Abstract
Field carcinogenesis detection represents a promising means for colorectal cancer (CRC) screening, although current techniques (e.g., flexible sigmoidoscopy) lack the requisite sensitivity. The novel optical technology low-coherence enhanced backscattering (LEBS) spectroscopy, allows identification of microscale architectural consequences of the field carcinogenesis in preclinical CRC models with unprecedented accuracy. To investigate the potential clinical translatability of this approach, we obtained biopsies from the normal-appearing rectal mucosa from patients undergoing colonoscopy (n = 219). LEBS signals were recorded through a bench-top instrument. Four parameters characterizing LEBS signal were linearly combined into a single marker. We found that LEBS signal parameters generally mirrored neoplasia progression from patients with no neoplasia, to 5 to 9 mm adenoma and to advanced adenomas. The composite LEBS marker calculated from the LEBS signal paralleled this risk status (ANOVA P < 0.001). Moreover, this was independent of CRC risk factors, benign colonic findings, or clinically unimportant lesions (diminutive adenomas, hyperplastic polyps). For advanced adenomas, the LEBS marker had a sensitivity of 100%, specificity of 80%, and area under the receiver operator characteristic curve of 0.895. Leave-one-out cross-validation and an independent data set (n = 51) supported the robustness of these findings. In conclusion, we provide the first demonstration that LEBS-detectable alterations in the endoscopically normal rectum were associated with the presence of neoplasia located elsewhere in the colon. This study provides the proof of concept that rectal LEBS analysis may potentially provide a minimally intrusive CRC screening technique. Further studies with an endoscopically compatible fiber optic probe are under way for multicenter clinical validation.
Introduction
Despite being eminently preventable, colorectal cancer (CRC) remains the second leading cause of cancer deaths among Americans underscoring the challenges involved in implementing screening regimens (1). The current screening options vary in both patient acceptability and accuracy, which are generally inversely correlated (2). For example, fecal occult blood test is well-tolerated but has poor sensitivity for clinically/biologically significant lesions (~10% for advanced adenomas and ~33% for CRCs; ref. 3). On the other hand, colonoscopy has outstanding (>97%) accuracy for advanced neoplasia (4). Moreover, colonoscopic identification and removal of precursor lesions (adenomatous polyps) affords a ~75% to 90% risk reduction for future CRC (2, 5). However, numerous obstacles including discomfort, cost, potential complications, and the unpleasant nature of bowel cleansing results in only a small proportion of the eligible population actually undergoing colonoscopy. Moreover, even if compliance could be improved, colonoscopic screening of the entire at-risk population (~87 million Americans older than 50 years) would be prohibitive from expense/resource perspectives. This is juxtaposed with the low prevalence of advanced neoplasia on screening colonoscopy (~5%). Thus, the vast majority of screening colonoscopies does not have any cancer preventive implications (i.e., polypectomy; ref. 6). A minimally invasive risk stratification prescreen would be of major public health importance by reserving colonoscopy to subjects likely to harbor neoplasia.
Many risk-stratification techniques exploit the “field effect”, the proposition that the genetic and environmental milieu that results in a focal lesion should be detectable, at least in some form, throughout the colonic mucosa (7, 8). Therefore, in principle, given its diffuse nature, any sample of colonic mucosa should allow discrimination of patients with and without neoplasia anywhere in their colon (9). The field effect is, in essence, the basis for the widespread use for flexible sigmoidoscopy for CRC screening, given that subjects with distal adenomas are at higher risk of harboring proximal neoplasia (10). However, two issues have stymied current approaches of CRC screening based on field effect exploitation: sensitivity and invasiveness. With regards to sensitivity, despite being a well-established marker, using the sentinel adenoma discovered flexible sigmoidoscopy would result in more than half the patients with advanced proximal neoplasia being missed (11). Furthermore, flexible sigmoidoscopy can be uncomfortable and requires a colonic purgative—one of the biggest obstacles in colon cancer screening (12).
Thus, restricting the exam to the most readily accessible part of the colon (i.e., the rectum) would be clearly advantageous. Although previous biomarkers [generally early events in colon carcinogenesis such as aberrant crypt foci (13), epithelial proliferation (14), apoptosis (15), alterations in gene expression (16), or protein profiles (17)] have corroborated the notion that rectal mucosal evaluation can predict neoplasia elsewhere in the colon, the performance characteristics have been suboptimal for CRC screening. Thus, more accurate biomarkers of colon carcinogenesis are needed to translate this clinically attractive approach to population screening.
Biomedical optics has been used for a variety of cancer screening applications. Traditionally biomedical optics has been used for determination of lesion histology (i.e., “optical biopsy”) or detecting dysplasia. However, our multidisciplinary group focuses on applying new light-scattering technologies such as low-coherence enhanced backscattering (LEBS) spectroscopy to CRC risk stratification (18, 19). LEBS provides heretofore unattainable information regarding the optical properties of the tissue and, therefore, its microscale cellular organization. We were interested in using LEBS to identify subtle microarchitectural consequences of the genetic/epigenetic perturbations that are the hallmark of field carcinogenesis. Our initial studies with two well-validated models of colon carcinogenesis, the azoxymethane-treated rat, and the MIN mouse showed that LEBS markers had excellent accuracy in predicting neoplastic risk (20). Importantly, this analysis was done in the histologically normal mucosa at a time point that far preceded development of conventional biomarkers (aberrant crypt foci, adenomas etc.).
