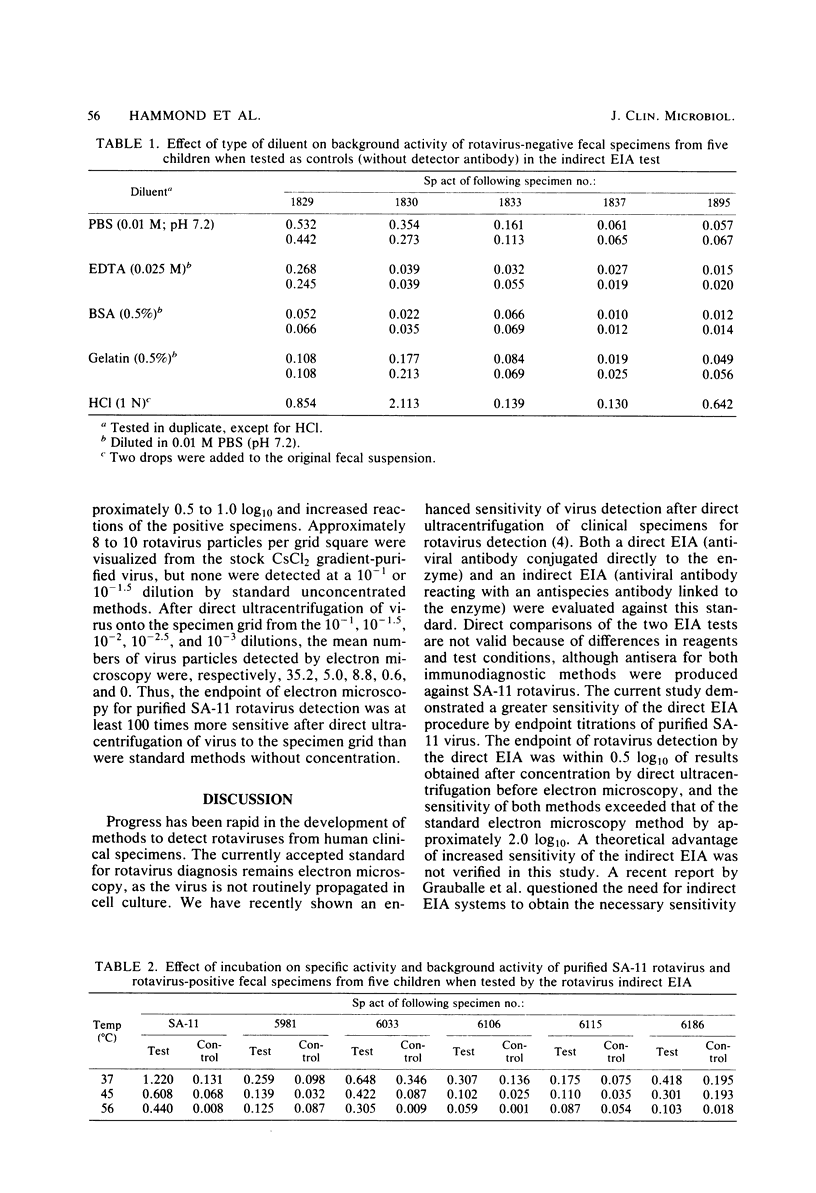
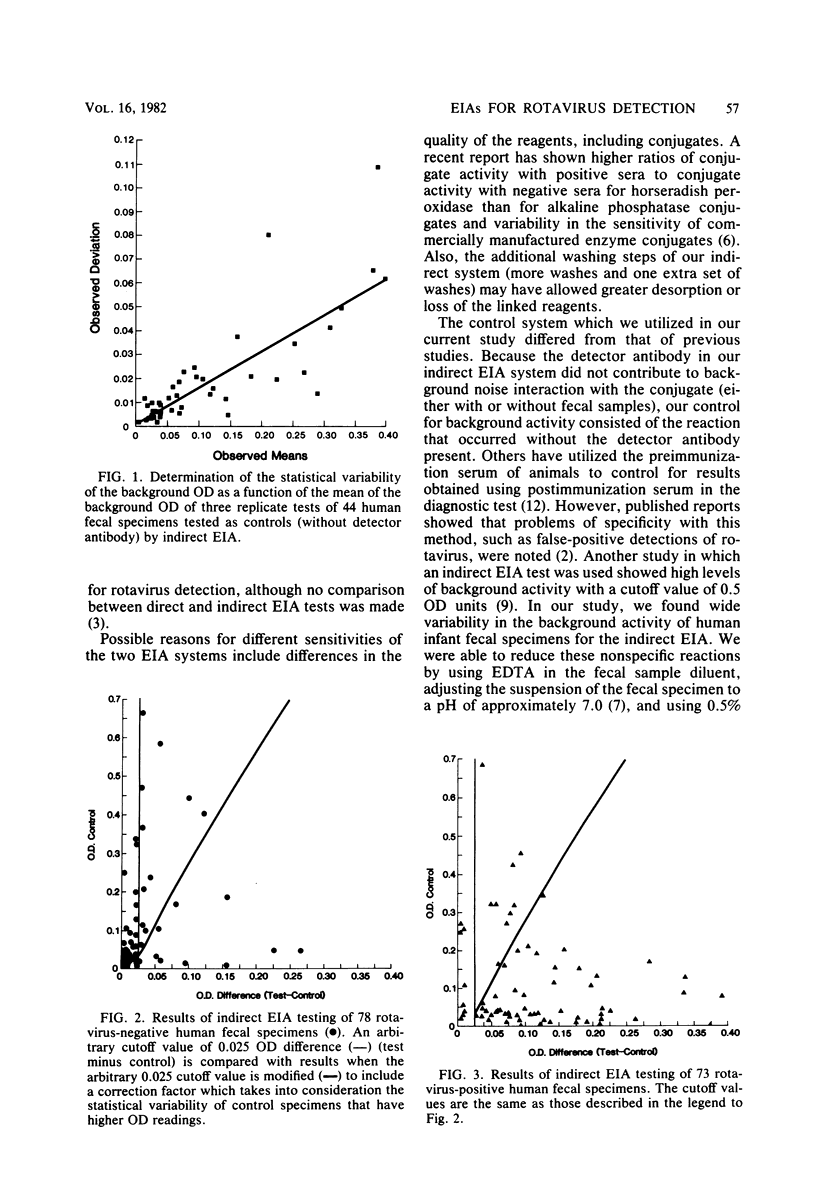
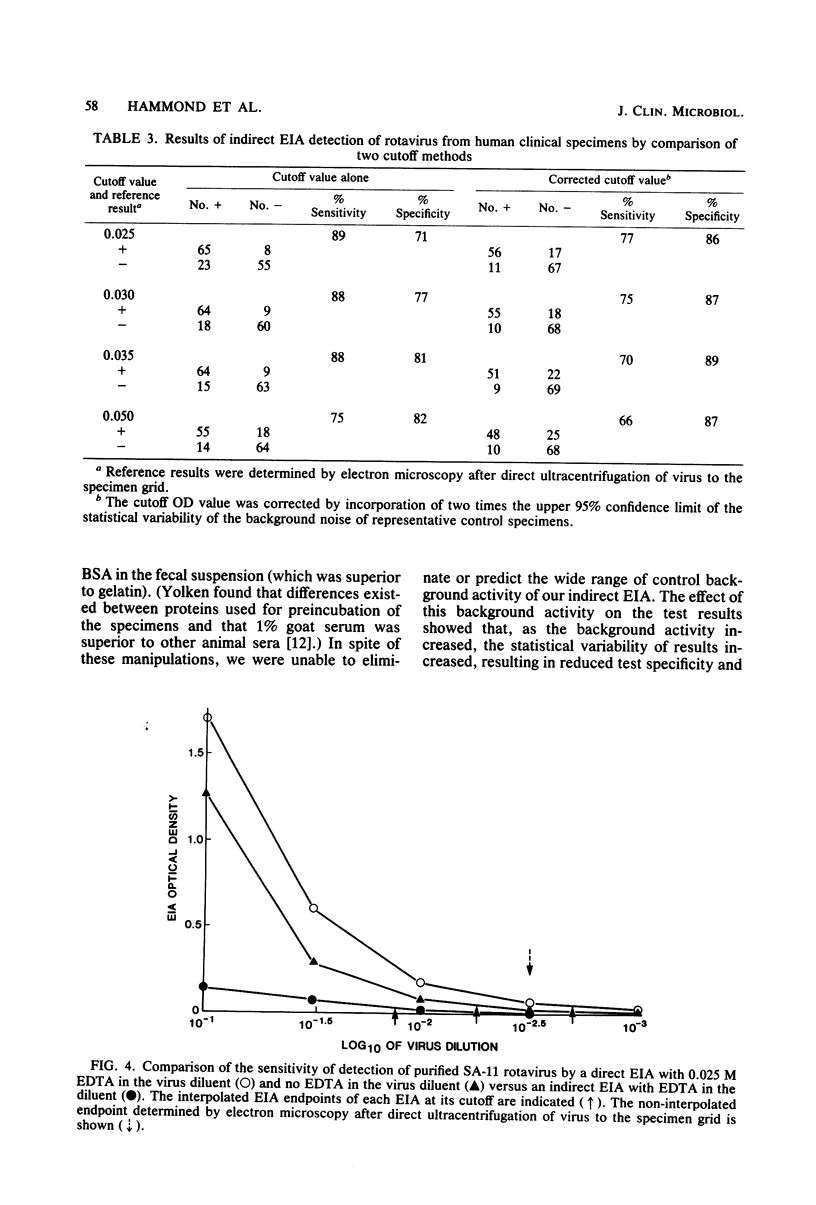
Abstract
A direct and an indirect enzyme immunoassay (EIA) were evaluated against a standard of electron microscopy after direct ultracentrifugation of the specimen for their performances in detecting rotaviruses. The indirect EIA had variable background activity which influenced test specificity. The indirect EIA control (test system without the detector antibody) plus a regression line (which reflected background noise) improved test specificity. However, the results of direct EIA (Rotazyme; Abbott Laboratories, North Chicago, Ill.) sensitivity (86%) and specificity (96%) were better than those of the indirect EIA in tests on 73 rotavirus-positive and 78 rotavirus-negative specimens. Endpoint titrations of purified SA-11 rotavirus showed greater sensitivity of the direct EIA test. Electron microscopy, performed after direct ultracentrifugation, and direct EIA were approximately 2 log10 more sensitive in the detection of purified SA-11 rotavirus than was electron microscopy with standard methods of unconcentrated specimen preparation. Direct EIA test are potentially sensitive, specific, and practical for the rapid detection of rotaviruses from human clinical specimens. Further studies are needed before EIA methods for detection of human rotaviruses can be equated with the level of reliability of results obtainable with sensitive electron microscopy techniques.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishai F. R., Spence L., Goodwin D., Petro R. Use of antisera against bovine (NCDV) and simian (SA11) rotaviruses in ELISA to detect different types of human rotavirus. Can J Microbiol. 