanced HSC formation (48 inc/85), while BayK8644 diminished HSC number (Fig.1D,J, 34 dec/79). The cardiac glycoside digoxin, a modulator of Na+/K+ fluxes, also increased HSCs (Fig.1G, 56 inc/79). NO is a well-established direct regulator of vascular tone and reactivity, thereby influencing blood flow. The NO donor SNAP (10μM) caused a significant increase in HSC development (69 inc/93). In contrast, the Nos inhibitor L-NAME (10μM) diminished runx1/cmyb expression (Fig.1E,K, 58 dec/90). Exposure to the angiotensin converting enzyme (ACE) inhibitor enalapril decreased HSC number (Fig.1F, 42 dec/81). These findings were corroborated by qPCR for runx1 (Fig.1M).
Figure 1. Modulation of vascular flow affects HSC formation in zebrafish.
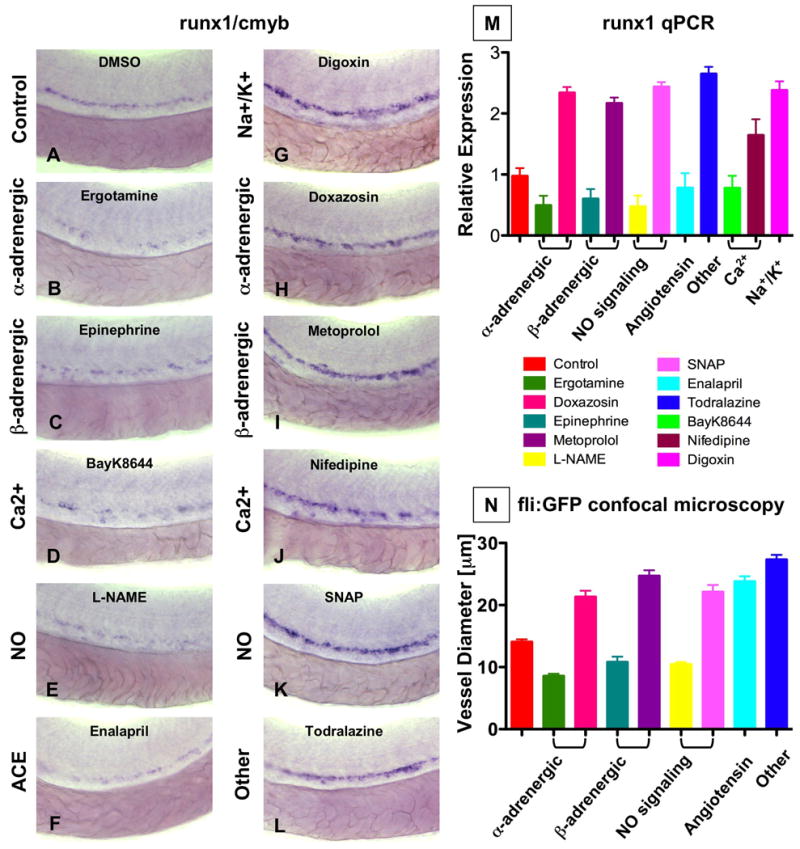
(A-M) Effect of blood flow modifiers on runx1/cmyb+ HSC formation. Zebrafish were exposed to chemicals (10 μM) from 10 somites to 36hpf and subjected to runx1/cmyb in situ hybridization. Photomicrographs were taken with Nomarski optics at 40× magnification. Representative examples following drug treatment are shown.
(L) Effect of todralazine (10 μM; 67 inc/84).
(M) Effect of drug treatment on runx1 expression, quantified by qPCR.
(N) Effect of drug treatment on the diameter of the dorsal aorta in vivo. Transgenic fli:GFP fish were treated with chemicals and imaged by confocal microscopy at 36hpf; all treatments were statistically significant from control, ANOVA, p<0.001, n=5).
Conserved vascular responses of each chemical class were demonstrated by in vivo confocal microscopy of fli:GFP; gata1:dsRed transgenic zebrafish (n=5/compound) at 36hpf (Fig.1N, Sup.Fig.2) (Eddy, 2005). These data correlated with prior zebrafish studies (Fritsche et al., 2000). Vasodilation of the artery and vein was accompanied by increased passage of total blood volume, as seen by digital motion analysis of gata1+ red blood cells (RBCs; data not shown); vasoconstriction caused RBCs to traverse only in single file. Together with the in situ hybridization studies, these experiments reveal that increases in vessel diameter typically were coincident with increased runx1 expression, and vice versa.
We have previously utilized microarray analysis of sorted cell populations isolated during various stages of embryogenesis to document cell-type and developmental specificity of genes of interest (North et al., 2007; Weber et al., 2005). We found components of the NO (nos1), angiotensin (ace2, agtrl1a, agt), and adrenergic signaling (adra2b, adra2da, adra2c) pathways expressed in the HSC compartment (Sup.Fig.1B). Most were more highly expressed during the definitive wave of hematopoiesis after the onset of the heartbeat and circulation, consistent with their role in regulating hemodynamic homeostasis. These data confirm that vascular tone and flow-modifying components are present and responsive to chemical manipulation in the AGM, and imply that modulation of blood flow could have a significant impact on HSC formation during embryonic development.
Absence of a heartbeat causes failures in definitive HSC development
In the zebrafish, the occurrence of vigorous blood circulation through the tail is coincident with HSC formation in this region (TEN, LIZ, unpublished observation). In order to establish the importance of blood flow for initiation of HSC formation, we examined sih mutant zebrafish embryos, which lack a heartbeat due to a mutation in cardiac troponin T (Sehnert et al., 2002) (Fig.2J,K). runx1/cmyb expression was dramatically reduced in sih-/- embryos (Fig.2A,E, 69 dec/77). In contrast, the vascular marker flk1 was minimally affected (Fig.2B,F), consistent with previous observations (Isogai et al., 2003). ephrinB2, a marker of arterial identity, was reduced in sih embryos (Fig.2C,G, 55 dec/74), while expression of the venous marker flt4 was increased (Fig.2D,H, 33 inc/61). These results were confirmed by qPCR (Fig.2I, p<0.05, n=3). In contrast, the erythroid marker globin and the myeloid marker myeloperoxidase (mpo) show distribution differences due to lack of blood circulation in sih mutants, but no gross quantitative changes (Sup.Fig.3A-H). Myosin heavy chain (mhc), a marker of somitogenesis, and the endodermal progenitor marker foxa3 were also not affected. These data demonstrate that the absence of a beating heart and subsequent failure to establish circulation specifically impairs arterial identity and HSC formation.
Figure 2. A beating heart is required for HSC formation and artery development.
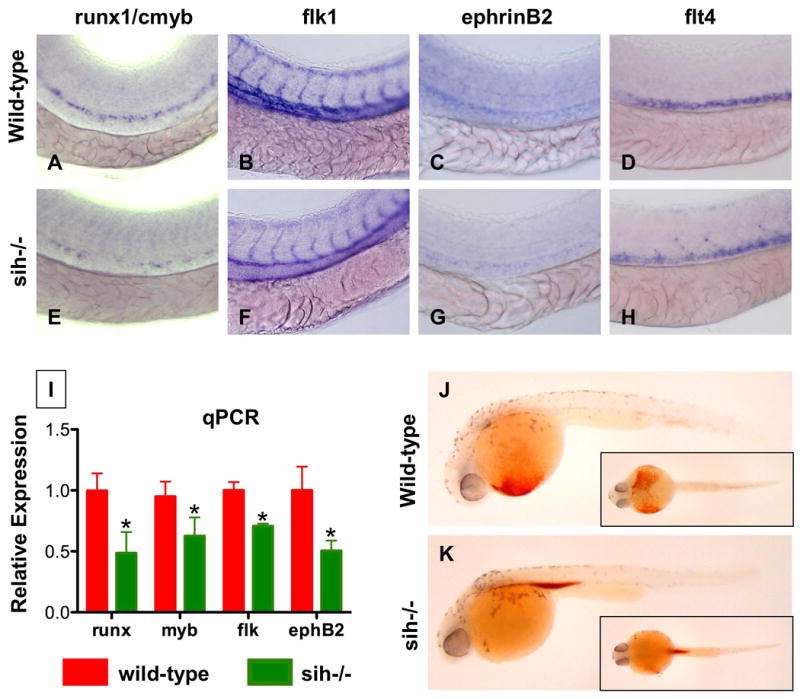
(A-H) Effect of sih mutation on HSC and vascular formation at 36hpf.