In the present study, we provide the first proof of concept that optical interrogation of the visually normal rectal mucosa can be used for CRC screening. Our studies show that the rectal LEBS signals were altered in patients with biologically significant neoplasia elsewhere in their colon and the performance characteristics seemed to be excellent.
Materials and Methods
Participants
All studies were conducted in accordance with the Institutional Review Board at Evanston-Northwestern Healthcare. The inclusion criteria included complete colonoscopic and pathologic information. Exclusion criteria included coagulopathy, colitis, and poor colonic cleansing or failure to intubate cecum. Polyp size was estimated using an open biopsy forceps as a comparator. For these studies, two rectal biopsies were taken from the endoscopically normal mucosa during colonoscope withdrawal. LEBS analysis was performed by investigators blinded to clinical findings.
LEBS analysis
Schematics of the bench-top LEBS instrument have been previously published (18, 20).
The backscattering signal was collected from the epithelial side of tissue. Enhanced backscattering (EBS) is manifested as a peak of intensity of light scattered by a sample centered around the backscattering direction with the typical full width at half maximum ~0.4° (18, 19, 21). Our instrument allowed acquisition of LEBS as a function of wavelength from 540 to 675 nm. Thus, LEBS peak was recorded as a two-dimensional data as a function of backscattering angle and wavelength from multiple spots per biopsy sample. Patients were classified into 1 of 4 categories: no neoplasia, diminutive adenoma (<5mm), 5- to 9-mm adenoma, or advanced adenoma (size, >10mm;>25% villous features; or high-grade dysplasia).
Conventionally, an EBS peak is characterized by its amplitude (termed enhancement factor) and width (Fig. 1A). In additional, elastic scattering spectra are frequently characterized by its spectral slope (22) with higher frequency oscillations characterized by a second-order statistic (correlation decay rate; Fig. 1B). Accordingly, we parameterized the LEBS signal by the use of these four fundamental characteristics. LEBS width was defined as the average full width at half maximum of an LEBS peak in the wavelength range of 550 to 600 nm. The LEBS enhancement factor was the average height of the LEBS peak over this same wavelength range. The LEBS spectral slope was obtained as the negative of the linear coefficient from a linear regression of the form ILEBS(λ) = A − B·λ, where ILEBS(λ) is the intensity at the LEBS peak for wavelength λ averaged within the range of angles (0 ± 0.23°). For correlation decay rate calculation, the fluctuating component of the spectrum is isolated by subtracting a third-order polynomial from the spectral profile of the top of the LEBS peak. This fitting removes the features contributed by the spectral slope and also aids in removing broad hemoglobin (Hb) absorption features. Due to the shallow penetration depth of the LEBS signal, Hb absorption is normally absent from the measurement. Correlation rate decay D is calculated from the autocorrelation function of LEBS spectra as follows: C (Δk) = ∫ IEBS (k) IEBS (k + Δk), where k is the wave number (k = 2Π/λ) and C (Δk) ∞ exp (−Δk2 D) for small Δk.
Figure 1.
Representative LEBS intensity signal IEBS(θ,λ) obtained from rectal biopsy. A, LEBS markers are defined as parameters that characterize this signal, such as spectral slope, LEBS enhancement factor, and the angular width of the LEBS peak. Bi, decay of the autocorrelation function obtained from the fluctuating component of the spectrum at the top of the LEBS peak. Bii, representative correlation decay profiles from a neoplasia-free patient compared with an adenoma-haboring patient.
Statistical analysis
Single factor ANOVA P values were calculated for each of the parameters and a multiple comparisons test using the Tukey-Kramer algorithm was used to determine which groups had significant differences from the control group. Additionally, a Student’s t test was also used to test for significant differences between each group from the controls.
The LEBS Marker was calculated as a linear combination of the LEBS parameters. A binary diagnostic variable (no neoplasia, 0; advanced adenoma, 1) was used to fit weighting coefficients, βi , using combination of logistic regression in STATA software package (vers. 8.0), and perturbations to the coefficients in Matlab such that:
Where P1-P4 are the four characteristic LEBS parameters and p is the probability of a positive binary outcome (advanced adenoma). Thus, the coefficients were determined empirically and defined the relationship between the LEBS Marker and the experimentally measured parameters. This weighting is analogous to that of standard logistic regression model fitting where the error between the model and the data are minimized. We modified the procedure to maximize for the diagnostic performance instead of minimizing the error. The resulting area under the receiver operator characteristic curve (AUROC) from both methods are identical; however, the shape of the ROC curve is improved by maximizing the diagnostic performance. Leave-one-out cross-validation was performed with logistic regression in Matlab by determining LEBS Marker values for each patient without including that patient in the fitting model.
Analysis of confounding factors was done for LEBS marker measurements by a single factor ANOVA with a multiple comparison test using the Tukey-Kramer method. Contributions of confounding factors toward the LEBS Marker were evaluated by performing an Analysis of Variance and Covariance test in STATA.
