Abstract
α,β-Difluoromethylene deoxynucleoside 5’-triphosphates (dNTPs, N = A or C) are advantageously obtained via phosphorylation of corresponding dNDP analogues using catalytic ATP, PEP, nucleoside diphosphate kinase (NDPK) and pyruvate kinase (PK). DNA pol β Kd values for the α,β-CF2 and unmodified dNTPs, α,β-NH dUTP, and the a,β-CH2 analogues of dATP and dGTP are discussed in relation to the conformations of α,β-CF2 dTTP v. α,β-NH dUTP bound into the enzyme active site.
In an ongoing multidisciplinary study of structure and function of DNA polymerase β, a eukaryotic enzyme primarily involved in filling short DNA gaps1, we required a series of α,β-methylene-substituted analogues of deoxynucleoside 5’-triphosphates (dNTPs). When the Pα-O-Pβ bridging oxygen in a natural mononucleotide substrate is replaced by an imido (NH)2–4 or methylene (CXY)4,5 group, the P-N or P-C bond should resist cleavage in the nucleotidyl transfer reaction catalyzed by the enzyme. As a result, these analogues will remain intact in stable ternary DNA complexes with the polymerase and therefore should be useful to probe pre-chemistry enzyme-complex function and structure, as recently shown with in an X-ray crystallographic study of α,β-NH dUTP with DNA pol β.6 Information about such complexes provides a reference point for theoretical analysis of the chemical mechanism7 for the complete transfer of a monophosphate nucleoside donor to the sugar acceptor in the active site. As probes for the mechanism of polymerase catalysis and its relationship to polymerase fidelity, α,β-methylene dNTP analogues permit exploration of stereoelectronic effects on active site interactions, by making appropriate substitutions X,Y on the adjacent Pα CXY bridging carbon. The largest obtainable electron-withdrawing effect with minimal steric perturbation can be achieved using X,Y = F, resulting in analogues in which the bisphosphonate group is expected to be less basic than the pyrophosphate moiety in the natural dNTPs.8,9
In this article, we describe the first synthesis of α,β-CF2 dCTP 6, using a modified chemical-enzymological approach that also can be applied to synthesis of α,β-CF2 dATP 3, affording these compounds in sufficient purity to virtually eliminate detectable contaminating substrate activity in polymerase inhibition kinetics assays. DNA pol β Kd values were determined for these analogues, and compared to Kd values for α,β-CF2 dTTP 9, the natural substrates dATP, dCTP and dTTP, dUTP, α,β-NH dUTP 10, and the dATP and dGTP α,β-CH2 analogues (11, 12). We also describe the ternary complex of 9 bound with template-primer DNA into the active site of DNA polymerase β, as determined by X-ray crystallography, and discuss its structural implications.
An obvious route to α,β-methylene-dNTP analogues entails coupling a particular methylenebis(phosphonate) derivative to the target nucleoside, followed by phosphorylation10 to add the terminal γ-phosphate. Several α,β-CXY NDPs (X,Y = H or F) were previously synthesized via tosylation of the 5’-OH in the protected ribonucleoside with tosyl chloride and (dimethylamino)pyridine, followed by deprotection and displacement of the tosyl moiety with the appropriate tris(tetrabutylammonium)11 bisphosphonate. A similar approach was used to prepare a α,β-CF2 dGDP (N2-(p-N-n-butylphenyl) derivative)12, dATP and dTTP,13 and related nucleotide analogues.14 In an synthesis of AZT 5’-carbonylbis(phosphonate), trimethyl (diazomethylene)bis(phosphonate) was coupled to AZT under Mitsunobu conditions, but subsequent deprotection (demethylation) at phosphorus was required.15 Direct 5’-coupling of two to six equivalents of methylenebis(phosphonic dichloride) with unprotected nucleosides also has been demonstrated.16
Chemical phosphorylation of α,β-CH2 dADP and dTDP has been carried out via the p-nitrobenzyl-phosphoromorpholidates. 13 Unfortunately, subsequent deprotection by hydrogenolysis of the p-nitrobenzyl-group restricts the pyrimidine substrate, because the cytosine ring of dC is prone to reduction under these conditions.17 Side products from the phosphorylation step typically contaminate the α,β-methylene dNTP analogue product. Carbonyldiimidazole (CDI) activation18 of the dGDP derivative referred to above has been utilized as an alternative route12. We found phosphorylation of our dNDP substrates using CDI to be problematic due to the reactivity of the imidazolate, which tended to give unwanted nucleoside phosphorylation at the 3’-OH and other side products.