(A,E) runx1/cmyb expression is greatly reduced in sih-/- embryos compared to WT siblings.
(B,F) flk1 expression reveals a grossly normal vascular pattern in sih-/- embryos; changes in the development of the intersomitic vessels and vascular plexus were noted in some animals.
(C,F) ephB2 expression is diminished in sih-/- embryos.
(D,H) flt4 expression is expanded in sih-/- embryos.
(L) The expression levels of HSC (runx1, cmyb), vascular (flk) and arterial (ephB2) markers are significantly decreased in sih-/- embryos compared to sibling controls (t-test, p<0.05, n=3), as measured by qPCR at 36hpf.
(J,K) The sih mutation has no effect on primitive hematopoiesis as seen by benzidine staining at 36hpf; in the absence of a heartbeat blood is pooled in the major vessels.
Nitric oxide signaling can affect HSC formation prior to the initiation of flow
To further investigate the role of circulation in the initiation of HSC development, zebrafish embryos were exposed to blood flow modulating agents either before (10 somites–23hpf) or after the onset of heartbeat (26–36hpf) and HSC development was assessed at 36hpf. All compounds examined increased HSC formation when used after the heartbeat began (Fig.3A,C,E,G). In contrast, only SNAP was capable of enhancing HSC number at 36hpf when treatment was completed before the heartbeat was established (Fig.3B,D,F). Likewise, the NO inhibitor L-NAME could reduce HSC formation when treatment occurred prior to the initiation of the heartbeat (Fig.3A). The effects of L-NAME and SNAP were dose-dependent over a range from 1-100 μM (Sup.Fig.4A-L) and specific to the HSC compartment, with mild effects on the vasculature, but not on globin, mpo, mhc, or foxa3 expression (Sup.Fig.5A-R). Additionally, changes were only observed during the definitive hematopoietic wave and were maintained into larval stages (Sup.Fig.6A-O). In addition to SNAP (Fig.3K, 18 inc/25), NO donors sodium nitroprusside (SNP, Fig.3N, 20 inc/31) and L-arginine (L-arg; Fig.3I, 15 inc/25;(Pelster et al., 2005; Pyriochou et al., 2006), enhanced runx1/cmyb expression (Fig.3I,K,N), while the non-specific nos inhibitor, N-monomethyl-L-arg acetate (L-NMMA, Fig.3O, 17 dec/29) diminished HSCs like L-NAME (Fig.3L, 16 dec/26). Inactive D-enantiomers had no effect (Fig.3J,M,P). To assess whether modification of runx1/cmyb expression correlated with a quantifiable effect on HSC number, we utilized cmyb:GFP; lmo2:dsRed reporter fish (North et al., 2007). Confocal microscopy revealed increased HSC (yellow) numbers after SNAP exposure, and a reduction after L-NAME treatment (Fig.3Q-S, Sup.Fig.5S, p<0.001, n=5). TUNEL analysis indicated that L-NAME could affect HSC by induction of apoptosis (Sup.Fig.7A-D). These analyses indicate that HSC modulation by the majority of flow modifying compounds requires the establishment of blood circulation, and that NO signaling is the mediator of blood flow in this process.
Figure 3. NO signaling prior to the onset of cardiac activity can affect HSC formation.
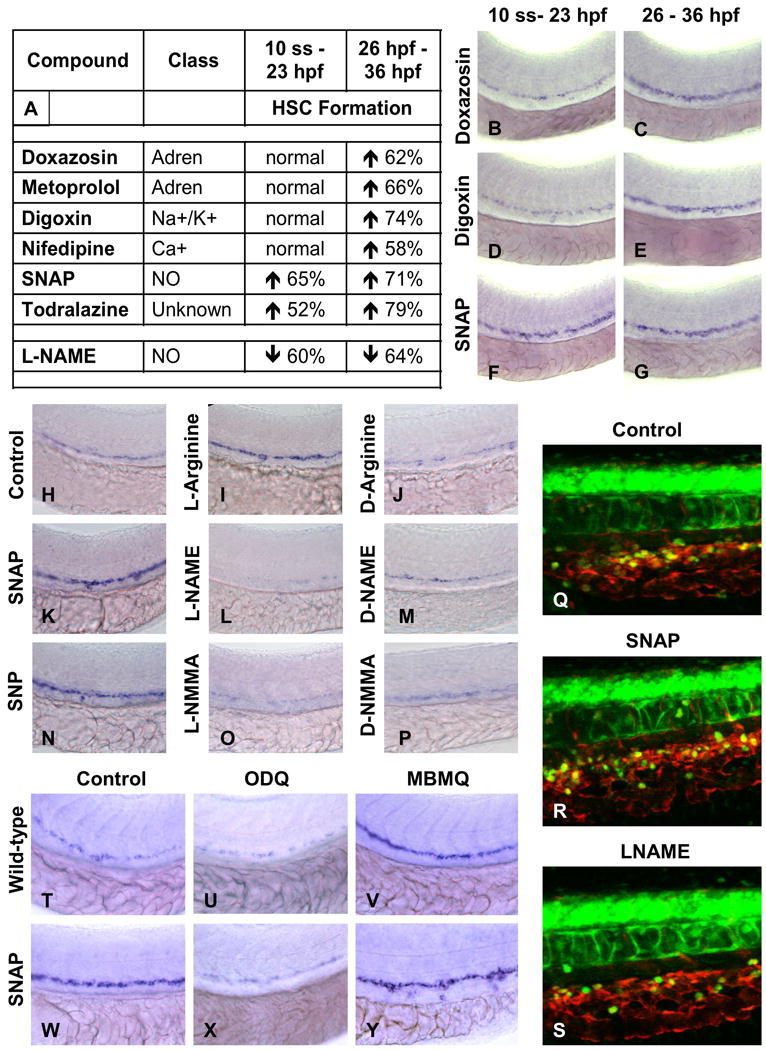
(A-G) Effect of vasoactive drugs (10 μM) on HSC formation before and after the onset of heartbeat at 24hpf, following exposure to chemicals from either 10 somites to 23hpf or from 26 – 36hpf.
(A) Most vasoactive drugs do not affect HSC formation when applied prior to the onset of heartbeat, while NO modifiers influenced HSC development even prior to heart beat initiation. The percentage of embryos (n>20) with altered runx1/cmyb expression is indicated.
(B-G) Representative examples of flow modifying drugs on runx1/cmyb expression.
(H-P) Specificity of NO signaling in HSC formation. NO donors enhanced and diminished HSCs; inactive D-enantiomers had no effect.
(Q-S) Effects of NO modulation on HSC number by in vivo confocal imaging in cmyb:GFP; lmo2:dsRed transgenic embryos.
(T-Y) Effects of downstream modifiers of NO signaling on runx1/cmyb expression.
(U,X) Inhibition of soluble guanyl cyclase by ODQ (10 μM) decreases runx1/cmyb expression in WT and SNAP treated embryos.
(V,Y) Inhibition of PDE V by MBMQ (10 μM) increases HSC formation in WT embryos and further enhances the effects of SNAP.