Results
Overview of LEBS
The premise of using elastically scattered light for tissue characterization is that light scattering depends on the spatial variations of tissue refractive index, which in turn, depends on the size, structure, and internal milieu of the scattering particles. EBS is an optical effect based on the principle that every photon scattered from tissue in the backward direction has a time-reversed photon traveling along the same path in the opposite direction. These photons have the same phase and thus interfere constructively with each other, resulting in an EBS peak. EBS is determined by the spatial variations of tissue density at length scales ranging from tens of nanometers to microns and thus provides insights into the statistical properties of tissue micro-architecture (18, 19, 21). We preferentially interrogated ~100 µm (approximating the base of crypts) because our animal data indicated that the field-effect changes were most pronounced at this depth (20). Figure 1 shows that the LEBS signal can be clearly identified in rectal biopsies.
Patient characteristics
Our data composed of a main and small independent validation data sets. The 219 patients for the main data set were composed of 47% female and had a mean age of 59.6 ± 9.8 years. Fifteen patients had advanced adenomas, 10 patients had diminutive adenomas (≤4 mm in size), and 27 patients had intermediate adenomas (5–9 mm in size). The independent testing set had 22 subjects with nonadvanced adenomas and 29 who were adenoma-free. Inclusion criteria were comparable with main data set with an enrichment for patients with a previous history of adenomas.
LEBS parameters from rectal mucosa
Figure 2 shows that rectal LEBS parameters tended to be altered in subjects who harbored a midrange (5–9 mm) and advanced adenoma when compared with subjects who were neoplasia free. This was statistically significant for LEBS correlation decay, LEBS slope, and LEBS width (ANOVA P values of 0.0092, 0.0015, and 0.017, respectively) but not LEBS enhancement (ANOVA P = 0.23). Importantly, the rectal LEBS parameters mirrored the neoplastic burden with patients whose adenomas were 5 to 9 mm, decreasing intermittently between those derived from neoplasia-free and advanced adenoma patients. However, no effect was noted in subjects who harbored diminutive (<5 mm) adenomas.
Figure 2.
LEBS parameters recorded from the histologically normal rectal mucosa correlated with the presence of adenomas (>5 mm) elsewhere in the colon. Advanced adenomas manifested a greater alteration than midrange adenomas (5–9 mm), with diminutive adenomas (1–4 mm) failing to show any effect. Each neoplasia category was also compared against control (adenoma free) with the statistical significance with t test (P < 0.01) indicated (*); **, significance from both t test and Tukey-Kramer test.
Evaluation of the LEBS marker
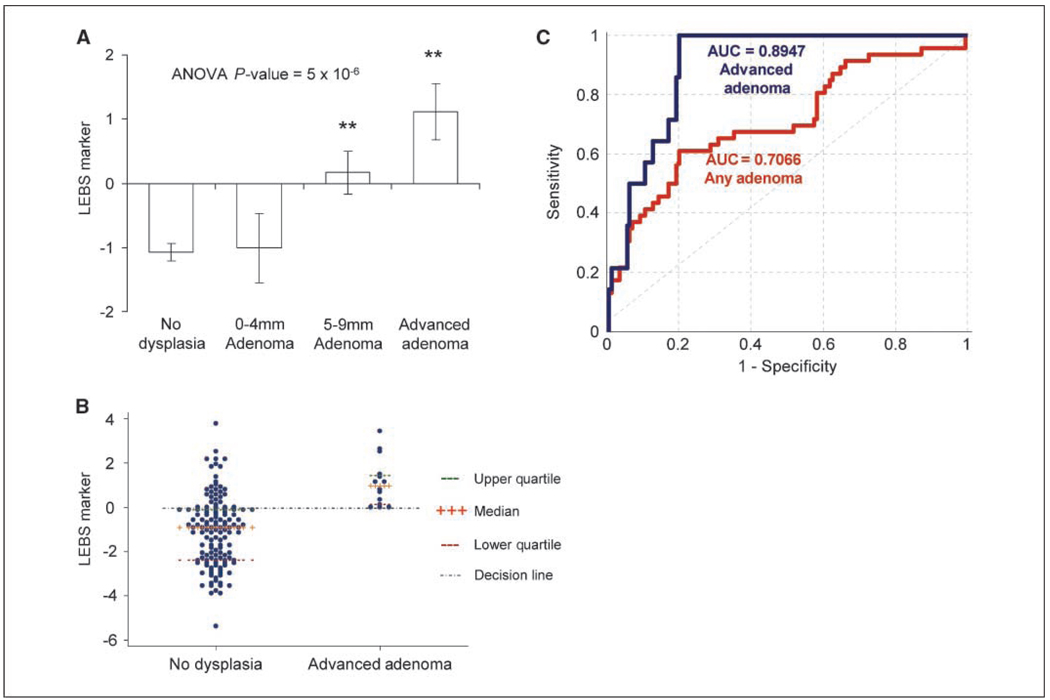
We evaluated the LEBS marker as a linear combination of the four LEBS signal parameters. As seen in Fig. 3A, there was a stepwise alteration in the LEBS marker with regards to adenomas that were 5 to 9 mm and those that were advanced when compared with patients who were adenoma-free. The LEBS marker was unaltered in those with diminutive adenomas (<5 mm).
Figure 3.
LEBS marker (linear combination of the 4 LEBS parameters) recorded from the histologically normal rectal mucosa was altered in patients harboring adenomas (>5 mm). A, there was a progressive significant alteration in LEBS marker among adenomas 5 to 9 mm and advanced adenomas. No association was noted for diminutive (<5 mm) adenomas. B, scatter plot comparing LEBS marker in the control and advanced adenoma patients with the decision line threshold detailed. C, ROC curve assessing the performance of rectal LEBS for all adenomas and advanced adenomas when compared with neoplasia-free patients. For advanced adenomas, the AUROC was 0.89, whereas for all adenomas (including diminutive) it was 0.71.