As an alternative to syntheses of α,β-imido dNTPs using CDI to activate the NDP analogue for coupling with tributylammonium phosphate,3,19 Kenyon proposed enzymatic phosphorylation by either creatine kinase (CK)2,19 or pyruvate kinase (PK) and phosphonenolpyruvate (PEP)19. However, inadequate CK and PK activity in attempted PEP phosphorylation of our dNDP intermediates, led us to consider adaptation of a method for phosphorylation of azole carboxamide riboxynucleoside 5’-diphosphates (NDPs) by ~stoichiometric ATP and nucleoside diphosphate kinase (NDPK)20a previously applied to synthesis of 11 20b, in which PEP and PK are included to recycle the ADP produced in the phosphoryl transfer and drive the reaction to completion.
Synthesis of α,β-dNTPs
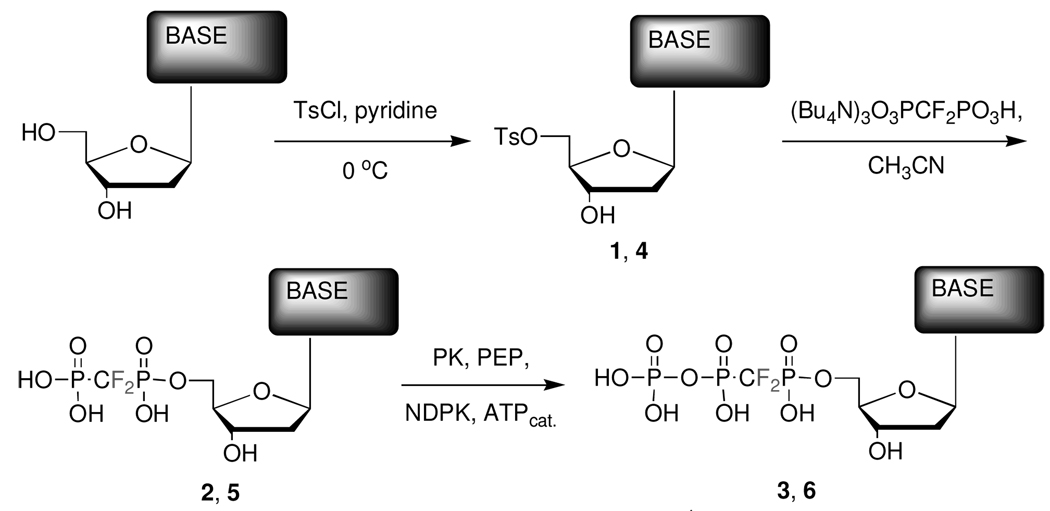
We first converted dA or N4-benzoyl-dC 7 to the corresponding 5’-tosylates, 1 or 4 respectively (see Scheme 1), by reaction with tosyl chloride in pyridine (75–80%).13
Scheme 1. Synthesis of α,β-methylene dNTP analogues.
1, 2, and 3: Base = adenine, 4: Base = N4-Bz-cytosine, 5 and 6: Base = cytosine. [Note: Before tosylation, dC was converted to N4-Bz-deoxycytidine 7 with benzoic anhydride, DIEA in pyridine, microwave irradiation (2 min – 300 W). Prior to the enzymatic phosphorylation, the N4-protected dCDP analogue 8 obtained directly from 4 was debenzoylated to 5 using methanolic ammonia.]
The NH2-group of dC was protected via facile microwave-induced reaction with benzoic anhydride and diisopropylethylamine (DIEA) in pyridine,21 to give 7 (76%). When purifying 4 from the reaction mixture, we intially used conventional extraction with aq. NaHCO3 (pH ~ 8.7), but obtained low isolated yields (~20%). The pKa of the N4-proton in 4 was estimated (ACDLabs pKaDB 8.01 software program) to be ~8. Accordingly, we changed to a slightly acidic aqueous work-up (citric acid, pH ~ 4.5), which increased the isolated yield to ~70%.
The purified tosylates were converted to the dNDP α,β-difluoromethylene analogues 2 or 5 via condensation with the tris(tetrabutylammonium) salt13 of difluoromethylenebis(phosphonic acid) (DFBP). The N4-benzoyl dCDP analogue 8 obtained as the direct tosylation product from 4 was then deprotected in methanolic ammonia to give 5 quantitatively, which was purified by preparative ion-exchange HPLC. DFBP was obtained by bromotrimethylsilane (BTMS) dealkylation22 of tetraisopropyl (difluoro)methylenebis(phosphonate) (TiPDFBP)8, synthesized by fluorination of the carbanion of tetraisopropylmethylenebisphosphonate.7,23
Phosphorylation to the dNTP analogues 3 or 6 was cleanly achieved using NDPK and a catalytic amount of ATP, regenerated with 2.5 eq. of PEP with PK in 50 mM HEPES buffer (75–90% by HPLC and 31P-NMR). Importantly, the latter modification renders unnecessary use of an affinity column20 to purify the product from excess ATP.