HSC formation is affected by signals downstream of NO
To clarify that the effect of flow on HSCs was mediated by NO signaling, downstream components of the NO signaling cascade were studied by chemical manipulation. The soluble guanyl cyclase inhibitor, 1H-oxadiazolo-quinoxalin-1-one (ODQ) prevents cGMP formation in response to NO signaling; it regulates vascular remodeling and blood flow in zebrafish in a dose and time-dependent manner (Pyriochou et al., 2006). ODQ (10μM) caused a profound decrease in HSCs (Fig.3T,U, 27 dec/43) and also blocked the effects of SNAP (Fig.3W,X, 8 inc/38). Phosphodiesterase (PDE) V converts cGMP to GTP. The PDEV inhibitor 4-{[3′,4′-methylene-dioxybenzyl]amino}-6-methoxyquinazoline (MBMQ, 10μM) increased HSCs (Fig.3V, 35 inc/43) and further enhanced the effects of SNAP (Fig.3Y, 40 inc/46). These data highlight the specificity of cGMP as a downstream effector of NO signaling in HSC formation.
NO signaling rescues HSCs in sih-/- embryos
To confirm a direct role for NO in HSC induction, we exposed sih embryos to SNAP. SNAP rescued runx1/cmyb expression toward wild-type (WT) levels in the majority of sih embryos examined (Fig.4A-D, 31 normal/51). These results were confirmed by qPCR (Fig.4E). SNAP also normalized ephB2defects in sih mutants (Fig.4F-I, 18 inc/27, Sup.Fig.8A). L-arg and SNP (data not shown) as well as bradykinin, a potent vasodilator that stimulates NO production, increased HSCs, and rescued the sih hematopoietic defect (Sup.Fig.8B-E). To further characterize the relationship between blood flow, NO signaling and HSC induction, we concomitantly exposed WT embryos to flow-modifying drugs and L-NAME (10 μM). As the majority of flow-regulating compounds that enhance HSCs also cause vasodilation and increase total blood flow through the aorta, they may directly trigger NO production by alterations in sheer stress, pulsatile flow or soluble signaling components. L-NAME treatment prevented the increase in HSC formation caused by most compounds tested (Fig.4J-U). These data further point to NO signaling as the direct link between blood flow and HSC development.
Figure 4. NO signaling affects zebrafish HSC formation independent of heartbeat.
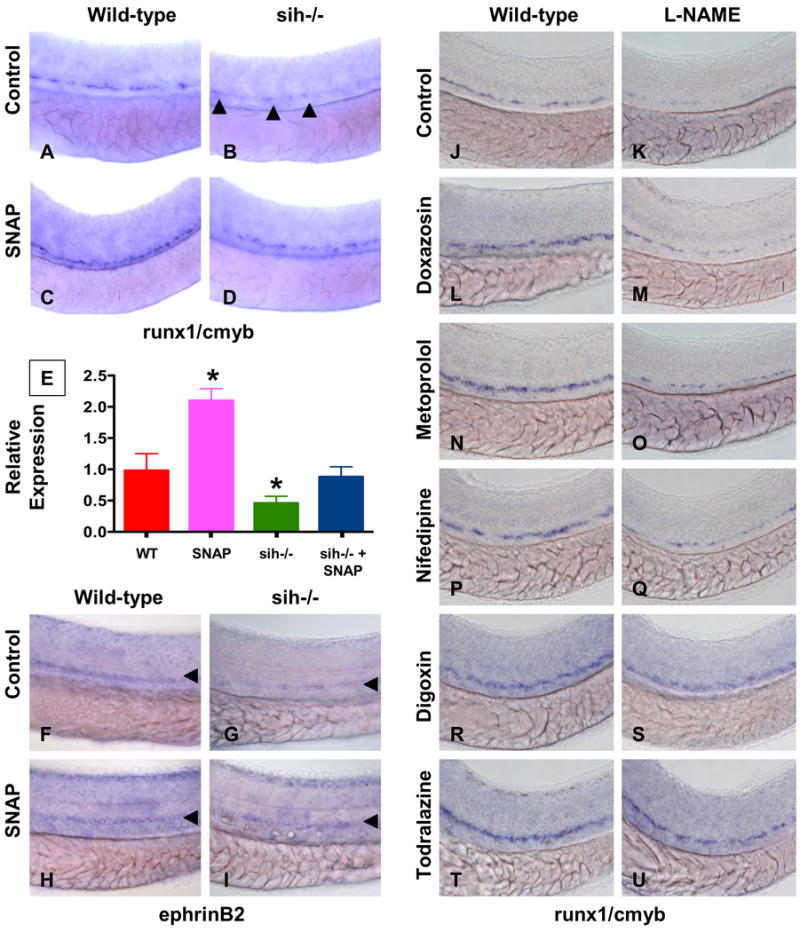
(A-I) WT and sih-/- mutants were exposed to DMSO and SNAP (10 μM) from 10 somites to 36hpf.
(A-D) In situ hybridization for runx1/cmyb. SNAP rescues HSC formation in sih-/- mutants. (B) runx1/cmyb+ cells are highlighted by arrowheads.
(E) qPCR for runx1.
(F-I) Effect of heartbeat and SNAP on ephrinB2 expression, highlighted by arrowheads.
(J-U) Effect of L-NAME on HSC formation in embryos concurrently treated with blood flow modifying agents. L-NAME inhibits the effects of doxazosin (M, 7 inc/36 observed), metoprolol (O, 3 inc/31) and nifedipine (Q, 4 inc/28), but not of digoxin (S, 16 inc/29) and todralazine (U, 20 inc/33).
Nos1 is required for HSC formation
Zebrafish lack genomic evidence for endothelial NO synthase (enos, nos3); however, we, and others (Pelster et al., 2005), observed eNos immunoreactivity in the tail region where the HSCs develop (Sup.Fig.9J-L). Phylogenetic and genomic examination demonstrates that neuronal nos (nnos, nos1) (Poon et al., 2003) and nos3 (enos) are highly related. Morpholino antisense oligonucleotide (MO) knockdown of nos1 had a profound dose-dependent impact on HSC development (Fig.5A,C,E; 63 dec/89 ATG MO, 48 dec/64 splice MO; Sup.Fig.9A-E), while knockdown of nos2 (inducible nos, inos) did not affect runx1/cmyb expression (Fig.5D,F; 9 dec/98 ATG MO, 10 dec/65; Sup.Fig.9F-I). The potent effect of nos1 was confirmed by chemical inhibition of NO synthesis (Fig.5B,G,H): selective inhibition of nos1 by S-methyl-L-thiocitrulline (10μM; 30 dec/44) severely diminished HSC number, whereas the nos2 (inos) specific inhibitor 1400W (10μM; 4 dec/49) only minimally affected HSCs. These data suggest that nos1 (nnos/enos) is required for HSC formation in zebrafish, which is supported by nos1 expression in both endothelial cells and HSCs (Sup.Fig.1B). Interestingly, in sih-/- embryos, nos1 was significantly decreased (Fig.5I; p<0.001); in contrast, nos2 was not significantly changed. Further, nos1, but not nos2, was significantly altered in response to chemical alteration of blood flow (Fig.5J,K; p<0.003). These data support the hypothesis that nos1 is the functionally relevant connection between blood flow and HSC development.
Figure 5. nos1 is required for HSC formation in zebrafish.
