Abstract
Fluctuations, inherent in flexible and biologically relevant lipid bilayers, make quantitative structure determination challenging. Shortcomings in older methods have been realized and new methodologies have been introduced that take fluctuations into account. The large uncertainty in literature values for structural parameters is being reduced.
Keywords: Diffraction, lipid bilayers, fluctuations, diffuse scattering, electron density, profiles, simulations, bilayer thickness, water of hydration.
Introduction
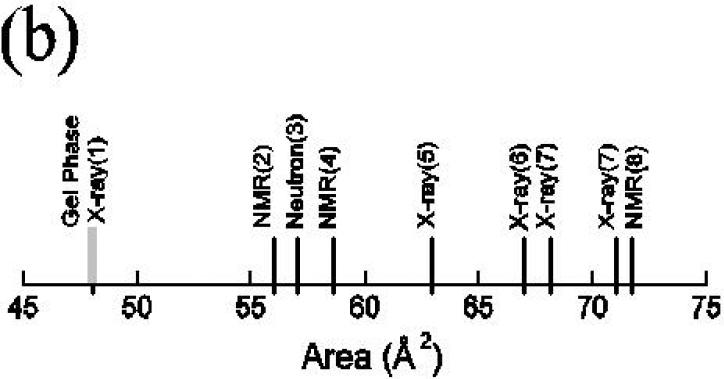
Lipid bilayers have been studied for so many years by so many researchers that it may be surprising to those not working directly in the field just how uncertain are structural quantities for the fully hydrated, fluid (Lα or liquid crystalline) phase. Some of these quantities include various thicknesses (see Fig. 1), such as; the hydrophobic thickness, relevant to hydrophobic matching of proteins; the steric thickness, relevant to determination of interbilayer interactions (see McIntosh, next review); the head-head separation measured from electron density profiles; and the Luzzati thickness (Fig. 2c), relevant to determination of water content in multilamellar vesicles. The most central structural quantity is the average area/molecule A along the surface of the bilayer, to which thickness is related through volumetric considerations. Fig. 1 shows the range of values of A that have been reported for the benchmark lipid DPPC. This is an enormous range, especially since the interesting number is the difference between the biologically relevant fluid phase area AF and the area AG of the ‘dead’ gel phase. The uncertainty in AF - AG in Fig. 1 for this most studied lipid is at the 100% level. This degree of uncertainty makes it difficult to compare bilayers of different lipids and it provides little guide to, or test of, quantitative theory or simulations. Modern structural efforts are directed to reducing this uncertainty and to providing, in collaboration with simulations, a quantitative basis for understanding biophysical interactions in bilayers and membranes.
Fig. 1.
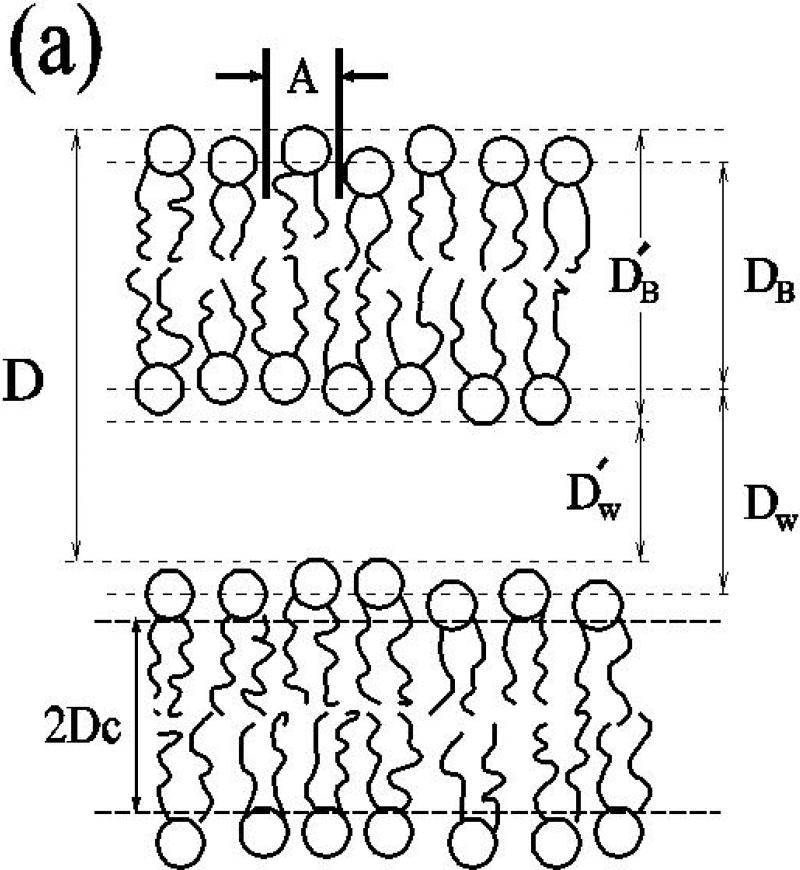
(a) The sketch of two bilayers in a multilamellar vesicle (MLV) identifies the primary lamellar repeat spacing D, the area A per molecule, the hydrophobic thickness 2DC the Luzzati thickness DB, the water thickness DW, the steric thickness , and the steric water thickness . (b) Prominent literature values for A for DPPC in the Lα phase (black) compared to the gel phase (grey).