1979 Sep;25(9):1118–1124. doi: 10.1139/m79-174. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Thomas L., Yolken R. H., Arrobio J. O., Kapikian A. Z., Parrott R. H., Chanock R. M. Comparison of direct electron microscopy, immune electron microscopy, and rotavirus enzyme-linked immunosorbent assay for detection of gastroenteritis viruses in children. J Clin Microbiol. 1981 May;13(5):976–981. doi: 10.1128/jcm.13.5.976-981.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grauballe P. C., Vestergaard B. F., Meyling A., Genner J. Optimized enzyme-linked immunosorbent assay for detection of human and bovine rotavirus in stools: Comparison with electron-microscopy, immunoelectro-osmophoresis, and fluorescent antibody techniques. J Med Virol. 1981;7(1):29–40. doi: 10.1002/jmv.1890070104. [DOI] [PubMed] [Google Scholar]
- Hammond G. W., Hazelton P. R., Chuang I., Klisko B. Improved detection of viruses by electron microscopy after direct ultracentrifuge preparation of specimens. J Clin Microbiol. 1981 Aug;14(2):210–221. doi: 10.1128/jcm.14.2.210-221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A. Z., Cline W. L., Greenberg H. B., Wyatt R. G., Kalica A. R., Banks C. E., James H. D., Jr, Flores J., Chanock R. M. Antigenic characterization of human and animal rotaviruses by immune adherence hemagglutination assay (IAHA): evidence for distinctness of IAHA and neutralization antigens. Infect Immun. 1981 Aug;33(2):415–425. doi: 10.1128/iai.33.2.415-425.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren M. L., Lillywhite J. E., Au A. C. Indirect enzyme linked immunosorbent assay (ELISA): practical aspects of standardization and quality control. Med Lab Sci. 1981 Jul;38(3):245–251. [PubMed] [Google Scholar]