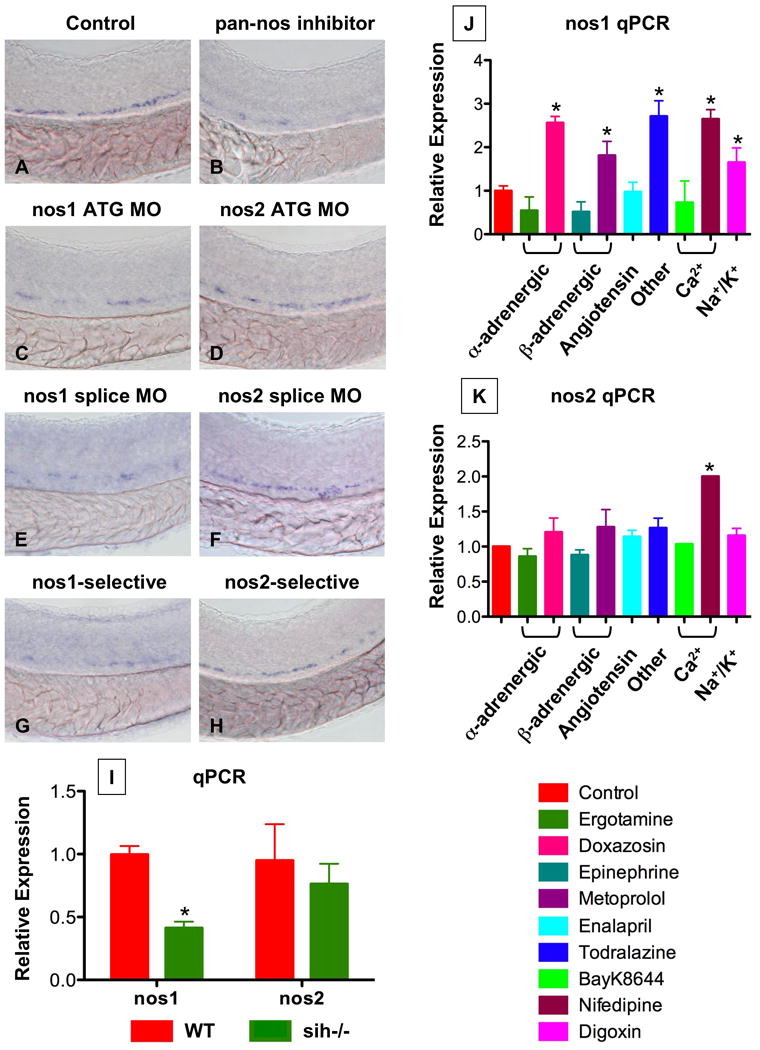
(A-H) In situ hybridization for runx1/cmyb at 36hpf.
(C,E) nos1 knockdown (40 μM) decreased HSC formation.
(D,F) MO (ATG and splice site) against nos2 (40 μM) had no effect on HSC development.
(B,G,H) Chemical nos inhibition confirmed the specific requirement for nos1: Embryos exposed to non-specific (L-NAME; 10 μM) and nos1-selective (S-methyl-L-thiocitrulline; 10 μM) inhibitors demonstrated decreased HSC formation; nos2-selective inhibition (1400W; 10 μM) had minimal impact.
(I) WT and sih-/- embryo extracts (n=20) were subjected to qPCR (* nos1; WT vs. sih, t-test, p<0.001, n=3; nos2; WT vs. sih, p=0.385, n=3).
(J,K) Effect of flow modifying chemicals (10 μM, 10 somites-36hpf) on nos1 and nos2 expression; nos1 is significantly regulated by most compounds tested. * significant vs. control, ANOVA, p<0.01, n=3.
The effect of NO signaling on HSC formation is cell-autonomous
In order to examine cell-autonomy and delineate the role of NO signaling in the HSC and surrounding hematopoietic niche, we employed a blastula transplant strategy. Cells harvested from cmyb:GFP embryos injected with control or nos1 MO were transplanted at the blastula stage into lmo2:dsRed recipients. In this transplant scheme, donor-derived HSCs appear green (Sup.Fig.10A) in the red fluorescent endothelial/HSC compartment (Fig.6A, Sup.Fig.10B). 62.5% of embryos examined had GFP+ HSC formation derived from control-injected donor cells (Fig.6B,D), while none of the nos1 MO injected donor cells gave rise to green HSCs (Fig.3C,D, p=0.0065); successful transplants were indicated by the contribution of cmyb+ donor cells to the recipient eye. In a reciprocal experiment, we found that uninjected lmo2:dsRed donor cells contributed to endothelial and HSC development in cmyb:GFP recipients (Sup.Fig.10C), particularly after MO knockdown in the recipient. These experiments formally demonstrate that nos1 acts in a cell-autonomous manner in the hemogenic endothelial cells.
Figure 6. The effect of NO signaling on HSC development is cell-autonomous.
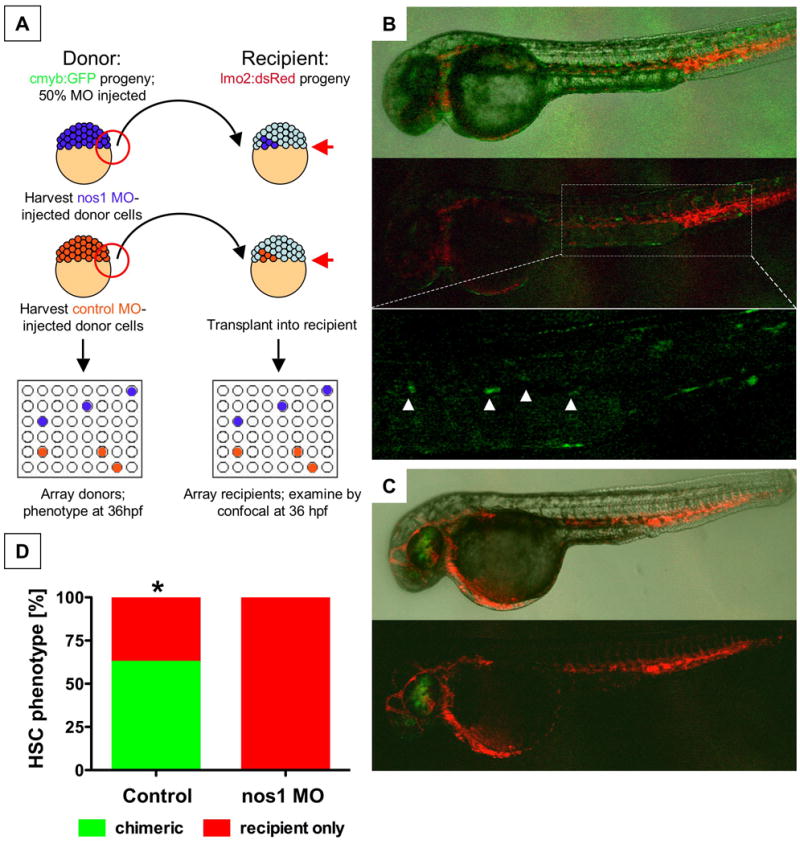
(A) Cells from cmyb:GFP transgenic donor embryos, injected with nos1 ATG MO or control MO, were transplanted into lmo2:dsRed recipients at the blastula stage.
(B) Donor contribution to HSC formation assessed by confocal microscopy at 36hpf. Shown are the merged picture on the top, red/green merge in the middle, and a high-magnification view of green fluorescence only on the bottom. cmyb:GFP donor-derived HSCs in recipients are highlighted by arrowheads.
(C) nos1 MO donors never contributed to HSC formation; presence of cmyb:GFP-derived donor cells in the eye is indicative of a successful transplant.
(D) HSC chimerism in transplanted embryos (control vs. nos1 MO, Fisher's exact, p=0.0065, n≥8).
Developmental signaling pathways interact with NO in HSC formation
Developmental regulators such as the notch and wnt pathways have been linked to HSC formation and self-renewal (Burns et al., 2005; Goessling et al., 2008a). Due to the effect of NO on HSC specification and expansion, potential interaction with notch and wnt signaling was examined. The notch pathway influences arterial/venous identity and functions upstream of runx1 in HSC specification; mindbomb (mib) mutants lack HSCs due to a deficiency of notch signaling (Burns et al., 2005) (33 dec/47). SNAP rescued HSC formation in these mutants (Sup.Fig.11A-D; 27 normal/43). Transgenic zebrafish embryos expressing an activated form of the notch intracellular domain (NICD) exhibit enhanced HSC numbers (55 inc/62); L-NAME blocked the HSC increase (Sup.Fig.11E-H; 16 inc/63) and inhibited NICD-mediated elevation of ephB2 expression in the aorta (Sup.Fig.12A-D). These studies imply that NO functions downstream of notch in regulation of arterial identity and/or in HSC induction.