Purification of α,β-dNTPs
In order to obtain product completely free of biochemically detectable nucleotide contaminants, we found that separation on DEAE Sephadex or Dowex3,5,9,12–14,24–30 or single-pass preparative HPLC using a C-18 or ion-exchange3,5,9,12–14,24–31 column was not sufficient. However, dual-HPLC (ion exchange, then C-18) provided highly purified products based on analytical HPLC and 1H, 31P and 19F NMR analysis.
This overall synthesis/purification route has the advantage of being applicable to both purine and pyrimidine examples, including the previously unavailable α,β-CF2 dCTP analogue 6. The reactions are relatively clean (particularly compared to standard CDI phosphorylation), do not require protection/deprotection chemistry in the phosphate moieties, eliminate the problem of excess ATP in the enzymatic phosphorylation, and after the dual-HPLC purification, provide exceptionally pure analogues suitable for polymerase inhibition studies (see below). The dual-HPLC purification protocol was also effective in reducing impurities in α,β-CF2 dTTP 9 prepared by the p-nitrobenzyl-phosphomorpholidate method (see below).
DNA Synthesis Assays
DNA synthesis was assayed on four single-nucleotide gapped DNA substrates where the templating base in the gap was complementary to the incoming dNTP. The DNA sequence was as described previously32 with the core sequence identical to that used for crystallization. Enzyme activities were determined using a reaction mixture containing 50 mM Tris-HCl, pH 7.4, 20 mM MgCl2, 200 nM single-nucleotide gapped DNA, and various dNTP concentrations.
Reactions were initiated with 0.5 nM enzyme at room temperature and stopped with EDTA mixed with formamide dye. For steady-state reactions, incubation times were chosen to insure that less than 20% of the primer was extended. The radiolabeled 15-mer primer substrate and 16-mer product were resolved by denaturing polyacrylamide gel electrophoresis [31 × 38.5 × 0.04 cm, 15% (v/v) polyacrylamide (19:1 acrylamide:bis–acrylamide), 8 M urea gels run at 70–80 W, 1 h 20 min - 1 h 40 min] and quantified in the dried gels by phosphorimagery.
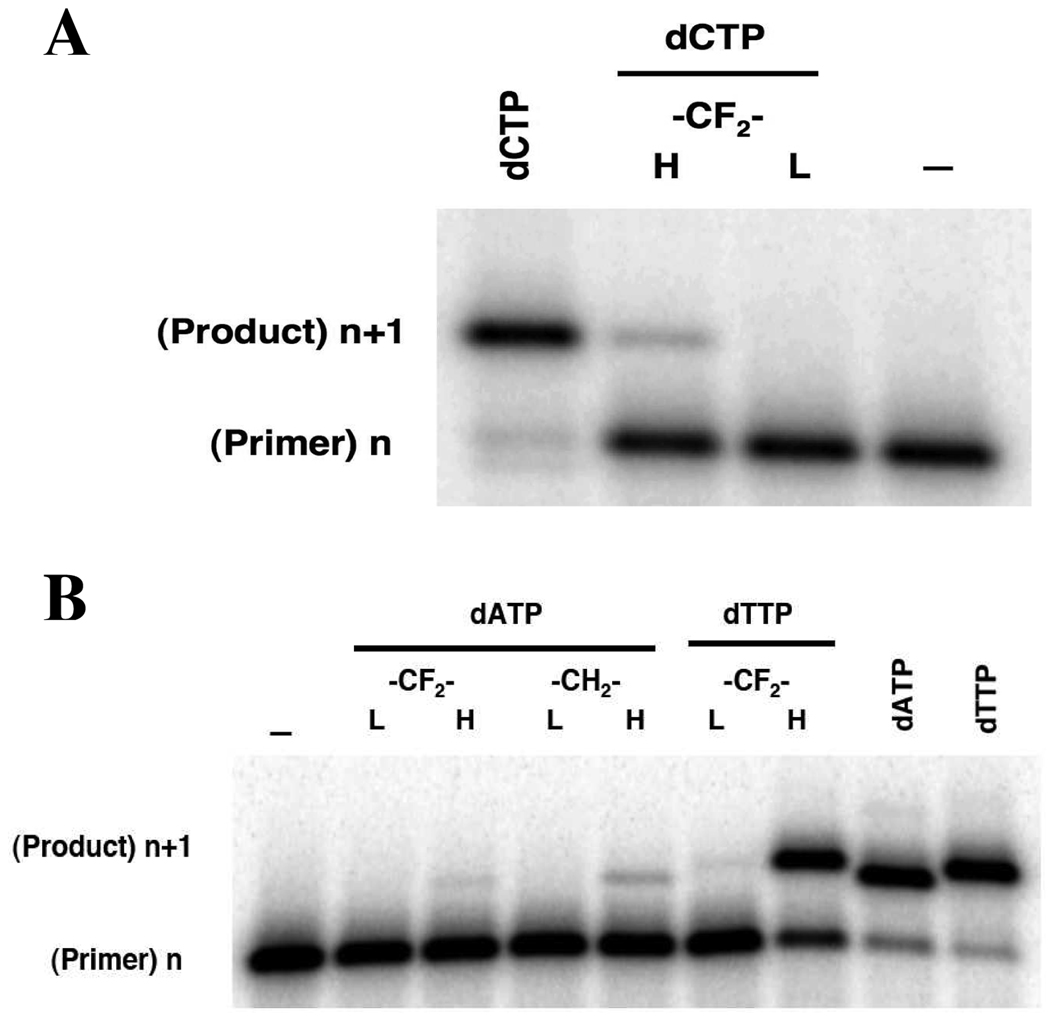
