Summary
We hypothesize that one mechanism of the anti-epileptic effect of the ketogenic diet is to alter brain handling of glutamate. According to this formulation, in ketotic brain astrocyte metabolism is more active, resulting in enhanced conversion of glutamate to glutamine. This allows for: (a) more efficient removal of glutamate, the most important excitatory neurotransmitter; and (b) more efficient conversion of glutamine to GABA, the major inhibitory neurotransmitter.
Keywords: glutamic acid, GABA, ketosis, brain amino acid metabolism
Our laboratory has addressed the relationship between ketosis and brain handling of amino acids, particularly glutamate (Erecinska, 1995; Yudkoff et al 1997; 2004; 2005; 2006; 2007). Glutamate is the major excitatory neurotransmitter (Meldrum 2000) but brain transports virtually no glutamate (or glutamine) from blood. Thus, brain must continually synthesize and deliver to neurons the glutamate that they release upon depolarization. It also is critically important that the brain rapidy and efficiently remove glutamate from the synaptic space, for two reasons: (a) maintaining low levels of glutamate in the synapse maximizes the signal-to-noise ratio upon release of this transmitter from nerve endings; and (b) a chronic elevation of glutamate in the synaptic space can excessively excite post-dendritic glutamate receptors (“excitotoxicity”) and injure susceptible neurons, a factor that may figure in phenomena such as hypoxic-ischemic brain injury, hypoglycemia, epilepsy, certain inborn errors of metabolism and other neurologic disorders (Olney, 2003, Schousboe and Waagepetersen 2005)
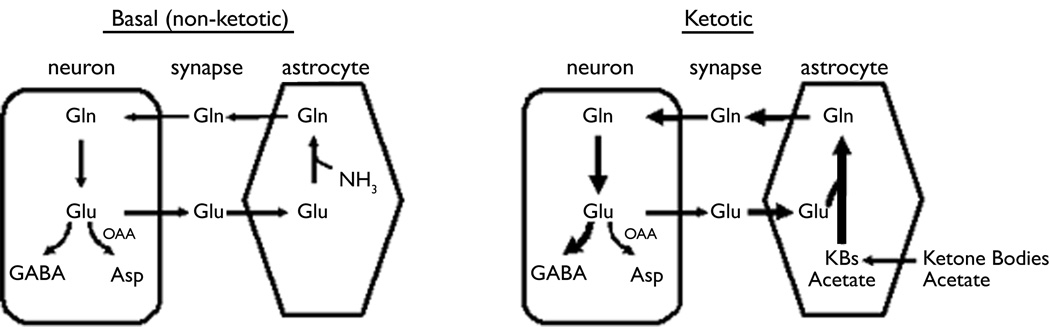
The brain maintains a low level of external glutamate and a high intra-neuronal concentration by assigning primarily to astrocytes the task of removing synaptic glutamate, a function they execute with remarkable efficiency. Astrocytes first convert glutamate to glutamine via glutamine synthetase, an exclusively glial enzyme, and then export glutamine to neurons. The latter cells hydrolyze glutamine to glutamate via the action of phosphate-dependent glutaminase, a mitochondrial enzyme that yields ammonia as well as glutamate. Such shuttling of glutamate and glutamine between astrocytes and neurons is termed the “Glutamate-Glutamine Cycle” (Fig. 1). This cycle constitutes the fundamental conceputalization in our thinking about brain amino acid metabolism (Bak et al , 2006; Albrecht et al 2007).
Fig. 1. The Glutamate-Glutamine Cycle.
The Glutamate-Glutamine Cycle. This mechanism cycle accomplishes (a) removal of glutamate from the synapse via uptake into astrocytes, which (b) convert glutamate to glutamine via glutamine synthetase, a glial enzyme; (c) glutamine export to neurons, which (d) hydrolyze this amino acid to glutamate, thereby replenishing the glutamate that neurons release to the synapse. In addition, glutamate can be converted to GABA, a major inhibitory neurotransmitter, and to aspartate. Left: function of the cycle in the basal state. Right: the cycle in ketosis. We hypothesize that in ketotic brain there occurs: (a) activation of astrocytic metabolism, resulting in enhanced conversion of glutamate to glutamine and providing more glutamine to serve as precursor to GABA; and (b) relatively less transamination of glutamate to yield aspartate and relatively more conversion of glutamate to GABA.
We hypothesized that an alteration in the dynamics of the Glutamate-Glutamine Cycle could constitute an important component of the anti-epileptic effect of the ketogenic diet (KD) (Fig. 1, right side). Our data suggests that ketosis induced the following metabolic changes: (a) Flux through the astrocytic glutamine synthetase pathway is intensified, thereby favoring the “buffering” of synaptic glutamate that is taken up by the glia; (b) More glutamine becomes accessible to GABA-ergic neurons, which then have at their disposal a larger precursor pool for the purpose of the synthesis of GABA, the major inhibitory neurotransmitter of the CNS. Indeed, we showed that GABA synthesis is greater in synaptosomes in the presence of acetoacetate (Erecinska et al, 1996); (c) A major metabolic fate of glutamate derived from glutamine by the action of phosphate-dependent glutaminase is transamination to aspartate via aspartate aminotransferase (Figure 1). In ketosis, less glutamate is metabolized and more becomes available to the glutamate decarboxylase reaction for the purpose of GABA synthesis.