Recent studies have shown that modulation of the wnt pathway affects HSCs (Goessling et al., 2008a; Reya et al., 2003). We used heat-shock inducible transgenic zebrafish embryos expressing negative (dkk) and positive (wnt8) regulators of wnt signaling to evaluate the interaction between the wnt and NO signaling cascades (Goessling et al., 2008b). Induction of dkk reduced HSC number (16 dec/8) and was rescued by SNAP (Sup.Fig.13A-D; 5 dec/33). In contrast, wnt8 enhanced HSC formation (22 inc/30), which was blocked by L-NAME (Sup.Fig.13E-H; 9 inc/28). In support of these findings, previous studies have shown an interaction of both the notch and wnt pathways with NO, although the directionality of these interactions varies (Du et al., 2006; Ishimura et al., 2005; Prevotat et al., 2006).
The relationship between NO and HSC induction is conserved in the mouse
To document a role for NO in murine HSC formation, we examined the Nos3:GFP expression in the AGM. Histological sections of e11.5 embryos showed endothelial cells lining the dorsal aorta expressing high levels of Nos3 (Sup.Fig.14A-D). Hematopoietic clusters and adjacent endothelium on the ventral wall of the aorta expressed Nos3 at a lower level; this expression pattern was reminiscent of the embryonic HSC markers such as runx1 and c-kit (North et al., 2002). FACS-based co-expression analysis confirmed that the majority of e11.5 ckithiCD34medCD45medVE-cadherinmed AGM HSCs (CR, ED, unpublished results) were Nos3med (Sup.Fig.14E). Transplantation of Nos3 AGM subfractions into irradiated adult recipients demonstrated that LTR-HSCs are enriched within the Nos3med population (Sup.Fig.14F).
To demonstrate a conserved functional requirement for NO signaling in HSC/progenitor formation, we exposed pregnant mice to L-NAME (2.5 mg/kg intraperitoneally) or vehicle control and compared effects on the AGM HSC and progenitor populations at e11.5. NO inhibition produces implantation defects in early pregnancy (Duran-Reyes et al., 1999), and can alter yolk sac angiogenesis (Nath et al., 2004); interestingly Nos3 deficiency caused significant lethality from e8.5 to 13.5 during the time when definitive HSCs are formed (Pallares et al., 2008). L-NAME treatment at e8.5 produced severely delayed embryos that lacked the majority of both extra- and intraembryonic blood vessels (data not shown). L-NAME treatment at e9.5 and e10.5 at prevented gross morphological abnormalities of the yolk sac, placenta or embryo. Histological analysis of the AGM region revealed L-NAME caused the disappearance of hemogenic endothelial clusters, which was confirmed by phenotypic FACS analysis (Fig.7A-H,I-L, Sup.Fig.15A-G). Similarly, analysis of e11.5 Nos3-/- embryos revealed a significant decrease in the AGM sca1+/ckit+ and CD45+/VE-Cadherin+ populations, which was confirmed histologically; Nos3-/- embryos displayed a reduction in the number and size of the hematopoietic clusters (Fig.7K). HSC induction was grossly normal in Nos1-/- animals (Fig.7L).
Figure 7. The effect of NO signaling on HSC development in the AGM is conserved in mice.
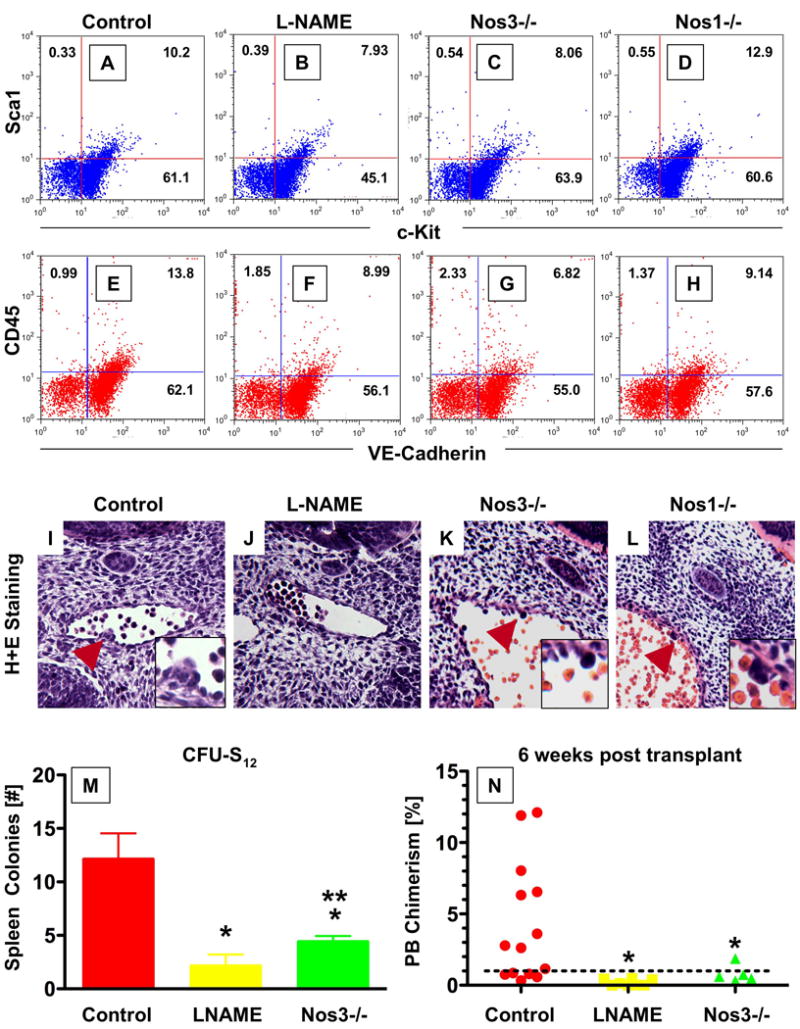
(A-H) FACS analysis of dissociated AGM cells in WT and Nos KO mice at e11.5. Nos3-/- mice exhibited a decrease in the Sca1/cKit+ and CD45/VE-Cadherin+ populations, while deletion of Nos1 had no significant effects.
(I-L) Histological sections through the AGM region of e11.5 embryos; the inset represents a high-magnification view around the hematopoietic clusters. L-NAME exposure causes absence of hematopoietic clusters; Nos3-/- mice exhibit smaller cluster size, while Nos1-/- does not impair cluster formation. Serial sections through the entire aorta of ≥10 embryos per genotype/treatment were analyzed.
(M,N) Effect of NO signaling on AGM HSC function. AGM regions of somite stage-matched WT, L-NAME treated or Nos3-/- progeny were subdissected at e11.5 and transplanted into sublethally irradiated recipients.
(M) L-NAME exposure or Nos3 deletion embryos significantly reduced CFU-S12 spleen colony formation (* sig vs. control; p<0.001; ** sig vs. L-NAME; p<0.05; ANOVA, n≥5).
(N) Diminished NO signaling significantly decrease embryonic donor cell chimerism rates in recipient mice at 6 weeks post transplant (* sig vs. control, p<0.05, ANOVA, n≥5).