Initial DNA polymerase inhibition studies on α,β-CF2 dCTP, 6 at 1 mM showed no detectable background DNA synthesis with 0.5 nM enzyme, and only a trace with 50 nM enzyme, representing < 0.01% incorporable contaminant (Figure 1A). Comparable absence of background synthesis was evident in the assay using α,β-CF2 dATP, 3 (Figure 1B). For comparison, with a preparation of inhibitor 9 obtained via the p-nitrobenzyl phosphoromorpholidate method, at 50 nM pol β most of the primer strand in the assay has been extended, with some incorporation still detectable using the lower concentration of enzyme (Figure 1B). After dual-HPLC purification, this artifact was no longer observable with 0.5 nM enzyme, although incorporation at 50 nM enzyme was more evident than for 6 and 3 (supplemental information).
| Equation 1 |
Figure 1.
Gapped DNA synthesis assay with DNA pol β for dNTPs (dCTP, dATP, dTTP) and α,β-CXY analogues. The purity of the analogues was assessed from their failure to be inserted into a gapped DNA substrate. Primer (n) extension was assayed in the presence of low (L, 0.5 nM) or high (H, 50 nM) pol β and 1 mM analogue for 5 or 10 min, respectively. A: dCTP vs. 6; B: 3 vs. 11, 9, dATP and dTTP. The mobility of the extension product (n+1) with dCTP, dATP or dTTP serves as a reference, where most of the primer (200 nM) is extended after a 21 min incubation with 50 nM enzyme and 100 µM dNTP.
Steady-state kinetic parameters (kcat, Km) were determined by fitting the rate data to the Michaelis equation (Equation 1). Inhibition of dNTP insertion by various α,β modified dNTPs was determined at various concentrations of inhibitor (I) and sub-saturating dNTP concentration (S). The inhibitor equilibrium binding constant, Ki, was determined by fitting the inhibition data to Equation 1 for competitive inhibition by nonlinear regression methods. Equilibrium binding constants for the natural incoming dNTPs were taken from previously published data [dATP33; dCTP34; dTTP35]. The Kis for 10, 11 and 12 were reported previously6,36.
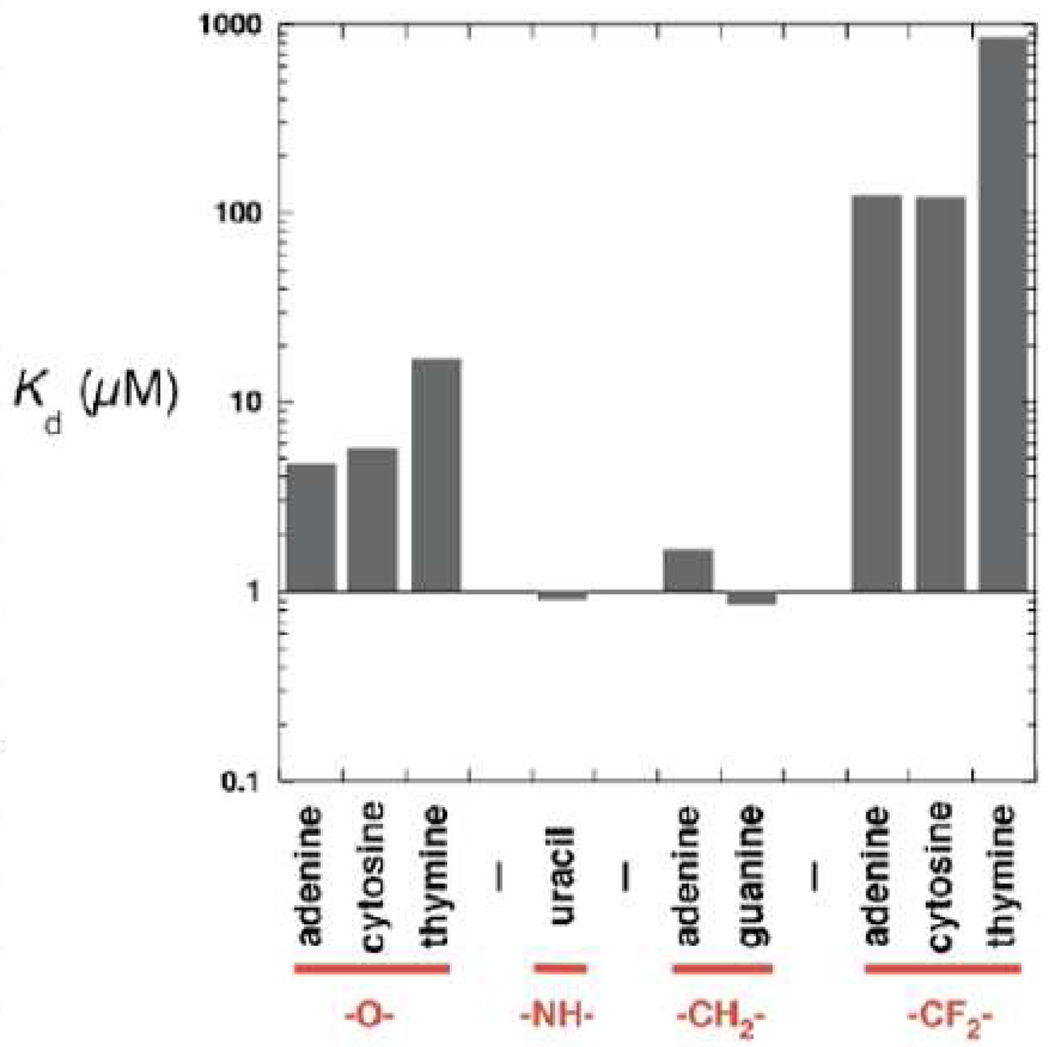