We focused our analysis on advanced adenomas because of the prevailing opinion that these are the most biologically/clinically significant precancerous lesions. A scatter plot presented in Fig. 3B showing that although there is some overlap, the mean LEBS marker was markedly altered in those with advanced adenoma patients when compared neoplasia-free patients. When compared with neoplasia-free patients, the rectal LEBS marker allowed excellent identification of those with advanced adenoma (100% sensitivity, 80% specificity) but performed less well against those with any adenomas (including diminutive; 61% sensitivity, 80% specificity). The AUROC (Fig. 3C) was 0.895 for advanced adenomas and 0.707 for all adenomas.
Influence of location
To evaluate whether the rectal LEBS marker was affected by the proximity to an adenoma, we dichotomized nondiminutive (≥5 mm) adenoma location into proximal or distal colon (defined at the level of the splenic flexure). Overall, although the rectal LEBS marker was altered in patients harboring neoplasia irrespective of location, the effect size seemed to be greater for distal rather than the proximal adenomas (Fig. 4A).
Figure 4.
Factors that could potentially modulate rectal LEBS markers. A, effect of location of adenoma on rectal LEBS markers. Adenomas (>5 mm) were divided into distal (descending, sigmoid, and rectum) and proximal (splenic flexure to cecum). The LEBS marker was altered in patients with distal (n = 14) as well as proximal adenomas (n = 22), although the effect was more pronounced for the distal adenomas. The ANOVA P value was 0.00000062. B, benign disease was not associated with rectal LEBS marker alteration. In adenoma-free subjects, the LEBS marker was not altered in patients with hyperplastic polysp (n = 38), hemorrhoids (n = 31), diverticulosis (n = 26) versus those without these findings (n = 44). C, effect of prior adenomas on rectal LEBS marker. In patients with a previous history of adenomas, there was no significant difference (P = 0.29) in LEBS marker between those with adenomas (n = 10) versus adenoma free (n = 12) on current colonoscopy (P = 0.29).
Potential confounders
To show that rectal LEBS markers represented presence of neoplasia and were not measuring confounders associated with CRC risk, we performed an Analysis of Variance and Covariance using the LEBS marker as the dependent variable and the presence of neoplasia, smoking and alcohol history, race, gender, body mass index, medication history (aspirin, nonsteroidal anti-inflammatory drugs, and statins), and age as predictors. The rectal LEBS marker remained significantly (P < 0.0001) related to adenoma presence even after the incorporation of these confounding factors (Table 1). All major confounding variables were nonsignificant except for alcohol and tobacco history. Alcohol/tobacco usage prevalence in our population, and hence, effect was marginal consistent with epidemiologic studies (23). Similar Analysis of Variance and Covariance results were obtained for LEBS width, slope, correlation decay rate, and enhancement factor (data not shown).
Table 1.
Analysis of confounding factors in subjects with neoplasia
| ANCOVA P value | |
|---|---|
| Presence of neoplasia | <0.0001 |
| Smoking history | 0.0209 |
| Race | 0.2235 |
| Alcohol history | 0.0443 |
| Gender | 05847 |
| BMI | 0.5857 |
| Medication history | 0.9278 |
| Age | 0.6526 |
NOTE: We performed an ANCOVA analysis with the LEBS marker as the dependent variable and the independent variables listed. After the addition of these confounding factors into the model, the LEBS marker remained a highly significant predictor for the presence of neoplasia (P < 0.0001).
Abbreviations: ANCOVA, Analysis of Variance and Covariance; BMI, body mass index.
We also wanted to see whether benign colonic findings could independently alter the LEBS marker. As shown in Fig. 4B, we noted that the presence of hyperplastic polyps (not premalignant), diverticular disease, or hemorrhoids did not effect LEBS marker in neoplasia-free patients, (ANOVA P = 0.91).
Cross-validation and prospective data set analysis
For independent prospective validation, we used a convenience set of patients undergoing rectal spectral analysis from a concurrent chemoprevention trial. We compared adenoma-free patients (n = 29) with those who harbored any adenoma (all nonadvanced, n = 22). Using empirically predetermined β coefficients from the original analysis, the AUROC for the validation set (patients with any sized adenomas versus those who were neoplasia free) was almost identical (0.69 versus 0.71 from the original training data set). Because the convenience set did not have a sufficient number of advanced adenomas, we performed leave-one-out cross-validation analysis from the original data set. The AUROC of 0.845 seemed to be a reasonable approximation of the initial study (0.895).
Effect of previous adenoma
We performed a subset analysis to investigate the effect of previous history of adenomas on rectal LEBS markers. We used both the main and validation set and required definitive pathologic documentation of previous adenoma. There were 22 patients who met this criterion. Ten of these patients had adenomas noted on the current colonoscopy (all nonadvanced) and 12 who were currently adenoma free. As noted in Fig. 4C, there was a slight alteration in LEBS markers but this failed to reach statistical significance (P = 0.29).