Fig. 2.
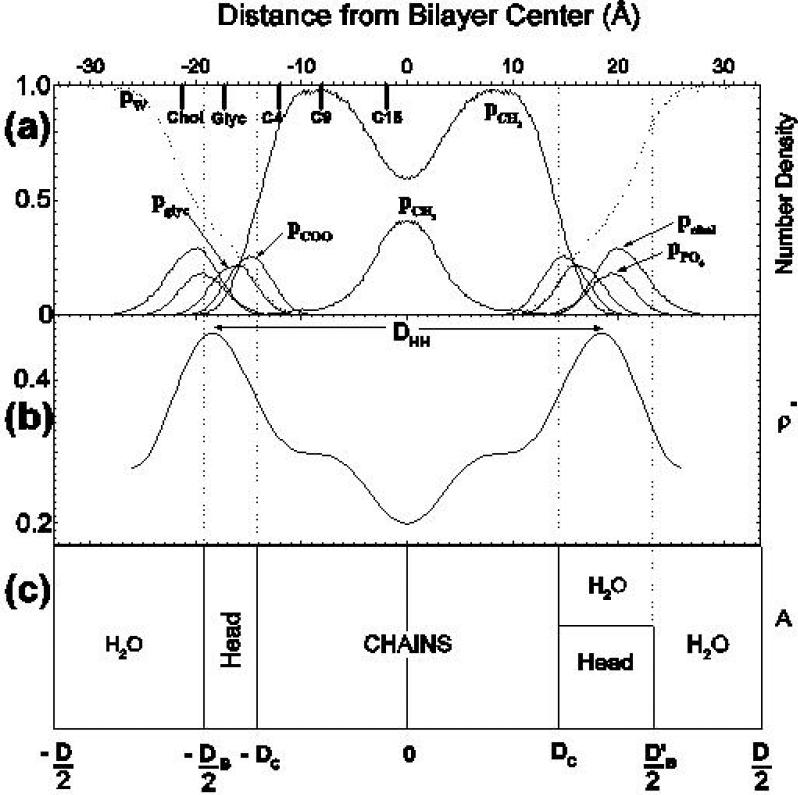
Several views of the structure of fully hydrated DPPC in the Lα phase (T = 50°C). (a) the curves show simulated probability distribution functions [9] for various component groups and the solid bars at the top left of the figure show average positions from neutron diffraction [3]. (b) shows an electron density profile [5]. (c) shows two volumetric views, employing Gibbs dividing surfaces, that relate lateral area A to various thicknesses. Both volumetric views in (c) show the hydrocarbon thickness 2DC. The view on the left shows the Luzzati bilayer thickness DB and the more realistic view on the right mixes water into the headgroup region to show the steric thickness . The Luzzati thickness is given by DB = VL/A, where for DPPC the lipid volume is accurately determined to be VL = 1232±2Å3 per molecule [5].
It is sometimes supposed that bilayer structure determination by diffraction means doing crystallography. While lipid crystallography has been pursued and has been illuminating, it is important to recognize that fully hydrated lipid bilayers are not even close to being in a crystalline state. The contrast is strongest for bilayers that are in the Lα, phase because the hydrocarbon chains are conformationally disordered in contrast to the all-trans chains in lipid crystals. (Even for the conformationally ordered gel and subgel bilayer phases, there are substantial differences compared to the crystal structures.) For fully hydrated fluid phase lipid bilayers it makes no sense to contemplate an atomic level structure because of the fluctuations. The absence of such structures in this field should not be blamed on poor diffraction technique or inadequate sample preparation. Such structures simply do not exist in the biologically relevant state.
The appropriate description for the positions of atoms in the lipid molecule is that of broad statistical distribution functions. Fig. 2a shows simulations for distribution functions for several of the component groups of DPPC along the direction of the bilayer normal [9]. Of course, these distributions do not even have to be Gaussians. Although Gaussians are a convenient form to use in data analysis, simulations show systematic differences from Gaussians [10.11]. Future analyses of diffraction data might benefit by using functional forms suggested by simulations, even though one might not trust precise numerical values which are subject to inaccuracies in the potentials used [5]. Experimental component distributions have been obtained as Gaussian approximations for samples that have been subjected to varying degrees of dehydration [3,12]. Even so, analysis is challenging. A noteworthy paper shows how the analysis of joint x-ray and neutron diffraction data can be improved by using volumetric constraints [11]. More typical x-ray data yield electron density profiles as shown in Fig. 2b; these indicate the location of the electron dense phosphate group. Fig. 2c combines lateral and transverse structure in a volumetric picture.