- Middleton P. J., Holdaway M. D., Petric M., Szymanski M. T., Tam J. S. Solid-phase radioimmunoassay for the detection of rotavirus. Infect Immun. 1977 May;16(2):439–444. doi: 10.1128/iai.16.2.439-444.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESSMAN D., YAGI Y., HIRAMOTO R. A comparison of fluorescein and I 131 as labels for determining the in vivo localization of anti-tissue antibodies. Int Arch Allergy Appl Immunol. 1958;12(3-4):125–136. doi: 10.1159/000228449. [DOI] [PubMed] [Google Scholar]
- Sarkkinen H. K., Tuokko H., Halonen P. E. Comparison of enzyme-immunoassay and radioimmunoassay for detection of human rotaviruses and adenoviruses from stool specimens. J Virol Methods. 1980;1(6):331–341. doi: 10.1016/0166-0934(80)90050-6. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Kim H. W., Clem T., Wyatt R. G., Kalica A. R., Chanock R. M., Kapikian A. Z. Enzyme-linked immunosorbent assay (ELISA) for detection of human reovirus-like agent of infantile gastroenteritis. Lancet. 1977 Aug 6;2(8032):263–267. doi: 10.1016/s0140-6736(77)90951-5. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Leister F. J. Evaluation of enzyme immunoassays for the detection of human rotavirus. J Infect Dis. 1981 Oct;144(4):379–379. doi: 10.1093/infdis/144.4.379. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Stopa P. J. Analysis of nonspecific reactions in enzyme-linked immunosorbent assay testing for human rotavirus. J Clin Microbiol. 1979 Nov;10(5):703–707. doi: 10.1128/jcm.10.5.703-707.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