Discussion
We provide the first demonstration that light scattering signatures in histologically normal rectal mucosa are altered in patients harboring neoplasia elsewhere in the colon. Individually, three of four parameters from the LEBS signal correlated with the presence of advanced neoplasia (ANOVA P < 0.05). The weighted sum of these parameters, the LEBS marker, showed dramatic alterations in patients with biologically significant adenomas (>5 mm), and this relationship was not confounded by the presence of common CRC risk factors. These studies are consistent with both our reports in preclinical models that LEBS markers from predyplastic epithelium can predict future neoplasia (20) and also with a pilot human study, which noted that a single spectral marker derived from an earlier generation technology (four-dimensional elastic light-scattering fingerprinting; ref. 24) was decreased in the midtransverse colon of patients harboring adenomatous polyps (25). The current report is a major step forward using a more accurate technology, markedly larger data set, four distinct spectral parameters, and most importantly, providing the first evidence of feasibility in a clinically relevant application (rectal mucosal assessment). Our future clinical vision is that rectal LEBS analysis could be performed with a fiberoptic probe inserted into the rectum (analogous to a rectal thermometer). If the LEBS marker threshold level is not met, the patient could forego immediate more intrusive CRC screening, whereas if the threshold were surpassed, immediate colonoscopy would be recommended.
We believe that the biological underpinning of the altered light scattering signatures in patients harboring adenomas is the microarchitectural consequenecs of the genetic/epigenetic perturbations that are the hallmarks of field carcinogenesis. Although the field effect concept is widely used in current clinical practice, the current markers are inadequate. For instance, flexible sigmoidoscopy is commonly used (1.2 million screening exams performed in 2002; ref. 26), identification of the sentinel adenoma (the intermediate biomarker for proximal neoplasia) is inadequate especially in women (identifying ~1/3; ref. 27). Earlier focal neoplastic lesions (e.g., rectal aberrant crypt foci) may also be somewhat insensitive to proximal neoplasia (13, 28). Therefore, research interest has focused on detecting diffuse field carcinogenesis in the predysplastic (histologically normal) mucosa. The techniques used have ranged from cellular markers (apoptosis and proliferation; ref. 15) to more powerful technologies such as microarray (16) or proteomic analysis (17). These approaches, while providing corroboration of the approach of detecting a field effect in the histologically normal distal colonic mucosa, are not suitable for population screening because of issues related to accuracy and practicality. The power of LEBS for identifying field carcinogenesis is not only its sensitivity for important lesions but also that the “bench-to-bedside” transition seems to be feasible.
These observations have been made possible by a breakthrough in optics technology from our laboratory (18). Our group first described EBS in tissue by using a low-coherence light to overcome challenges of speckle, narrow peak width, and lack of spectroscopic information. The premise of using elastically scattered light for tissue characterization is based on the fact that light scattering depends on the spatial variations of tissue refractive index, which is proportional to the local concentration of tissue constituencies (18, 19, 21). LEBS signatures are governed not simply by the size and shape of scattering particles but also the relationship with the internal cellular milieu. Indeed, our work with two well-validated models of colon carcinogenesis (azoxymethane-treated rat and the MIN mouse) indicated that LEBS markers from the histologically normal mucosa had remarkable accuracy in identifying risk for future neoplasia (20). Thus, LEBS optical markers can be exquisitely sensitive to the earliest genetic/epigenetic changes of field carcinogenesis (20).
Preliminary performance characteristic estimates for the rectal LEBS analysis were very encouraging for advanced adenomas. The cut-points were designed to maximize sensitivity to have an outstanding negative predictive value. Because we envision use of rectal LEBS as a precolonoscopic screening, the negative predictive value is of paramount importance. It is critical that all patients who are deemed to be negative by LEBS in actuality do not harbor significant neoplasms. We believe that a lower positive predictive value is acceptable because without the prescreening tests, all these subjects should have undergone colonoscopy. Furthermore, the LEBS results need to be kept in perspective of other tests that have been advocated to prescreen for colonoscopy. For instance, a guaiac-based fecal occult blood test, one of the most common colon cancer screening test, had a sensitivity of 10.8% for advanced neoplasia (cancer or advanced adenoma; ref. 3). Fecal DNA analysis has a minimum improvement (18.2% sensitivity for advanced neoplasia) but at a large increase in costs (3). Studies on novel markers of field carcinogenesis in the colon (genomic and proteomic) have generally been more phenomenological and not geared for performance characteristics. However, one may glean insight about the promise of his approach through comparable studies in other malignancies. For instance, using a similar approach in lung cancer (microarray analysis from the endoscopically normal right mainstem bronchus) yielded a sensitivity and specificity of 80% and 84%, respectively (29). These performance characteristics for our field carcinogenesis-based approach would seem to be clinically relevant as judged by the fact that although computed tomography colography (virtual colonoscopy) had a sensitivity for advanced adenoma of 84% (per patient analysis of 90%) with a positive predictive value of only 23% (30), this was sufficient to be sanctioned for population screening (2). Others reports have suggested that computed tomography colography may be more useful as a precolonoscopic risk stratification, although the need for bowel purge, radiation exposure, and expense may make this approach less desirable (31). When viewed in this context, rectal LEBS may be quite attractive for CRC population screening given its minimally intrusive nature and accuracy. Moreover, our preclinical data would suggest that this approach may also have utility in assessment of response to chemopreventive agents or determination of risk in individuals with a family history of CRC, although clinical confirmation is clearly required (32).