How might ketosis cause these changes? We and others found that ketosis activates mitochondrial metabolism and flux through the tricarboxylic acid cycle (Melo et al, 2006; Yudkoff et al, 2005; 2006). This occurs because in the non-ketotic state the brain uses only glucose as a metabolic substrate. In contrast, ketotic brain avidly consumes ketone bodies (3-OH-butyrate and acetoacetate) as well as acetate itself. These compounds, particularly acetate, are oxidized primarily in glia, not neurons (Waniewski and Martin, 1998). The metabolism of ketone bodies and acetate must engage the tricarboxylic acid cycle of the mitochondria. In contrast, the consumption of glucose is partially an anaerobic process that starts in the cytosol and results in the formation of pyruvate and lactate. Indeed, to a variable degree astrocytes even may release lactate to neurons. Thus, the ketotic state intensifies mitochondrial metabolism and flux through the tricarboxylic acid cycle (Melo et al, 2006; Yudkoff et al, 2005; 2006). A consequence of this phenomenon in astrocytes is enhanced formation of glutamine, thereby allowing increased “buffering” of glutamate and increased synthesis of an important GABA precursor (Sonnewald et al, 1993).
Ketosis also may alter neuronal handling of glutamate, at least in nerve endings. We found, using stable isotope probes such as [15N]glutamate and [2-15N]glutamine, that a major metabolic fate of glutamate in synaptosomes is conversion to aspartate via transamination with oxaloacetate (glutamate + oxaloacetate ↔ α-ketoglutarate + aspartate) (Yudkoff et al, 1994). The oxidation of ketone bodies necessarily produces acetyl-CoA (3-OH-butyrate → → acetoacetyl-CoA → acetyl-CoA), which is a substrate for the citrate synthetase reaction (oxaloacetate + acetyl-CoA → citrate), a very active pathway in brain. Our data suggest that augmented flux through citrate synthetase could limit the rate of transamination of glutamate to aspartate. As a result, more glutamate could become available to the glutamate decarboxylase reaction in nerve endings and more glutamate could be available to the glutamine synthetase reaction in astrocytes.
The changes described above undoubtedly comprise only a portion of the adaptations that ketosis evokes in order to assert an anti-epileptic effect. What matters most is the positive use we can make of such information in order to control epilepsy in affected patients. In this regard, some evidence suggests that even modest caloric restriction or a low-carbohydrate regimen could improve seizure control. If, as we hypothesize, an important component of the therapeutic effect of the KD is a shift toward ketone bodies and acetate as cerebral metabolic substrates, it seems plausible to consider whether supplementation with ostensibly innocuous nutritionals such as acetylcarnitine might prove beneficial.
Acknowledgement
This work was supported by grants NS054900 and HD26979 from the National Institutes of Health.
Footnotes
Disclosures:
The authors have read the journal’s policy on ethical publishing and agree that this article is consistent with those guidelines.
The authors of this article disclose that there are no conflicts of interest
References
- Albrecht J, Sonnewald U, Waagepetersen HS, Schousboe A. Glutamine in the central nervous system: function and dysfunction. Front Biosci. 2007;12:332–343. doi: 10.2741/2067. [DOI] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Nelson D, Daikhin Y, Yudkoff M. Regulation of GABA level in rat brain synaptosomes: fluxes through enzymes of the GABA shunt and effects of glutamate, calcium and ketone bodies. J. Neurochem. 1996;67:2325–2334. doi: 10.1046/j.1471-4159.1996.67062325.x. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J. Nutr. 2000;130:1007–1015. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- Melo TM, Nehlig A, Sonnewald U. Neuronal-glial interactions in rats fed a ketogenic diet. Neurochem. Int. 2006;48:498–507. doi: 10.1016/j.neuint.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Olney JW. Excitotoxicity, apoptosis and neuropsychiatric disorders. Curr. Opin. Pharmacol. 2003;3:101–109. [PubMed] [Google Scholar]
- Schousboe A, Waagepetersen HS. Role of astrocytes in glutamate homeostasis: implications for excitotoxicity. Neurotox. Res. 2005;8:221–225. doi: 10.1007/BF03033975. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Westergaard N, Schousboe A, Svendsen JS, Unsgard G, Petersen SB. Direct demonstration by [13C]NMR spectroscopy that glutamine from astrocytes is a precursor for GABA synthesis in neurons. Neurochem. Int. 1993;22:19–29. doi: 10.1016/0197-0186(93)90064-c. [DOI] [PubMed] [Google Scholar]
- Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J. Neurosci. 1998;18:5225–5233. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkoff M, Nelson D, Daikhin Y, Erecinska M. Tricarboxylic acid cycle in rat brain synaptosomes. Fluxes and interactions with aspartate aminotransferase and malate/aspartate shuttle. J. Biol. Chem. 1994;269:27414–27420. [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Grunstein R, Nissim I. Effects of ketone bodies on astrocyte amino acid metabolism. J. Neurochem. 1997;69:682–692. doi: 10.1046/j.1471-4159.1997.69020682.x. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Lazarow A, Luhovyy B, Wehrli S, Nissim I. Ketogenic diet, brain glutamate metabolism and seizure control. Prostaglandins Leukot. Essential Fatty Acids. 2004;70:277–285. doi: 10.1016/j.plefa.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Horyn O, Lazarow A, Nissim I. Response of brain amino acid metabolism to ketosis. Neurochem. Int. 2005;47:119–128. doi: 10.1016/j.neuint.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Horyn O, Luhovyy B, Lazarow A, Nissim I. Short-term fasting, seizure control and brain amino acid metabolism. Neurochem. Int. 2006;48:650–656. doi: 10.1016/j.neuint.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Melø TM, Nissim I, Sonnewald U, Nissim I. The ketogenic diet and brain metabolism of amino acids: relationship to the anticonvulsant effect. Annu Rev Nutr. 2007;27:415–430. doi: 10.1146/annurev.nutr.27.061406.093722. [DOI] [PMC free article] [PubMed] [Google Scholar]