To examine effects on HSC function, transplantation studies were performed using single cell suspensions of subdissected AGM tissue from WT, L-NAME exposed and Nos3-/- embryos at e11.5. Progenitor activity as measured by spleen colony formation at days 8 and 12 post transplantation was diminished in L-NAME exposed (Sup.Fig.15J, p<0.001) and Nos3-/- embryos (Fig.7M, p<0.001). Multilineage repopulation after 6 weeks revealed significantly diminished PB chimerism (Fig.7N, Sup.Fig.15K, p>0.05) and engraftment rates >1% (Sup.Fig.15L) for recipients of both L-NAME exposed and Nos3-/- AGM cells. These results indicate a conserved role for NO signaling in the regulation of hematopoietic stem/progenitor formation and function during embryonic development. Although at reduced numbers, Nos3-/- embryos develop to adulthood and lack significant steady-state peripheral blood abnormalities; our data suggest, that while impaired initially, some functional HSCs do arise in Nos3-/- embryos. Interestingly, while the Nos3-/- animals exhibit some residual HSC production, L-NAME exposed embryos do not possess AGM HSCs, implying that functional redundancy with other Nos family members must occur.
Discussion
The purpose of a beating heart and circulation at embryonic stages where diffusion is still sufficient for oxygenation of developing tissue has long been a source for speculation (Burggren, 2004; Pelster and Burggren, 1996). Through a chemical screen in zebrafish, small molecules that regulate vascular dynamics were found to influence HSC development; intriguingly, changes in HSC formation were coupled to blood flow and NO production. Our data imply that circulation itself, through NO induction, signals to trigger the onset of definitive hematopoiesis, thereby ensuring proper timing of blood cell development to support additional hematopoietic requirements during accelerated growth in fetal/larval stages. Significantly, we found that the enhancing role of NO in HSC induction is conserved from fish to mammals.
NO production can be induced by sheer stress and alterations in blood flow (Fukumura et al., 2001). The coincident timing of HSC induction with the achievement of vigorous pulsatile flow implies that the latter may serve as the physiologic inductive signal for NO in the AGM. Pulsatile flow achieved by a regular heartbeat has been shown to trigger NO production in the endothelium (White and Frangos, 2007). The data from the silent heart embryos, as well as observations in Ncx1-/- mice (Lux et al., 2008; Rhodes et al., 2008), which also fail to establish circulation due to heart-specific defects, indicate that in the absence of flow there are alterations in specification, budding and shedding of HSCs from endothelial hematopoietic clusters. It will be intriguing to further decipher the correlation between flow rate and total AGM HSC number; MO knock down of tnnt2 (sih) (Bertrand et al., 2008; Murayama et al., 2006; Jin et al., 2009) and analysis of incompletely penetrant sih mutants with occasional heartbeats (data not shown) show less severe reductions in HSC number, implying small bursts of NO production may be sufficient to trigger HSC induction. As NO can regulate endothelial cell movement and processes resembling HSC budding, such as podokinesis, by altering cell-cell adhesions and actin conformation (Noiri et al., 1998), it could directly control the formation and stability of hematopoietic clusters once flow is established. Confirming this conjecture, we determined that there is a cell-autonomous role of NO signaling during hematopoietic development, where the hemogenic endothelial population must be capable of NO production to support subsequent HSC formation in the AGM.
We found that NO may additionally function to establish the AGM vascular niche prior to HSC formation; our data showing significant alterations in ephrinB2 staining in the absence of flow support the concept that flow itself plays a role in maintaining vascular identity. NO is a well-characterized regulator of angiogenesis and is required for murine yolk sac vasculogenesis (Nath et al., 2004). Prior studies in the zebrafish embryo showed that chemical inhibition of NO production/signaling by L-NAME or ODQ during somitogenesis produces vascular abnormalities (Pyriochou et al., 2006). Since definitive HSCs are formed within the major embryonic arteries (de Bruijn et al., 2000), any alterations in NO signaling and subsequent vessel development would negatively impact HSC number. As ephrinB2 and arterial identity are established by notch signaling (Lawson et al., 2001), the interaction of the notch and NO pathways may be particularly relevant for HSC formation. NO may initiate arterial specification early during development and may maintain arterial identity once flow is established. This is in agreement with reports that demonstrate arterialization is an ongoing and flow dependent process, influenced by NO (Teichert et al., 2008). Similarly, vascular endothelial growth factor, VEGF, a potent vascular mitogen regulated by both notch and wnt is a well-characterized inducer of NO production (Fukumura et al., 2001). In the dorsal aorta, VEGF may increase NO production and signaling to cause the vascular remodeling required for the production of the hematopoietic clusters.
Our data demonstrate both a requirement for and enhancing response to NO signaling for AGM HSC development. Several nos isoforms have been identified in zebrafish: nos1, which is expressed in developing neural tissues as well as the gut, kidney and major vessels (Holmqvist et al., 2004; Poon et al., 2003) and two isoforms of nos2. While genomic evidence for the presence in zebrafish of nos3 is lacking (Pelster et al., 2005), immunoreactivity to eNos antibodies suggests the conservation of the functional epitope (Fritsche et al., 2000). As NO-mediated vascular reactivity is clearly present in fish, and nos1 and nos3 are highly related at both the sequence and structural levels, nos1 likely assumes the role of vascular NO production in fish. Nos1 is genetically complex with individual splice forms showing tissue-specific expression, and it is likely that one form of nnos acts enos-like in zebrafish. In support of this hypothesis, our microarray analysis demonstrated nos1 expression in CD41+ HSCs and the vascular niche.
In the murine AGM, phenotypic and histological analysis showed that Nos3 (eNos) is expressed in HSCs and required for stem cell function. Conversely, we found Nos1 (nNos) not to be essential under normal developmental conditions. Interestingly, Nos3 and Nos1 are both expressed in the fetal liver shortly after AGM HSC formation and could play a role in the developmental regulation and expansion of HSC and progenitor populations (Krasnov et al., 2008). Their co-expression suggests a functional redundancy in mammalian HSCs that could explains the impaired, but present, HSC formation and adult viability of Nos3-/- embryos. Consequently, global NO inhibition by L-NAME had a much more severe effect on HSC formation. It remains to be determined if differences in hematopoietic development occur in mice in which all Nos isoforms are disrupted.
NO donors positively affect multipotent hematopoietic progenitors in vitro (Michurina et al., 2004); additionally, the ability of stromal cell lines to support stem cell maintenance corresponds with NO production (Krasnov et al., 2008). In contrast, others have shown that NO inhibition enhances HSC engraftment following transplantation (Krasnov et al., 2008; Michurina et al., 2004). While these studies imply that NO may have a negative effect on adult HSCs, parallel work has shown that NO is induced by ionizing irradiation, and that the absence of Nos diminishes superoxide and peroxide damage (Epperly et al., 2007). These data preclude a clear interpretation of transplantation/repopulation studies where the hematopoietic niche is cleared via irradiation. After 5-fluorouracil bone marrow injury, Nos3-/- mice show impaired regeneration, indicating an important role for Nos3 in stem and progenitor cell function in vivo after marrow injury (Aicher et al., 2003). Taken together with the results presented here, these studies indicate that the effects of NO are likely highly time and context dependent, and further work is needed to decipher the role of NO in regulating adult hematopoietic homeostasis and maintaining both the stromal and vascular niche.
Our study demonstrates that definitive hematopoietic stem cell formation in the developing embryo is dependent on the induction of the heartbeat and establishment of circulation. Two models have been proposed for the relationship of blood formation in murine extraembryonic tissues and the embryo proper: in one model, the stem cells arise independently in discrete locations in the embryo and extraembryonic tissues and subsequently colonize the fetal liver (Dzierzak and Speck, 2008), whereas the other proposes that cells from the extraembryonic tissues traverse circulation to colonize the intraembryonic hematopoietic sites (Palis and Yoder, 2001; Rhodes et al., 2008). A recent study using the Ncx1-/- mouse showed that yolk sac hematopoietic progenitors could form in the absence of blood flow, while the appearance of progenitors in the embryo proper was greatly impaired; these data were interpreted to show that it is yolk sac-derived embryonic progenitors traverse the circulation and seed the fetal liver (Lux et al., 2008). Our data imply that the contemporaneous establishment of circulation and the appearance of HSCs within the embryo proper may not simply reflect the transit of HSCs formed in extraembryonic tissues to colonize the aorta and fetal liver, but rather that the circulation functions directly to provide inductive signals to specific regions of the embryonic vasculature, making it competent to produce HSCs de novo.