Our results indicated that rectal LEBS was altered in patients harboring advanced adenomas. For those with intermediate adenomas (5–9 mm), the effect size seemed reasonable, which may be relevant given that these lesions have a 1 in 15 chance of harboring advanced features (33). The clinical implications for the insensitivity for diminutive adenomas are unclear. One could argue that the risk of advanced features in these lesions are negligible (34). On the other hand, the realization that lesions such as flat and depressed lesion can harbor advanced features at smaller diameter is of concern (35). Although this remains to be resolved, it bears emphasis that computed tomography colography received approval for CRC screening, yet this test is, in general, unable to detect diminutive adenomas (2, 30).
Rectal LEBS measurements were sensitive to lesions in both the distal and proximal colon, although the magnitude of alterations was clearly greater in the distal colon. This may simply be a distance effect. On the other hand, this may reflect regional biological differences with the proximal tumors more likely to be microsatellite unstable (36). Moreover, because there is emerging evidence that some of the biological basis of field changes (e.g., promotor hypermethylation in the uninvolved mucosa) may be distinct between proximal and distal neoplasia, it seems reasonable to conjecture that the rectal LEBS marker may reflect these differences (36).
For rectal LEBS analysis to be a practical screening test, it would need to be performed in situ and preferably without colonic preparation. Our group has recently reported that for other markers of field carcinogenesis (i.e., microvascular blood content), in situ testing seemed to improve performance characteristics over the ex vivo approach (37, 38). Preliminary studies using a recently designed endoscopically compatible LEBS probe indicates that not only can LEBS markers be assessed from the rectum, but the ability to predict proximal neoplasia seems promising (39). Larger scale studies using patients with and without colonic purges are currently ongoing.
There are several limitations of this study. The number of advanced adenomas in this screening population was modest. In any biomarker study, it is mandatory to use a robust independent validation set before pronouncements over performance characteristics can be confidently made. While awaiting completion of a comprehensive validation study, we were able to partially mitigate these concerns by analyzing a different cohort. As described, this small convenience set (n = 51) unfortunately lacked advanced adenomas. However, it was reassuring that the AUROC for total adenomas was almost comparable between the initial and validation data sets. In addition, we performed a leave-one-out cross validation for advanced adenomas in the initial data set, which further assuages these concerns.
Another issue is the modest specificity (80% for advanced adenomas) in this trial. An important caveat is that the gold standard (colonoscopy) is clearly not infallible. Tandem colonoscopy studies estimate that 22% of all adenomas are missed (4). These can occur for advanced adenomas as suggested by a ~6-fold difference in detection rate among well-trained endoscopists (40). Therefore, our preliminary report on performance characteristics is probably an underestimate of the true accuracy of rectal LEBS for both total and advanced adenomas. Additionally, endoscopic adenoma size estimation may be somewhat inaccurate although this should not bias our results (41). The issue of whether rectal LEBS reflects immediate or long-term risk is unclear. There did seem to be a slight association with tobacco and alcohol use, which are factors that our group (23, 42), and others (43) have reported to be modest risk factors for CRC. Our studies on the effect of previous adenomas are inconclusive but potentially suggest contributions from both longer term field carcinogenesis and tumor-related factors analogous to our data for microvascular blood content (38). Other groups have shown that rectal apoptosis rate predicts future development of neoplasia, suggesting that the markers of field carcinogenesis may reflect immediate as well as long-term risk (44). Thus, the utility of rectal LEBS for postpolypectomy surveillance will need to be determined.
Finally, it bears mentioning that the biological determinants of these alterations in spectral markers remain incompletely understood. One could argue that the molecular pathogenesis of many biomarkers (serum proteomics, microarrays, etc.) is poorly understood, but that this is irrelevant to its cancer screening applications. In principle, the only requirement is an excellent correlation between biomarker level and presence of the disease of interest (45). On the other hand, this approach, although clinically appropriate, seems somewhat intellectually unsatisfying. We, therefore, have conducted preliminary studies demonstrating that specific genetic alterations in colon carcinogenesis can alter optical nanoscale signatures both in cell culture (46) and the MIN mouse intestinal mucosa (47). Systematic studies are under way to more precisely elucidate the molecular and ultrastructural etiologies of the LEBS spectral marker alterations in field carcinogenesis.
In conclusion, we show, for the first time, that microarchitecture of the uninvolved rectal mucosa was altered in subjects harboring neoplasia elsewhere in their colon. These alterations were detectable by a novel, highly sensitive optical technique, LEBS. This represents a proof-of-principle that optical interrogation of the endoscopically normal rectal mucosa may allow risk-stratification for colon neoplasia. In the future, one could potentially envision that during the annual rectal examination, the primary care physician could insert this thin, flexible probe to determine the need/timing for more intrusive tests such as colonoscopy. Furthermore, this work may herald the role for biomedical optics as a powerful cancer risk-stratification tool.
Acknowledgments
Grant support: NIH grants U01CA111257, R01CA128641, R33CA122017, R01CA112315, R42CA130508, and R01EB003682, and NSF grant CBET-0733868.
We thank Beth Parker for excellent manuscript preparation.