Gravimetric methods
A method for obtaining A without obtaining electron density profiles is the gravimetric x-ray method (GX), frequently called the Luzzati method [17]. The key formula,
| (1) |
shows how A can be obtained from the highly accurate lipid volume VL and lamellar repeat D, once the number of water molecules per lipid nW is known. The problem with applying this formula using the GX method is that the gravimetrically determined value of nw includes water molecules that go into defect regions between individual MLVs, but the number that is required by the equation should include only the water that goes neatly between well-stacked bilayers. Consequently, this method, which has been much used for many different lipids, tends to overestimate A. To compensate for this methodological problem, Rand and Parsegian [7] supposed that the defect regions could be squeezed out by applying 10 atmospheres of osmotic pressure. The A so obtained was then extrapolated to A0 at full hydration using the formula,
| (2) |
where ADW is the water volume under osmotic pressure Posm and KA is the area compressibility. The values of A0 obtained were indeed lower - see Table 1 in the row labelled GXC for gravimetric x-ray compressibility method. Of course, the GXC method depends upon having experimental values for KA. In an important new paper [13], the values of KA that were previously used [7] have been revised upward and extended to other lipids. This revision will add a negative correction (although only of order −0.4Å2) to the values shown in Table 1.
TABLE 1.
Comparison of Literature Values for Area/Lipid
Electron density profile method
A method that obtains A using electron density profiles (EDP) is due to McIntosh and Simon and was applied by them to DLPE [14]. The method uses gel phase structure which can be accurately determined using wide angle chain packing diffraction. Then, differences in volume and thickness, as measured by DHH in Fig. 2b, are used to obtain A in the Lα, phase. This method has more recently been applied to four phosphatidylcholines. DPPC [5], DOPC [15], DAMPC and EPC [16] with results for A shown in Table 1 in the EDP row. The values are somewhat smaller than the GXC values, which is reasonable if defect water has not been completely removed by the GXC method. One reason for delay in obtaining the EDP results was the necessity of obtaining gel phase structure for DPPC; this is more complicated than for DLPE because the chains are tilted [1]. The other reason is related to the fundamental role that fluctuations play in lipid bilayer diffraction, which we discuss next.
Liquid crystallography
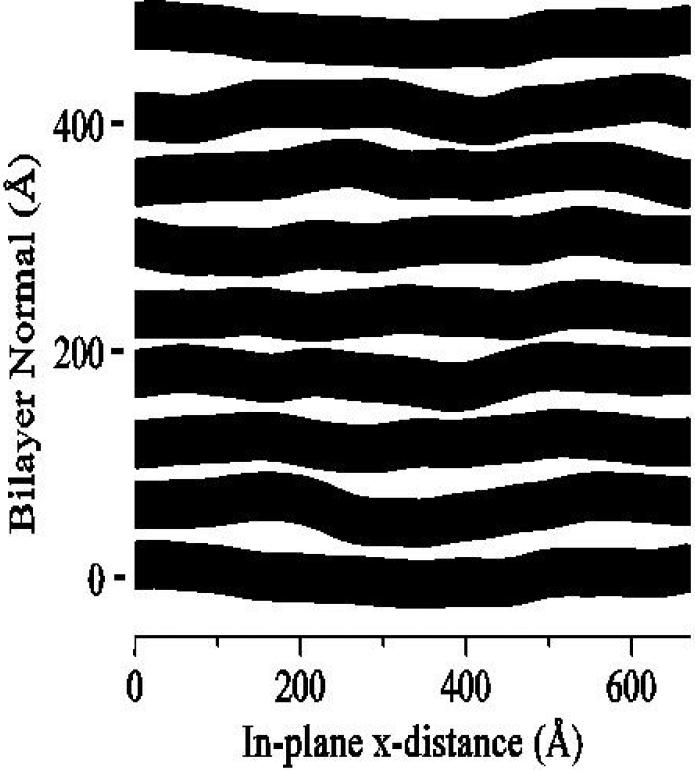
If the distribution functions shown in Fig. 2a are broad, then the electron density profile will be smooth and the higher orders of Fourier decomposition will be negligible. This disorder of the first kind is intrinsic to bilayer structure. Such disorder is accounted for in previous bilayer diffraction analysis which essentially treats the system as a disordered one-dimensional crystal [3,12]. In contrast, disorder of the second kind is not inherent in the structure of the bilayer but affects the regularity of stacking of bilayers relative to each other. Fig. 3 shows this kind of disorder which intimately involves bilayer undulations. This kind of disorder destroys the true long range order that all crystals have and replaces it with ’quasi long range order’ which is a characteristic feature of smectic liquid crystals. Disorder or the second kind also reduces the number of orders of diffraction that are observable. It has been shown that not taking this into account would wrongly imply, using standard diffraction analysis, that there is rapid structural change as full hydration is approached [5]. Disorder of the second kind takes intensity from the diffraction peaks, in higher proportion as the diffraction order increases, and distributes it broadly into diffuse scattering in the tails of the peaks where it is difficult to distinguish from background without the aid of good theory and high resolution detection. A scattering theory developed for liquid crystals [21,22] has been shown experimentally, using synchrotron x-rays [23], to be superior to the older paracrystalline theory used occasionally in membrane biophysics. The liquid crystal analysis uses the measured shapes of the diffraction peaks together with the theory to determine the fluctuation parameters that can then be used to recover the lost intensity. Although the name ’liquid crystallography’ has been used before for analysis of bilayer diffraction data [12], we suggest that this name is more appropriate for the more modern analysis that includes the quasi long range order feature that actually characterizes liquid crystals [22].