Here, we established a conserved role for NO in the developing hematopoietic system. NO can function in vessel formation and specification, blood flow regulation and hematopoietic cluster formation, suggesting that it is required in the vascular niche for HSC production. Although the function of NO in the adult marrow is complex, our findings during embryogenesis indicate that modulation of blood flow or NO signaling might be therapeutically beneficial for patients undergoing stem cell transplantation.
Methods
Zebrafish husbandry
Zebrafish were maintained according to IACUC protocols. fli:GFP, hs:gal4;uas:NICD, wnt8:GFP, dkk1:GFP transgenic and sih and mib mutant fish were described previously (Burns et al., 2005; Goessling et al., 2008b; Itoh et al., 2003; Lawson and Weinstein, 2002; Sehnert et al., 2002). Embryonic heat-shock was conducted as described (Goessling et al., 2008b).
Mice
Embryos were generated from C57Bl/6, Runx1:lacZ (North et al., 1999), Nos3:GFP transgenic (van Haperen et al., 2003), Nos1-/- and Nos3-/- mice. Vaginal plug identification was considered embryonic day 0.5 (e0.5). Animals were handled according to institutional guidelines.
In situ hybridization
Paraformaldehyde-fixed embryos were processed for in situ hybridization using standard zebrafish protocols (http://zfin.org/ZFIN/Methods/ThisseProtocol.html). The following RNA probes were used: runx1, cmyb, flk1, ephrinB2, flt4, globin, mpo, mhc, foxa3. Changes in expression compared to WT controls are reported as the # altered/# scored per genotype/treatment (North et al., 2007); a minimum of 3 independent experiments was conducted per analysis.
Chemical exposure
Zebrafish embryos were exposed to chemicals at the doses indicated; DMSO carrier content was 0.1%. To evaluate HSC development, exposure ranged from early somitogenesis (5+ somites) until 36hpf, unless otherwise noted.
Morpholino injection
MO (GeneTools) designed against the ATG and exon1 splice sites of nos1 (5′-ACGCTGGGCTCTGATTCCTGCATTG; 5′-TTAATGACATCCCTCACCTCTCCAC) and nos2 (5′-AGTGGTTTGTGCTTGTCTTCCCATC; 5′-ATGCATTAGTACCTTTGATTGCACA) and mismatched controls were injected into one-cell stage embryos.
Confocal microscopy
Fluorescent reporter embryos were exposed to blood flow modulators (10μM, unless otherwise noted) as indicated, live embedded in 1% agarose and imaged using a Zeiss LSM510 Meta confocal microscope at 36hpf (North et al., 2007).
qPCR
qPCR was performed on cDNA obtained from whole embryos at 36hpf (n=20/variable), (primers listed in Supplementary Table 1) as previously described (North et al., 2007), using SYBR Green Supermix on the iQ5 Multicolor RTPCR Detection System (BioRad).
Blastula transplantation
cmyb:GFP embryos were injected with nos1 or control MO at the one-cell stage. At the blastula stage, 100 cells were removed from the donor embryo and transplanted into stage-matched recipients. Embryos were analyzed by confocal microscopy at 36hpf.
Murine AGM histology
At e11.5 following timed mating, embryos dissected from the uterus and processed for histological evaluation. Paraffin serial sections were stained with H+E; cryosections were assessed by fluorescence microscopy for GFP. X-Gal staining was performed as indicated.
Nos3:GFP AGM FACS analysis
Embryos (e11.5) from Nos3:GFP transgenic animals were isolated and AGM tissue dissected and disaggregated. Flow cytometric analysis was performed for Nos3:GFP, VE-Cadherin, CD34, Sca-1, c-kit, and CD45 (BD Pharmingen).
Nos3:GFP AGM transplantation
Transgenic AGM cells were sorted into Nos3: fractions. AGM (1 embryo equivalent) cell suspensions were injected into irradiated (9 Gy) FVB recipient mice with adult spleen carrier cells (2 × 105 per recipient). Recipient peripheral blood was analyzed at 4 months post transplantation for donor-derived cells by DNA PCR for GFP (donor marker) and myogenin (normalization control). Recipients with > 10% donor marked cells were considered positive.
AGM transplantation and progenitor and LTR HSC analysis
AGM transplantations were performed using the CD45.1/45.2 allelic system. Pregnant C57Bl/6 females were injected with DMSO or L-NAME (2.5 mg/kg) intraperitoneally on e9.5 and e10.5. WT, L-NAME, Nos1-/- and Nos3-/- AGM regions were dissected and disaggregated at e11.5 then injected into 8-week old C57Bl/6 sublethally irradiated recipients. For CFUS8 and 12 analyses, spleens were dissected, weighed, fixed with Bouin's solution, and hematopoietic colonies were counted. For long-term transplants, PB obtained from recipient mice at 6 weeks was analyzed for donor chimerism and multilineage engraftment by FACS.
Supplementary Material
Acknowledgments
We thank M. Lin and J. Loscalzo for helpful suggestions. This work was supported by the National Institutes of Health (T.E.N., W.G., E.D. and L.I.Z) and a Netherlands BSIK award (E.D.). L.I.Z. is a HHMI investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- Bertrand JY, Kim AD, Teng S, Traver D. CD41+ cmyb+ precursors colonize the zebrafish pronephros by a novel migration route to initiate adult hematopoiesis. Development. 2008;135:1853–1862. doi: 10.1242/dev.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren WW. What is the purpose of the embryonic heart beat? Or how facts can ultimately prevail over physiological dogma. Physiol Biochem Zool. 2004;77:333–345. doi: 10.1086/422230. [DOI] [PubMed] [Google Scholar]
- Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005;19:2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. Embo J. 2000;19:2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing JR, Head DR, Curcio-Brint AM, Hulshof MG, Motroni TA, Raimondi SC, Carroll AJ, Drabkin HA, Willman C, Theil KS, et al. An AML1/ETO fusion transcript is consistently detected by RNA-based polymerase chain reaction in acute myelogenous leukemia containing the (8;21)(q22;q22) translocation. Blood. 1993;81:2860–2865. [PubMed] [Google Scholar]
- Du Q, Park KS, Guo Z, He P, Nagashima M, Shao L, Sahai R, Geller DA, Hussain SP. Regulation of human nitric oxide synthase 2 expression by Wnt beta-catenin signaling. Cancer Res. 2006;66:7024–7031. doi: 10.1158/0008-5472.CAN-05-4110. [DOI] [PubMed] [Google Scholar]
- Duran-Reyes G, Gomez-Melendez MR, Morali-de la Brena G, Mercado-Pichardo E, Medina-Navarro R, Hicks-Gomez JJ. Nitric oxide synthesis inhibition suppresses implantation and decreases cGMP concentration and protein peroxidation. Life Sci. 1999;65:2259–2268. doi: 10.1016/s0024-3205(99)00491-9. [DOI] [PubMed] [Google Scholar]
- Dzierzak E, Medvinsky A. The discovery of a source of adult hematopoietic cells in the embryo. Development. 2008;135:2343–2346. doi: 10.1242/dev.021279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy FB. Role of nitric oxide in larval and juvenile fish. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:221–230. doi: 10.1016/j.cbpb.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Epperly MW, Cao S, Zhang X, Franicola D, Shen H, Greenberger EE, Epperly LD, Greenberger JS. Increased longevity of hematopoiesis in continuous bone marrow cultures derived from NOS1 (nNOS, mtNOS) homozygous recombinant negative mice correlates with radioresistance of hematopoietic and marrow stromal cells. Exp Hematol. 2007;35:137–145. doi: 10.1016/j.exphem.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Fritsche R, Schwerte T, Pelster B. Nitric oxide and vascular reactivity in developing zebrafish, Danio rerio. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2200–2207. doi: 10.1152/ajpregu.2000.279.6.R2200. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2008a doi: 10.1016/j.cell.2009.01.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W, North TE, Lord AM, Ceol C, Lee S, Weidinger G, Bourque C, Strijbosch R, Haramis AP, Puder M, et al. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev Biol. 2008b;320:161–174. doi: 10.1016/j.ydbio.2008.05.526. [DOI] [PubMed] [Google Scholar]