Footnotes
Disclosure of Potential Conflicts of Interest
H.K. Roy, M.J. Goldberg, and V. Backman are cofounders/shareholders in American BioOptics LLC. The other authors disclosed no potential conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med. 2004;351:2704–2714. doi: 10.1056/NEJMoa033403. [DOI] [PubMed] [Google Scholar]
- 4.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343–350. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 6.Roy HK, Backman V, Goldberg MJ. Colon cancer screening: the good, the bad, and the ugly. Arch Intern Med. 2006;166:2177–2179. doi: 10.1001/archinte.166.20.2177. [DOI] [PubMed] [Google Scholar]
- 7.Kopelovich L, Henson DE, Gazdar AF, et al. Surrogate anatomic/functional sites for evaluating cancer risk: an extension of the field effect. Clin Cancer Res. 1999;5:3899–3805. [PubMed] [Google Scholar]
- 8.Braakhuis BJ, Tabor MP, Kummer J, Leemans CR, Brakenhoff RH. A genetic explanation of Slaughter's concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727–1730. [PubMed] [Google Scholar]
- 9.Roy HK, Liu Y, Wali RK, et al. Four-dimensional elastic light-scattering fingerprints as preneoplastic markers in the rat model of colon carcinogenesis. Gastroenterology. 2004;126:1071–1081. doi: 10.1053/j.gastro.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Lewis JD, Ng K, Hung KE, et al. Detection of proximal adenomatous polyps with screening sigmoidoscopy: a systematic review and meta-analysis of screening colonoscopy. Arch Intern Med. 2003;163:413–420. doi: 10.1001/archinte.163.4.413. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G Veterans Affairs Cooperative Study Group 380. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. N Engl J Med. 2000;343:162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 12.Beebe TJ, Johnson CD, Stoner SM, Anderson KJ, Limburg PJ. Assessing attitudes toward laxative preparation in colorectal cancer screening and effects on future testing: potential receptivity to computed tomographic colonography. Mayo Clin Proc. 2007;82:666–671. doi: 10.4065/82.6.666. [DOI] [PubMed] [Google Scholar]
- 13.Seike K, Koda K, Oda K, et al. Assessment of rectal aberrant crypt foci by standard chromoscopy and its predictive value for colonic advanced neoplasms. Am J Gastroenterol. 2006;101:1362–1369. doi: 10.1111/j.1572-0241.2006.00578.x. [DOI] [PubMed] [Google Scholar]
- 14.Anti M, Marra G, Armelao F, et al. Rectal epithelial cell proliferation patterns as predictors of adenomatous colorectal polyp recurrence. Gut. 1993;34:525–530. doi: 10.1136/gut.34.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernstein C, Bernstein H, Garewal H, et al. A bile acid-induced apoptosis assay for colon cancer risk and associated quality control studies. Cancer Res. 1999;59:2353–2357. [PubMed] [Google Scholar]
- 16.Chen L, Hao C, Chiu Y, et al. Alteration of gene expression in normal-appearing colon mucosa of APCmin mice and human cancer patients. Cancer Res. 2004;64:3694–3600. doi: 10.1158/0008-5472.CAN-03-3264. [DOI] [PubMed] [Google Scholar]
- 17.Polley AC, Mulholland F, Pin C, et al. Proteomic analysis reveals field-wide changes in protein expression in the morphologically normal mucosa of patients with colorectal neoplasia. Cancer Res. 2006;66:6553–6562. doi: 10.1158/0008-5472.CAN-06-0534. [DOI] [PubMed] [Google Scholar]
- 18.Kim YL, Liu Y, Turzhitsky V, et al. Coherent backscattering spectroscopy. Opt Lett. 2004;29:1906–1908. doi: 10.1364/ol.29.001906. [DOI] [PubMed] [Google Scholar]
- 19.Kim YL, Liu Y, Turzhitsky VM, et al. Depth-resolved low-coherence enhanced backscattering. Opt Lett. 2005;30:741–743. doi: 10.1364/ol.30.000741. [DOI] [PubMed] [Google Scholar]
- 20.Roy HK, Kim YL, Liu Y, et al. Risk stratification of colon carcinogenesis through enhanced backscattering spectroscopy analysis of the uninvolved colonic mucosa. Clin Cancer Res. 2006;12:961–968. doi: 10.1158/1078-0432.CCR-05-1605. [DOI] [PubMed] [Google Scholar]
- 21.Kim YL, Liu Y, Wali RK, et al. Low-coherent backscattering spectroscopy for tissue characterization. Appl Opt. 2005;44:366–377. doi: 10.1364/ao.44.000366. [DOI] [PubMed] [Google Scholar]
- 22.Bigio I, Mourant J. Ultraviolet and visible spectroscopies for tissue diagnostics: fluorescence spectroscopy and elastic-scattering spectroscopy. Phys Med Biol. 1997;42:803–814. doi: 10.1088/0031-9155/42/5/005. [DOI] [PubMed] [Google Scholar]
- 23.Zisman AL, Nickolov A, Brand RE, Gorchow A, Roy HK. Associations between the age at diagnosis and location of colorectal cancer and the use of alcohol and tobacco: implications for screening. Arch Intern Med. 2006;166:629–634. doi: 10.1001/archinte.166.6.629. [DOI] [PubMed] [Google Scholar]