Inhibition of natural (O α-β) nucleotide insertion by the respective analogues was determined at various concentrations of the analog and sub-saturating concentrations of the natural nucleotide. The inhibition constant, Ki (=Kd, dissociation constant), was determined by Dixon analysis. The CF2-analogues readily inhibit pol β single-nucleotide insertion (Figure 2). However, their binding affinities are at least an order of magnitude lower than those of the natural nucleotides. Interestingly, the -NH- and -CH2- analog exhibited the tightest binding (~10-fold tighter than natural dNTPs), despite polarity differences between the α,β-NH and α,β -CH2 groups. This pattern contrasts with the results obtained by O’Hagan for NADH-dependent glycerol 3-phosphate dehydrogenase with -CH2- and -CF2- phosphonate analogues of sn-glycerol-e-phosphate,37 which respectively had the same and 3.5-fold greater Km values than the natural substate. In a study by Berkowitz on substrate mimics for glucose 6-phosphate dehydrogenase, the Km of the α-CF2 analogue was an order of magnitude greater than the Km of glucose 6-phosphate, but that of the α-CH2 derivative was also higher (4-fold).38
Figure 2.
DNA pol β dissociation constants (Kd) for three natural dNTPs (dATP, dCTP, dTTP), for α,β-NH dUTP (10), the α,β-CH2 analogues of dATP and dGTP (11, 12), and the α,β-CF2 analogues of dATP, dCTP and dTTP (3, 6, 9). Kd values determined by single-turnover analysis.
Crystallization and analysis of α,β-modified dNTP analogues complexed with DNA primer and DNA pol β
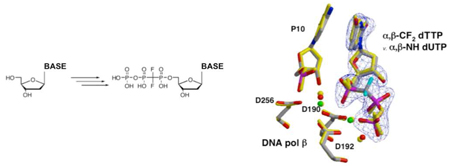
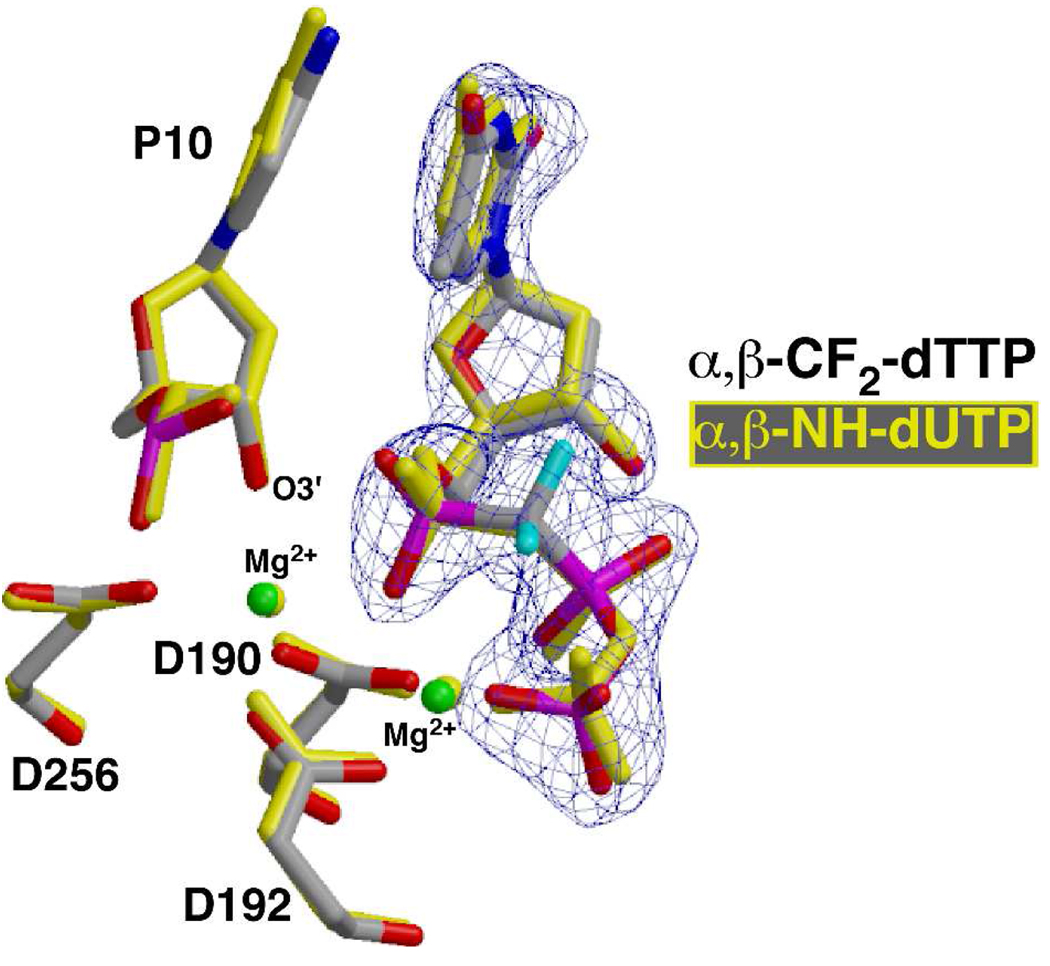
The crystal structure of the ternary complex of pol β with the incoming analog 9 opposite dA represents the precatalytic state of the nucleotidyl transfer reaction for correct incorporation, containing all atoms required for catalysis including the two catalytic metal ions. As expected, the substitution of the CF2 for the bridging oxygen prevented dissociation of the pyrophosphate leaving group, trapping the complex. All correlated atoms in the active site of this structure superimpose well with previously determined ternary complex structures of pol β where the reaction was trapped by deletion of the nucleophilic 3’-OH on the primer terminus39 or by using a nitrogen in place of the α,β bridging oxygen1,4,6 (Figure 3).
Figure 3.