- Golub TR, Barker GF, Bohlander SK, Hiebert SW, Ward DC, Bray-Ward P, Morgan E, Raimondi SC, Rowley JD, Gilliland DG. Fusion of the TEL gene on 12p13 to the AML1 gene on 21q22 in acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 1995;92:4917–4921. doi: 10.1073/pnas.92.11.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist B, Ellingsen B, Forsell J, Zhdanova I, Alm P. The early ontogeny of neuronal nitric oxide synthase systems in the zebrafish. J Exp Biol. 2004;207:923–935. doi: 10.1242/jeb.00845. [DOI] [PubMed] [Google Scholar]
- Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase up-regulates Notch-1 in mouse cholangiocytes: implications for carcinogenesis. Gastroenterology. 2005;128:1354–1368. doi: 10.1053/j.gastro.2005.01.055. [DOI] [PubMed] [Google Scholar]
- Isogai S, Lawson ND, Torrealday S, Horiguchi M, Weinstein BM. Angiogenic network formation in the developing vertebrate trunk. Development. 2003;130:5281–5290. doi: 10.1242/dev.00733. [DOI] [PubMed] [Google Scholar]
- Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Jin H, Sood R, Xu J, Zhen F, English MA, Liu PP, Wen Z. Definitive hematopoietic stem/progenitor cells manifest distinct differentiation output in the zebrafish VDA and PBI. Development. 2009;136:647–654. doi: 10.1242/dev.029637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnov P, Michurina T, Packer MA, Stasiv Y, Nakaya N, Moore KA, Drazan KE, Enikolopov G. Neuronal nitric oxide synthase contributes to the regulation of hematopoiesis. Mol Med. 2008;14:141–149. doi: 10.2119/2007-00011.Krasnov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J, Yoder MC. All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood. 2008;111:3435–3438. doi: 10.1182/blood-2007-08-107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michurina T, Krasnov P, Balazs A, Nakaya N, Vasilieva T, Kuzin B, Khrushchov N, Mulligan RC, Enikolopov G. Nitric oxide is a regulator of hematopoietic stem cell activity. Mol Ther. 2004;10:241–248. doi: 10.1016/j.ymthe.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Murayama E, Kissa K, Zapata A, Mordelet E, Briolat V, Lin HF, Handin RI, Herbomel P. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity. 2006;25:963–975. doi: 10.1016/j.immuni.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Nath AK, Enciso J, Kuniyasu M, Hao XY, Madri JA, Pinter E. Nitric oxide modulates murine yolk sac vasculogenesis and rescues glucose induced vasculopathy. Development. 2004;131:2485–2496. doi: 10.1242/dev.01131. [DOI] [PubMed] [Google Scholar]
- Noiri E, Lee E, Testa J, Quigley J, Colflesh D, Keese CR, Giaever I, Goligorsky MS. Podokinesis in endothelial cell migration: role of nitric oxide. Am J Physiol. 1998;274:C236–244. doi: 10.1152/ajpcell.1998.274.1.C236. [DOI] [PubMed] [Google Scholar]
- North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, Marin-Padilla M, Speck NA. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126:2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- North TE, de Bruijn MF, Stacy T, Talebian L, Lind E, Robin C, Binder M, Dzierzak E, Speck NA. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH, Grosser T, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palis J, Yoder MC. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp Hematol. 2001;29:927–936. doi: 10.1016/s0301-472x(01)00669-5. [DOI] [PubMed] [Google Scholar]
- Pallares P, Garcia-Fernandez RA, Criado LM, Letelier CA, Esteban D, Fernandez-Toro JM, Flores JM, Gonzalez-Bulnes A. Disruption of the endothelial nitric oxide synthase gene affects ovulation, fertilization and early embryo survival in a knockout mouse model. Reproduction. 2008;136:573–579. doi: 10.1530/REP-08-0272. [DOI] [PubMed] [Google Scholar]
- Pelster B, Burggren WW. Disruption of hemoglobin oxygen transport does not impact oxygen-dependent physiological processes in developing embryos of zebra fish (Danio rerio) Circ Res. 1996;79:358–362. doi: 10.1161/01.res.79.2.358. [DOI] [PubMed] [Google Scholar]
- Pelster B, Grillitsch S, Schwerte T. NO as a mediator during the early development of the cardiovascular system in the zebrafish. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:215–220. doi: 10.1016/j.cbpb.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Poon KL, Richardson M, Lam CS, Khoo HE, Korzh V. Expression pattern of neuronal nitric oxide synthase in embryonic zebrafish. Gene Expr Patterns. 2003;3:463–466. doi: 10.1016/s1567-133x(03)00063-2. [DOI] [PubMed] [Google Scholar]
- Prevotat L, Filomenko R, Solary E, Jeannin JF, Bettaieb A. Nitric oxide-induced down-regulation of beta-catenin in colon cancer cells by a proteasome-independent specific pathway. Gastroenterology. 2006;131:1142–1152. doi: 10.1053/j.gastro.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Pyriochou A, Beis D, Koika V, Potytarchou C, Papadimitriou E, Zhou Z, Papapetropoulos A. Soluble guanylyl cyclase activation promotes angiogenesis. J Pharmacol Exp Ther. 2006;319:663–671. doi: 10.1124/jpet.106.108878. [DOI] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, Conway S, Orkin SH, Yoder MC, Mikkola HK. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2:252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehnert AJ, Huq A, Weinstein BM, Walker C, Fishman M, Stainier DY. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat Genet. 2002;31:106–110. doi: 10.1038/ng875. [DOI] [PubMed] [Google Scholar]
- Teichert AM, Scott JA, Robb GB, Zhou YQ, Zhu SN, Lem M, Keightley A, Steer BM, Schuh AC, Adamson SL, et al. Endothelial nitric oxide synthase gene expression during murine embryogenesis: commencement of expression in the embryo occurs with the establishment of a unidirectional circulatory system. Circ Res. 2008;103:24–33. doi: 10.1161/CIRCRESAHA.107.168567. [DOI] [PubMed] [Google Scholar]
- van Haperen R, Cheng C, Mees BM, van Deel E, de Waard M, van Damme LC, van Gent T, van Aken T, Krams R, Duncker DJ, et al. Functional expression of endothelial nitric oxide synthase fused to green fluorescent protein in transgenic mice. Am J Pathol. 2003;163:1677–1686. doi: 10.1016/S0002-9440(10)63524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Stacy T, Binder M, Marin-Padilla M, Sharpe AH, Speck NA. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci U S A. 1996;93:3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber GJ, Choe SE, Dooley KA, Paffett-Lugassy NN, Zhou Y, Zon LI. Mutant-specific gene programs in the zebrafish. Blood. 2005;106:521–530. doi: 10.1182/blood-2004-11-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CR, Frangos JA. The shear stress of it all: the cell membrane and mechanochemical transduction. Philos Trans R Soc Lond B Biol Sci. 2007;362:1459–1467. doi: 10.1098/rstb.2007.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