- 24.Chang SY, Roy HK, Kim Y, et al. Four-dimensional elastic light-scattering fingerprinting (4D-ELF) provides accurate risk stratification for human colonic neoplasia. Gastroenterology. 2004;126:A342. doi: 10.1053/j.gastro.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Roy HK, Turzhitsky V, Kim YL, et al. Spectral slope from the endoscopically-normal mucosa predicts concurrent colonic neoplasia: a pilot ex-vivo clinical study. Dis Colon Rectum. 2008;51:1381–1386. doi: 10.1007/s10350-008-9384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seeff LC, Richards TB, Shapiro JA, et al. How many endoscopies are performed for colorectal cancer screening? Results from CDC’s survey of endoscopic capacity. Gastroenterology. 2004;127:1670–1677. doi: 10.1053/j.gastro.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 27.Schoenfeld P, Cash B, Flood A, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med. 2005;352:2061–2068. doi: 10.1056/NEJMoa042990. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Ng J, Arozulllah A, et al. Aberrant crypt focus size predicts distal polyp histopathology. Cancer Epidemiol Biomarkers Prev. 2008;17:1155–1162. doi: 10.1158/1055-9965.EPI-07-2731. [DOI] [PubMed] [Google Scholar]
- 29.Spira A, Beane JE, Shah V, et al. Airway epithelial gene expression in the diagnostic evaluation of smokers with suspect lung cancer. Nat Med. 2007;13:361–366. doi: 10.1038/nm1556. [DOI] [PubMed] [Google Scholar]
- 30.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207–1217. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin OS, Kozarek RA, Schembre DB, et al. Risk stratification for colon neoplasia: screening strategies using colonoscopy and computerized tomographic colonography. Gastroenterology. 2006;131:1011–1019. doi: 10.1053/j.gastro.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Roy HK, Kim YL, Wali RK, et al. Spectral markers in preneoplastic intestinal mucosa: an accurate predictor of tumor risk in the MIN mouse. Cancer Epidemiol Biomarkers Prev. 2005;14:1639–1645. doi: 10.1158/1055-9965.EPI-04-0837. [DOI] [PubMed] [Google Scholar]
- 33.Lieberman D, Moravec M, Holub J, Michaels L, Eisen G. Polyp size and advanced histology in patients undergoing colonoscopy screening: implications for CT colonography. Gastroenterology. 2008;135:1100–1105. doi: 10.1053/j.gastro.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butterly LF, Chase MP, Pohl H, Fiarman GS. Prevalence of clinically important histology in small adenomas. Clin Gastroenterol Hepatol. 2006;4:343–348. doi: 10.1016/j.cgh.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Soetikno R, Friedland S, Kaltenbach T, Chayama K, Tanaka S. Nonpolypoid (flat and depressed) colorectal neoplasms. Gastroenterology. 2006;130:566–576. doi: 10.1053/j.gastro.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Niv Y. Microsatellite instability and MLH1 promoter hypermethylation in colorectal cancer. World J Gastroenterol. 2007;13:1767–1769. doi: 10.3748/wjg.v13.i12.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wali RK, Roy HK, Kim YL, et al. Increased micro-vascular blood content is an early event in colon carcinogenesis. Gut. 2005;54:654–660. doi: 10.1136/gut.2004.056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy HK, Gomes A, Turzhitsky V, et al. Spectroscopic microvascular blood detection from the endoscopically normal colonic mucosa: biomarker for neoplasia risk. Gastroenterology. 2008;135:1069–1078. doi: 10.1053/j.gastro.2008.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roy HK, Turzhitsky V, Gomes A, et al. Prediction of colonic neoplasia through spectral marker analysis from the endoscopically-normal rectum: an ex vivo and in vivo study. Gastroenterology. 2008;134:109. [Google Scholar]
- 40.Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–2541. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 41.Gopalswamy N, Shenoy VN, Choudhry U, et al. Is in vivo measurement of size of polyps during colonoscopy accurate? Gastrointest Endosc. 1997;46:497–402. doi: 10.1016/s0016-5107(97)70003-8. [DOI] [PubMed] [Google Scholar]
- 42.Roy HK, Gulizia JM, Karolski WJ, Ratashak A, Sorrell MF, Tuma D. Ethanol promotes intestinal tumorigenesis in the MIN mouse. Multiple intestinal neoplasia. Cancer Epidemiol Biomarkers Prev. 2002;11:1499–1402. [PubMed] [Google Scholar]
- 43.Potter JD. Colorectal cancer: molecules and populations. J Natl Cancer Inst. 1999;91:916–932. doi: 10.1093/jnci/91.11.916. [DOI] [PubMed] [Google Scholar]
- 44.Keku TO, Amin A, Galanko J, Martin C, Schliebe B, Sandler RS. Apoptosis in normal rectal mucosa, baseline adenoma characteristics, and risk of future adenomas. Cancer Epidemiol Biomarkers Prev. 2008;17:306–310. doi: 10.1158/1055-9965.EPI-07-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roy HK, Khandekar JD. Biomarkers for the early detection of cancer: an inflammatory concept. Arch Intern Med. 2007;167:1822–1824. doi: 10.1001/archinte.167.17.1822. [DOI] [PubMed] [Google Scholar]
- 46.Subramanian H, Pradhan P, Liu Y, et al. Optical methodology for detecting histologically unapparent nanoscale consequences of genetic alterations in biological cells. Proc Natl Acad Sci U S A. 2008;105:20124–20129. doi: 10.1073/pnas.0804723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roy HK, Iversen P, Hart J, et al. Down-regulation of SNAIL suppresses MIN mouse tumorigenesis: modulation of apoptosis proliferation, and fractal dimension. Mol Cancer Ther. 2004;3:1159–1165. [PubMed] [Google Scholar]