Fig.3.
Snapshot of a Monte Carlo simulation of eight liquid crystalline bilayers (in black) with fluctuating water spacings (in white) [20]. The mesoscale Monte Carlo simulation incorported bilayer bending energy and van der Waals and hydration force interactions between the bilayers (see McIntosh review - next paper).
Comparison with some other results
We turn now to the results listed in the last row of Table 1. The entry for DMPC combined the GXC method for moderately low levels of hydration with an NMR method to obtain KA [18]. Although KA has since increased [13], the value of A does not change much. Concerning the entry for DPPC, it has been suggested [5] that there are two better ways to obtain A that use the primary results shown in Fig. 2a for distances of component groups along the bilayer normal [3]; this revision raises A close to 63Å2 instead of the originally reported value for A in Table 1 [5]. The entry for EPC was also obtained using the GX method [19]; it is an exception to the rule that the GX method gives larger values than the GXC or EDP methods. The entry for DOPC in the ’other’ row comes from the important joint refinement method developed by Wiener and White [12]. Unfortunately, this lipid was very dry, nW = 5.4 at 66% relative humidity, which corresponds to Posmotic = 570 atm. Even with the compressibility correction in Eq. 2 and a temperature adjustment, the predicted fully hydrated A would still only be 65Å2, significantly lower than the GXC and EDP values for DOPC in Table 1. More recent work from White's lab [24] explains this discrepancy as an abrupt structural change as a function of increasing hydration near nW = 12. Indeed, a recent simulation indicated that about 12 water molecules are needed to provide the first strong hydration shell for DOPC [25] and an earlier simulation suggested about 15 [26]. It is not surprising that the strong forces that arise from stripping off essential water should cause drastic structural changes that can not be handled by any kind of extrapolation such as Eq. 2. For comparison, there was a minimum nW that safely exceeded 12 for the other samples reported in Table 1.
Let us turn briefly to the use of NMR order parameters to obtain A. Fig. 1 indicates that the literature values for A have spanned a slightly greater range than the diffraction values. This range is due, not to different primary data for the SCD order parameters, but from different methods of interpretating those data to give A. A recent attempt to improve the interpretation employed simulations and a method emerged that fit the simulations very well [27]. The new method gives nearly identical numerical results as the method employed earlier for DPPC by Brown's group [8] that gave A = 71.7Å2 . When applied to DMPC data [18], it gives A = 65.4Å2 [27]. Both these values are considerably higher than the values given by the EDP method and even higher than the GXC values, which should provide an upper bound. Some NMR, practitioners refuse to attempt to determine absolute values of A [18] and that may be a reasonable, if disappointing, conclusion for this use of NMR. On the other hand, a new NMR method that involves magic angle spinning has been shown to give good agreement with the EDP and GXC results for DOPC [28].
Temperature dependence
Temperature dependence of bilayer thickness has been obtained for EPC with the result that the bilayer becomes thinner by about 0.08Å/°C over a temperature range from 10−50°C [29]. The temperature dependence of D in DMPC and DPPC accelerates as the temperature is lowered towards the main transition [30-33]. It appears that half this increase is due to thickening of the bilayer caused by a pretransitional straightening of the hydrocarbon chains as T is reduced [32-34]. The cause of the other half of the increase is still not clear and may involve interactions between the bilayers that cause the water space to increase anomalously [32].
Chain ordered phases
Although chain ordered phases are not usually directly biologically relevant, the EDP method emphasizes the value of gel phase structure as a stepping stone to obtaining Lα phase structure. Chain ordered phases are also valuable to elucidate molecular interactions and to test simulations; only recently has a molecular dynamics simulation [36] been able to match the experimental hydrocarbon chain tilting pattern established for gel phase DPPC bilayrers [1]. The reason that gel phase structure is directly obtainable is that the ordering of the hydrocarbon chains produces wide angle reflections that can be directly indexed to give lateral chain packing area Ac. Although it is challenging to obtain chain tilt angle θt, once that is done [35], A = 2Ac/cosθ and many other quantities follow directly [1]. Temperature and chain length dependence of the gel phase for di-saturated lecithins show great regularity up to chains with 20 carbons [37], but new types of gel phases form in lecithins with longer same chain lipids [38,39]. Curiously, when the lipid is varied so that the chain lengths differ by two carbons, there is no gel phase at all [40], as has recently been reconfirmed for MPPC [44]. Instead, the subgel phase that was first found in DPPC becomes more stable than the gel phase and melts directly into the ripple phase. Understanding this difference between same-chain and mixed-chain phase behavior requires more detailed structure of the subgel phase. In addition to the hydrocarbon chains becoming more ordered [41,42], it also appears that the subgel phase involves headgroup ordering in DPPC [43] and in DPPG [42]. A recent study of one of the members of the glycosphingolipid family illustrates some of the variety of different ordered chain structures that can occur in different lipids of special biological relevance, [45].