Superposition of the active site of the ternary substrate complex of pol β with incoming 9, α,β-CF2-dTTP (gray carbons) and the corresponding ternary complex with 10, α,β-NH-dUTP (yellow atoms; pdb ID 2FMS). Active site carboxylates (D190, D192, D256) that coordinate two magnesium ions are shown as well as the 3’-OH of the primer terminus (P10). The fluorine atoms of 9 are cyan. Electron density for the 2Fo-Fc simulated annealing map (blue) is contoured to 5s illustrating the high quality of the electron density.
In summary, we have synthesized a series of α,β-CF2 dNTP analogues. Two of these, 3 and 6, were synthesized by an advantageous chemical-enzymatic method that was used to obtain the previously unavailable dCTP analog, 6.40 The isolation method provides the inhibitors essentially free of contaminating ‘dNTP’ activity. Kd values for these and comparable analogues, combined with an X-ray crystallographic study of 9 bound into a pol β ternary complex indicates that CXY modification in the P α-O-Pβ bridging position of dNTPs where X,Y = F,F or H,H can modulate binding properties over a wide range of Kd while substantially retaining a dNTP-like configuration.
Supplementary Material
Acknowledgment
We thank Dr. Ron New (Mass Spectrometry Facility, University of California, Riverside) for assistance in obtaining high resolution mass spectra of nucleotide analogues, Ms. Keriann Oertell (University of Southern California) for verifying the DNA synthesis background activity in 9 after dual-HPLC purification and Prof. George L. Kenyon (University of Michigan) for helpful discussions. This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences and by NIH program project grant 5-U19-CA105010.
Footnotes
Supporting Information Available General methods used; detailed synthetic procedures and characterization data for 1 – 9; analytical and preparative HPLC methods; HPLC studies of conversions of 1 to 2, 2 to 3, 7 to 4, 4 to 5, 5 to 6; 1H NMR spectra of 1–7 and 9; 31P and 19F NMR spectra of 2, 3, 5, 6, 9; 13C NMR spectrum of 9; analytical HPLC traces for 3, 6 and 9; HRMS spectra for 3 and 6, and LRMS spectrum for 9; DNA synthesis gel analysis of 9 before and after HPLC purification; protocol and inhibition plot for inhibition of pol β dCTP incorporation by 6; crystallographic methods and statistics for the ternary pol β complex with 9; and supplementary literature references are available online at http://pubs.acs.org.