Enigmatic ripple phase
The ripple phase that occurs in the lecithins continues to attract attention. Freeze fracture electron microscopy and diffraction studies have for many years indicated that the ripple is not sinusoidal and this has been positively confirmed by solving the x-ray phase problem [46] for high resolution intensities from DMPC [47] with the result shown in Fig. 4. The packing of the hydrocarbon chains within this structure is still not known although a recent suggestion has been made which, however, would require that the thickness of the major M side be greater than the thickness of the gel phase [48], in contradiction to the result in Fig. 4. Determining the detailed molecular structure would seem to be prerequisite to understanding the interactions that are responsible for the formation of this enigmatic phase.
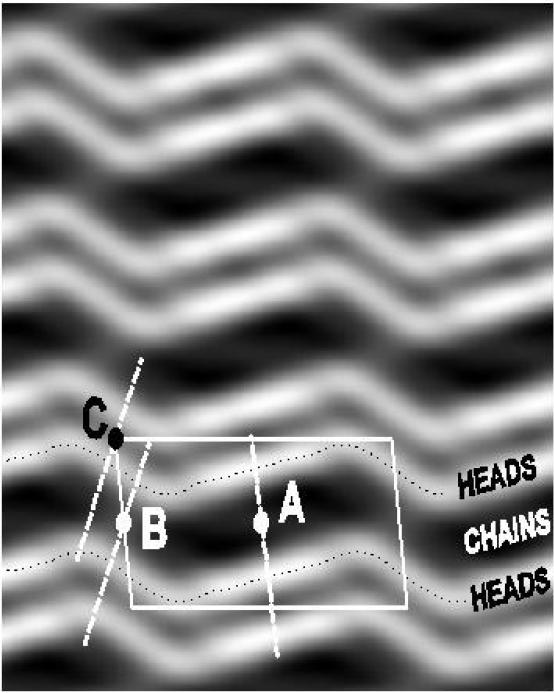
Fig. 4.
Electron density map obtained using x-ray phases from [46] and intensity data from [47] for the ripple thermodynamic phase of DAMPC with 2.5% water (nW = 13) at 18°C. The rippling repeat period is 142Å (length of unit cell) and the lamellar repeat is 58Å (height of unit cell). The profiles show a major M side (across A) that has the same thickness as the gel phase and a thinner minor m side (across B). The presence of a thin water layer between bilayers (across C) indicates complete inner shell hydration of the headgroups.
The ripple phase in DPPC is more complicated because a different ripple pattern occurs when the phase is formed by cooling from the Lα phase than when it is formed by heating from the gel phase [49]. The nature of the cooling phase was somewhat ambiguous from powder diffraction data, but recent data on aligned samples [51] unambiguously confirms the original suggestion [49] that this phase is a mixture of short ripples of the kind in Fig. 4 and of ripples that are nearly twice as long (2.55Å). The occurrence of a rectangular unit cell [51] and images from freeze fracture electron microscopy [50] are consistent with the long ripples consisting of an MmmM repeating motif instead of the MmMm motif of the short ripples. If this is the case, then the free energies of the two ripple phases ought to be very similar, and this is consistent with the recent result that the two patterns melt into the Lα phase within 0.3°C of each other [51].
Conclusions and future directions
Modern structural determinations are reducing the uncertainty suggested in Fig. 1. The older, purely gravimetric x-ray method (GX) (which has been used for many results in the literature) has been improved by the GXC method. Except for DPPC, the GXC method gives results for A only a little larger than those obtained by the completely independent EDP method, and the difference can be rationalized as residual amounts of weighed water not involved in the structure. Although the EDP method requires rather arduous liquid crystallography, this method also provides information about interactions between bilayers (see McIntosh, next paper). Now that the vapor pressure paradox has been resolved (also see McIntosh, next paper), it is very likely that fully hydrated aligned samples will be employed to obtain data to higher spatial resolution and better values for A.
Once A has been determined, many other bilayer structural quantities follow (as shown in Fig. 2c for DPPC). The joint use of the experimental determinations, as in Figs. 2b and 2c, and simulations, as in Fig. 2a, promises to lead to better quantitative determination of bilayer structure and eventually to the molecular interactions that determine biologically interesting differences in structure of different lipid bilayers.
Acknowledgment
Support from NIH Grant GM44976 is gratefully acknowledged.
Abbreviations
- DPPC
dipalmitoylphosphatidylcholine
- DMPC
dimyristoylphosphatidylcholine
- DOPC
dioleoylphosphatidylcholine
- EPC
egg phosphatidylcholine
- EDP
electron density profile method
- GXC
gravimetric x-ray compressibility method
Footnotes
REFERENCES
- 1.Sun W-J, Suter RM, Knewtson MA, Worthington CR, Tristram-Nagle S, Zhang R, Nagle JF. Order and disorder in fully hydrated unoriented bilayers of gel phase DPPC. Phys Rev E. 1994;49:4665–4676. doi: 10.1103/physreve.49.4665. [DOI] [PubMed] [Google Scholar]
- 2.Pace RJ, Chan SI. Molecular motions in lipid bilayers I. Statistical mechanical model of acyl chain motion. J Chem Phys. 1982;76:4217–4227. [Google Scholar]
- 3.Buldt B, Gally HU, Seelig J, Zaccai G. Neutron diffraction studies on phosphatidylcholine model membranes I: Head group conformation. J Mol Biol. 1979;134:673–691. doi: 10.1016/0022-2836(79)90479-0. [DOI] [PubMed] [Google Scholar]
- 4.Schindler H, Seelig J. Deuterium order parameters in relation to thermodynamic properties of a phospholipid bilayer. Biochemistry. 1975;14:2283–2287. doi: 10.1021/bi00682a001. [DOI] [PubMed] [Google Scholar]
- 5.Nagle JF, Zhang R, Tristram-Nagle S, Sun W-S, Petrache HI, Suter RM. X-ray structure determination of fully hydrated Lα, phase dipalmitoylphosphatidylcholine bilayers. Biophys J. 1996;70:1419–1431. doi: 10.1016/S0006-3495(96)79701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis BA, Engelman DM. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J Mol Biol. 1983;166:211–217. doi: 10.1016/s0022-2836(83)80007-2. [DOI] [PubMed] [Google Scholar]
- 7.Rand RP, Parsegian VA. Hydration forces between phospholipid bilayers. Biochim Biophys Acta. 1989;988:351–376. [Google Scholar]
- 8.Thurmond R, Dodd SW, Brown MF. Molecular areas of phospholipids as determined by 2H NMR spectroscopy. Biophys J. 1991;59:108. doi: 10.1016/S0006-3495(91)82203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrache HI, Feller SE, Nagle JF. Determination of component volumes of lipid bilayers from simulations. Biophys J. 1997;72:2237–2242. doi: 10.1016/S0006-3495(97)78867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feller SE, Yin D, Pastor RW, MacKerrell AD. Molecular dynamics simulation of unsaturated lipid bilayers at low hydration: Parameterization and comparison with diffraction studies. Biophys J. 1997;73:2269–2279. doi: 10.1016/S0006-3495(97)78259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Armen RS, Uitto OD, Feller SE. Phospholipid component volumes: Determination and application to bilayer structure calculations. Biophys J. 1998;75:734–744. doi: 10.1016/S0006-3495(98)77563-0. [In addition to the improved analysis of diffraction data, this paper provides the volumes of the component groups in the lipid molecule from simulations. The analysis also indicates the errors involved in using the Gaussian approximation to distribution functions (but notice that the labellings of the two curves in Fig. 4b are reversed).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiener MC, White SH. Structure of a fluid dioleoylphosphatidylcholine bilayer determined by joint refinement of x-ray and netron diffraction data. III. Complete structure. Biophys J. 1992;61:434–447. doi: 10.1016/S0006-3495(92)81849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Rawicz W, Olbrich KC, McIntosh TJ, Needham D, Evans EA. Elastic properties of polyunsaturated lipid bilayers. Biophys J. 2000 doi: 10.1016/S0006-3495(00)76294-1. accepted. [This paper reports ‘true’ values of KA that are crucial for many of the methods of structure determination, as well as other important elastic moduli and some theory to rationalize them. The distinction between the true KA and the previously reported app)Jarent KA is related to the fluctuations that affect so much of bilayer structure determination.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McIntosh TJ, Simon SA. Area per molecule and distribution of water in fully hydrated dilaurylphosphatidylethanolamine bilayers. Biochemistry. 1986;25:4948–4952. doi: 10.1021/bi00365a034. [DOI] [PubMed] [Google Scholar]