References
- 1.Beard WA, Wilson SH. Chem. Rev. 2006;106:361–382. doi: 10.1021/cr0404904. [DOI] [PubMed] [Google Scholar]
- 2.Ma QF, Kenyon GL, Markham GD. Biochemistry. 1990;29:1412–1416. doi: 10.1021/bi00458a011. [DOI] [PubMed] [Google Scholar]
- 3.Ma QF, Bathurst IC, Barr PJ, Kenyon GL. J. Med. Chem. 1992;35:1938–1941. doi: 10.1021/jm00089a002. [DOI] [PubMed] [Google Scholar]
- 4.Barrie SE, Paine RM, Stock JA, Vincent RB, Harrap KR. Adv. Exp. Med. Biol. 1984;165:371–374. doi: 10.1007/978-1-4757-0390-0_70. [DOI] [PubMed] [Google Scholar]
- 5.Wang G, Boyle N, Chen F, Rajappan V, Fagan P, Brooks JL, Hurd T, Leeds JM, Rajwanshi VK, Jin Y, Prhavc M, Bruice TW, Cook PD. J. Med. Chem. 2004;47:6902–6913. doi: 10.1021/jm040116w. [DOI] [PubMed] [Google Scholar]
- 6.Batra VK, Beard WA, Shock DD, Krahn JM, Pedersen LC, Wilson SH. Structure. 2006;14:757–766. doi: 10.1016/j.str.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Sucato CA, Upton TG, Kashemirov BA, Batra VK, Martinek V, Xiang Y, Beard WA, Pedersen LC, Wilson SH, McKenna CE, Florian J, Warshel A, Goodman MF. Biochemistry. 2007;46:461–471. doi: 10.1021/bi061517b. [DOI] [PubMed] [Google Scholar]; b) Sucato CA, Upton TG, Kashemirov BA, Osuna J, Oertell K, Beard WA, Wilson SH, Florian J, Warshel A, McKenna CE, Goodman MF. Biochemistry. 2008;47:870–879. doi: 10.1021/bi7014162. [DOI] [PubMed] [Google Scholar]
- 8.McKenna CE, Shen P-D. J. Org. Chem. 1981;46:4573–4576. [Google Scholar]
- 9.Blackburn GM, England DA, Kolkmann F. J. Chem. Soc., Chem. Commun. 1981:930–932. [Google Scholar]
- 10.McKenna CE, Kashemirov B, Blazewska KM. Science of Synthesis Houben-Weyl Methods of Molecular Transformations, 42. In: Mathey F, editor. Phosphoric acids and derivatives. Stuttgart: Georg Thieme Verlag; 2009. pp. 779–921. [Google Scholar]
- 11.Davisson VJ, Davis DR, Dixit VM, Poulter CD. J. Org. Chem. 1987;52:1794–1801. [Google Scholar]
- 12.Arabshahi L, Khan NN, Butler M, Noonan T, Brown NC, Wright GE. Biochemistry. 1990;29:6820–6826. doi: 10.1021/bi00481a010. [DOI] [PubMed] [Google Scholar]
- 13.Blackburn GM, Langston SP. Tetrahedron Lett. 1991;32:6425–6428. [Google Scholar]
- 14.Spelta V, Mekhalfia A, Rejman D, Thompson M, Blackburn GM, North RA. Br. J. Pharmacol. 2003;140:1027–1034. doi: 10.1038/sj.bjp.0705531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKenna CE, Kashemirov BA, Roze CN. Bioorg. Chem. 2002;30:383–395. doi: 10.1016/s0045-2068(02)00521-7. [DOI] [PubMed] [Google Scholar]
- 16.Kalek M, Jemielity J, Stepinski J, Stolarski R, Darzynkiewicz E. Tetrahedron Lett. 2005;46:2417–2421. [Google Scholar]
- 17.Otmar M, Masojfdkova M, Votruba I, Holy A. Collect. Czech. Chem. Commun. 2001;66:500–506. [Google Scholar]
- 18.Hoard DE, Ott DG. J. Am. Chem. Soc. 1965;87:1785–1788. doi: 10.1021/ja01086a031. [DOI] [PubMed] [Google Scholar]
- 19.Li R, Muscate A, Kenyon GL. Bioorg. Chem. 1996;24:251–261. [Google Scholar]
- 20.a) Wu W, Bergstrom DE, Davisson VJ. J. Org. Chem. 2003;68:3860–3865. doi: 10.1021/jo020745i. [DOI] [PubMed] [Google Scholar]; b) Rezende MC, Vallejos G, Osorio-Olivares M, Sepulveda-Boza S. Synth. Commun. 2001;31:3699–3705. [Google Scholar]