- 15**.Tristram-Nagle S, Petrache HI, Nagle JF. Structure and Interactions of Fully Hydrated Dioleoylphosphatidylcholine Bilayers. Biophys J. 1998;75:917–925. doi: 10.1016/S0006-3495(98)77580-0. [This paper initiated a Fourier truncation correction which enabled a rough estimate of KA which agrees, within error, with the recent value [13]. This was then used with Eq. 2 to obtain better value of A and various bilayer thickness for fully hydrated DOPC. Interactions between bilayers were also studied (McIntosh, next review).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Petrache HI, Tristram-Nagle S, Nagle JF. Fluid phase structure of EPC and DMPC bilayers. Chem. Phys. Lipids. 1998;95:83–94. doi: 10.1016/s0009-3084(98)00068-1. [Further refined the electron density profile method to obtain A and found the various thicknesses of two Lα phase bilayers. Absolute electron density profiles and continuous transforms were obtained.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tardieu A, Luzzati V, Reman FC. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water systems. J Mol Biol. 1973;75:711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- 18**.Koenig BW, Strey HH, Gawrisch K. Biophys. J. 1997;73:1954–66. doi: 10.1016/S0006-3495(97)78226-2. [The order parameters from NMR are used, not to obtain A directly but to estimate changes in A to obtain the area modulus KA, which is combined with the GXC method to obtain A. Fig. 3 in this paper graphically emphasizes why the gravimetric method overestimates A near full hydration.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McIntosh TJ, Magid AD, Simon SA. Range of the solvation pressure between lipid membranes: Dependence on the packing density of solvent molecules. Biochemistry. 1989;28:7904–7912. doi: 10.1021/bi00445a053. [DOI] [PubMed] [Google Scholar]
- 20.Gouliaev N, Nagle JF. Simulations of interacting membranes in the soft confinement regime. Phys Rev Lett. 1998;81:2610–2613. doi: 10.1103/PhysRevLett.81.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caillé A. Remarques sur la diffusion des rayons X dans les smectiques A. C. R. Acad. Sc. Paris Série B. 1972;274:891–893. [Google Scholar]
- 22.Zhang R, Suter RM, Nagle JF. Theory of the structure factor of lipid bilayers. Phys Rev E. 1994;50:5047–5060. doi: 10.1103/physreve.50.5047. [DOI] [PubMed] [Google Scholar]
- 23.Zhang R, Tristram-Nagle S, Sun W-J, Headrick RL, Irving TC, Suter RM, Nagle JF. Small angle x-ray scattering from lipid bilayers is well described by modified Caille theory, but not by paracrystalline theory. Biophys J. 1996;70:349–357. doi: 10.1016/S0006-3495(96)79576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Hristova K, White SH. Determination of the hydrocarbon core structure of fluid DOPC bilayers by x-ray diffraction using specific bromination of the double-bonds: Effect of hydration. Biophys J. 1998;74:2419–2433. doi: 10.1016/S0006-3495(98)77950-0. [Hydration was varied from the low nW = 5.4 value studied earlier [12] to the gravimetrically determined nW = 16, which is nearly half the full hydration value [15,28.7]. An abrupt structural change was indicated by a distinct shift in the location of the bromine labels near nW = 12 at which the first hydration shell becomes filled [25]. This is a warning that too much dehydration will not yield biologically relevant fully hydrated structural quantities.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mashl RJ, Scott HL, Subramaniam S, Jakobsson E. Molecular simulation of DOPC bilayers at differing levels of hydration. Biophys. J. 2000 doi: 10.1016/S0006-3495(01)75941-3. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perera L, Essman U, Berkowitz ML. The role of water in the hydration force - molecular dynamics simulations. Progr Colloid Polym Sci. 1997;103:107–113. [Google Scholar]
- 27.Petrache HI, Tu K, Nagle JF. Analysis of simulated NMR order parameters for lipid bilayer structure determination. Biophys. J. 1999;76:2479–2487. doi: 10.1016/S0006-3495(99)77403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagle JF, Liu Y, Tristram-Nagle S, Epand RM, Stark RW. Re-analysis of magic angle spinning NMR determination of interlamellar waters. Biophys J. 1999;77:2062–2065. doi: 10.1016/S0006-3495(99)77047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon SA, Advani S, McIntosh TJ. Temperature dependence of the repulsive pressure between phosphatidylcholine bilayers. Biophys J. 1995;69:1473–1483. doi: 10.1016/S0006-3495(95)80017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honger T, Mortensen K, Ipsen JH, Lemmich J, Bauer R, Mouritsen OG. Anomalous swelling of multilamellar bilayers in the transition region by renormalization of curvature elasticity. Phys Rev Lett. 1994;72:3911–3914. doi: 10.1103/PhysRevLett.72.3911. [DOI] [PubMed] [Google Scholar]
- 31.Chen FY, Hung WC, Huang HW. Critical swelling of phospholipid bilayers. Phys Rev Lett. 1997;79:4026–4029. [Google Scholar]
- 32.Nagle JF, Petrache HI, Gouliaev N, Tristram-Nagle S, Liu Y, Suter RM, Gawrisch K. Multiple Mechanisms for Critical Behavior in the Biologically Relevant Phase of Lecithin Bilayers. Phys. Rev. E. 1998;58:7769–7776. [Google Scholar]
- 33.Richter F, Finegold L, Rapp G. Sterols sense swelling in lipid bilayers. Phys Rev E. 1999;59:3483–3492. [Google Scholar]
- 34.Mason PC, Gaulin BD, Epand RM, Katsaras J. Critical swelling in single phospholipid bilayers. Phys Rev E. 2000 doi: 10.1103/physreve.61.5634. (submitted) [DOI] [PubMed] [Google Scholar]
- 35.Tristram-Nagle S, Zhang R, Suter RM, Worthington CR, Sun W-J, Nagle JF. Measurement of chain tilt angle in fully hydrated bilayers of gel phase lecithins. Biophys J. 1993;64:1097–1109. doi: 10.1016/S0006-3495(93)81475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venable RM, Brooks BR, Pastor RW. Molecular dynamics simulations of gel (LβI) phase lipid bilayers in constant pressure and constant surface area ensembles. J Chem Phys. 2000;112:4822–4832. [Google Scholar]
- 37.Sun W-J, Tristram-Nagle S, Suter RM, Nagle JF. Structure of gel phase saturated lecithin bilayers: Temperature and chain length dependence. BiophyIs J. 1996;71:885–891. doi: 10.1016/S0006-3495(96)79290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun W, Tristram-Nagle S, Suter RM, Nagle JF. Anomalous phase behavior of long chain saturated lecithin bilayers. Biochim Biophys Acta. 1996;1279:17–24. doi: 10.1016/0005-2736(95)00236-7. [DOI] [PubMed] [Google Scholar]
- 39.Snyder RG, Liang GL, Strauss HL, Mendelsohn R. IR spectroscopic study of the structure and phase behavior of long-chain diacylphosphatidylcholines in the gel state. Biophys J. 1996;71:3186–3198. doi: 10.1016/S0006-3495(96)79512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serrallach EN, deHaas GA, Shipley GG. Structure and Thermotropic Properties of Mixed-Chain Phosphatidylcholine Bilayer Membranes. Biochemistry. 1984;23:713–720. [Google Scholar]
- 41.Ruocco MJ, Shipley GG. Characterization of the sub-transition of hydrated DPPC bilayers; kinetic, hydration and structural study. Biochem Biophys Acta. 1982;691:309–320. [Google Scholar]
- 42.Blaurock AE, McIntosh TJ. Structure of crystalline bilayer in the subgel phase of dipalmitoylphosphatidylglycerol. Biochemistry. 1986;25:299–305. doi: 10.1021/bi00350a003. [DOI] [PubMed] [Google Scholar]
- 43.Katsaras J, Raghunathan VA, Dufourc EJ, Dufourcq J. Evidence for a two-dimensional molecular lattice in subgel phase DPPC bilayers. Biochemistry. 1995;34:4686–4688. doi: 10.1021/bi00014a023. [DOI] [PubMed] [Google Scholar]
- 44.Tristram-Nagle S, Isaacson Y, Lyatskaya Y, Liu Y, Brummond K, Katsaras J, Nagle JF. Polymorphism in MPPC. Chem Phys Lipids. 1999;100:101–113. doi: 10.1016/s0009-3084(99)00041-9. [DOI] [PubMed] [Google Scholar]
- 45*.Saxena K, Zimmerman P, Schmidt RR, Shipley GG. Bilayer properties of totally synthetic C16:0-Lactosyl-Ceramide. Biophvs J. 2000;78:306–312. doi: 10.1016/S0006-3495(00)76593-3. [Polymorphism of chain ordered phases of this fully hydrated lipid of biological relevance, is found and compared to others of the glycosphingolipid and glycoglycerolipid families. These systematic studies are aimed at understanding the role of oligosaccharide complexity in regulating structure and function.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun W, Tristram-Nagle S, Suter RM, Nagle JF. Structure of the ripple phase in lecithin bilayers. Proc Natl Acad Sci (USA) 1996;93:7008. doi: 10.1073/pnas.93.14.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wack CD, Webb WW. Synchrotron x-ray study of the modulated lamellar phase P′β in the lecithin-water system. Phys Rev A. 1989;40:2712. doi: 10.1103/physreva.40.2712. [DOI] [PubMed] [Google Scholar]
- 48.Sengupta K, Raghunathan VA, Katsaras J. Novel structural features of the ripple phase of phospholipids. Europhys Lett. 2000 (in press) [Google Scholar]
- 49.Yao H, Matuoka S, Tenchov B, Hatta I. Metastable ripple phase of fully hydrated dipalmitoylphosphatidylcholine as studied by small angle x-ray scattering. Biophys J. 1991;59:252–255. doi: 10.1016/S0006-3495(91)82216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodward JT, Zasadzinski JA. High-resolution scanning tunneling microscopy of fully hydrated ripple=phase bilayers. Biophys J. 1997;72:964. doi: 10.1016/s0006-3495(97)78731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51*.Katsaras J, Tristram-Nagle S, Lin Y, Headrick RL, Fontes E, Mason PC, Nagle JF. Clarification of the ripple phase of lecithin bilayers using fully hydrated, aligned samples. Phys Rev E. 2000 doi: 10.1103/physreve.61.5668. accepted. [Now that the vapor pressure paradox has been resolved, aligned fully hydrated samples were produced that give scattering patterns that unambiguously show ripple phase patterns, including the mixture of long and short ripples produced upon cooling.] [DOI] [PubMed] [Google Scholar]