- 21.Rao NS, Kumar P, Chauhan VK, Garg BS, Gupta KC. Nucleos. Nucleot. Nucl. 2002;21:393–400. doi: 10.1081/NCN-120006833. [DOI] [PubMed] [Google Scholar]
- 22.McKenna CE, Higa MT, Cheung NH, McKenna M-C. Tetrahedron Lett. 1977:155–158. [Google Scholar]
- 23.Marma MS, Khawli LA, Harutunian V, Kashemirov BA, McKenna CE. J. Fluorine Chem. 2005;126:1467–1475. [Google Scholar]
- 24.Blackburn GM, Kent DE, Kolkmann F. J. Chem. Soc., Chem. Commun. 1981:1188–1190. [Google Scholar]
- 25.Victorova L, Sosunov V, Skoblov A, Shipytsin A, Krayevsky A. FEBS Lett. 1999;453:6–10. doi: 10.1016/s0014-5793(99)00615-8. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton CJ, Roberts SM, Shipitsin A. Chem. Commun. 1998:1087–1088. [Google Scholar]
- 27.Shipitsin AV, Victorova LS, Shirokova EA, Dyatkina NB, Goryunova LE, Beabealashvilli RS, Hamilton CJ, Roberts SM, Krayevsky A. J. Chem. Soc., Perkin Trans. 1: Org. Bioorg. Chem. 1999:1039–1050. doi: 10.1080/15257779908041639. [DOI] [PubMed] [Google Scholar]
- 28.Martynov BI, Shirokova EA, Jasko MV, Victorova LS, Krayevsky AA. FEBS Lett. 1997;410:423–427. doi: 10.1016/s0014-5793(97)00577-2. [DOI] [PubMed] [Google Scholar]
- 29.Blackburn GM, Kent DE, Kolkmann F. J. Chem. Soc., Perkin Trans. 1: Org. Bioorg. Chem. 1984:1119–1125. [Google Scholar]
- 30.Moffatt JG, Khorana HG. J. Am. Chem. Soc. 1961;83:649–658. [Google Scholar]
- 31.a) Mohamady S, Jakeman DL. J. Org. Chem. 2005;70:10588–10591. doi: 10.1021/jo0518598. [DOI] [PubMed] [Google Scholar]; b) Marlow AL, Kiessling LL. Organic Letters. 2001;3:2517–2519. doi: 10.1021/ol016170d. [DOI] [PubMed] [Google Scholar]
- 32.Beard WA, Shock DD, Wilson SH. J. Biol. Chem. 2004;279:31921–31929. doi: 10.1074/jbc.M404016200. [DOI] [PubMed] [Google Scholar]
- 33.Radhakrishnan R, Arora K, Wang YL, Beard WA, Wilson SH, Schlick T. Biochemistry. 2006;45:15142–15156. doi: 10.1021/bi061353z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vande Berg BJ, Beard WA, Wilson SJ. J. Biol. Chem. 2001;276:3408–3416. doi: 10.1074/jbc.M002884200. [DOI] [PubMed] [Google Scholar]
- 35.Beard WA, Shock DD, Yang XP, DeLauder SF, Wilson SH. J. Biol. Chem. 2002;277:8235–8242. doi: 10.1074/jbc.M107286200. [DOI] [PubMed] [Google Scholar]
- 36.Batra VK, Beard WA, Shock DD, Pedersen LC, Wilson SH. Mol. Cell. 2008;30:315–324. doi: 10.1016/j.molcel.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieschalk J, Batsanov AS, OHagan D, Howard JAK. Tetrahedron. 1996;1:165–176. [Google Scholar]
- 38.Berkowitz DB, Bose M, Pfannenstiel TJ, Doukov T. J. Org. Chem. 2000;65:4498–4508. doi: 10.1021/jo000220v. [DOI] [PubMed] [Google Scholar]
- 39.Pelletier H, Sawaya MR, Kumar A, Wilson SH, Kraut J. Science. 1994;264:1891–1903. [PubMed] [Google Scholar]
- 40.Preliminary accounts of this work were presented byUpton TG, Kashemirov BA, McKenna CE, Goodman MF, Prakash GKS, Kultyshev R, Batra VK, Shock DD, Pedersen LC, Beard WA, Wilson SH. #P148, XVIIth International Conference on Phosphorus Chemistry Xiamen; China. 2007. Apr 15–19, and byUpton TJ, Osuna J, Kashemirov BA, McKenna CE. ORGN-278, 235th National Meeting of the American Society of the American Chemical Society; New Orleans: 2008. Apr 6–10,
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.