Abstract
Recent studies have demonstrated that plasma nitrite (NO2-) reflects endothelial nitric oxide synthase activity and it has been proposed as a prognostic marker for cardiovascular disease. In addition, NO2- itself has been shown to have biological activities thought to be triggered by reduction back to NO in blood and tissues. The development of sensitive and reproducible methods for the quantitative determination of plasma NO2- is, therefore, of great importance. Ozone-based chemiluminescence assays have been shown to be highly sensitive for the determination of nanomolar quantities of NO and NO related species in biological fluids. We report here an improved direct chemiluminescence method for the determination of plasma NO2- without interference of other nitric oxide related species such as nitrate, S-nitrosothiols, N-nitrosamines, nitrated proteins and nitrated lipids. The method involves a reaction system consisting of glacial acetic acid and ascorbic acid in the purge vessel of the NO analyzer. Under these acidic conditions NO2- is stoichiometrically reduced to NO by ascorbic acid. Fasting human plasma NO2- values were found in the range of 56-210 nM (mean =110 ± 36 nM). This method has high sensitivity with an accuracy of 97% and high precision (C.V <10%) for determination of plasma nitrite. The present method is simple and highly specific for plasma NO2-. It is particularly suited to evaluate vasculature endothelial NO production that predicts the risks for cardiovascular disease.
Keywords: Plasma, Nitrite, Nitric oxide, Chemiluminescence method, Ascorbic acid, Endothelial nitric oxide synthase
Introduction
Nitric oxide (NO) synthesized by endothelial nitric oxide synthase (eNOS) diffuses both into smooth muscle cells and blood circulation playing a crucial role in regulating blood flow and vascular homeostasis. Reduced NO synthesis in the endothelium is a strong risk factor for the development of atherogenesis, vascular thrombosis, reperfusion injury and hypertension. [1, 2]. The in vivo production of nitric oxide can not be determined directly because of the short life span of NO in blood milieu (less than 2 milliseconds) [3]. In blood, NO is used up predominantly by the reaction with erythrocytic hemoglobin producing nitrate. In addition, the interaction of NO with other blood components produces nanomolar levels of nitrite as well as S-nitrosothiols (RSNOs), N-nitrosamines (RNNOs), iron-nitrosyls, nitrated lipids and nitrated proteins. The predominant species of plasma nitrate and nitrite are being measured to assess the in vivo production of NO. Similarly nitrite and nitrate determinations are used in cell culture medium to assess NO production by cells [4].
The reliability of the nitrate measurement has been questioned, because nitrate levels are influenced by several NOS independent sources such as diet, intestinal bacteria, renal function and inhalation of atmospheric NO and NO2 gases. In addition to these factors, the expected small increase in nitrate attributed to a small change in eNOS activity is difficult to quantitate in the presence of the high background values of plasma nitrate (~ 25μM). For these reasons, recent studies designed to assess eNOS activity have utilized measurements of the nanomolar levels of plasma nitrite alone, instead of measuring both nitrate and nitrite [5-7].
Support for the use of nitrite alone to assess eNOS activity comes from studies that have shown [5, 8] [9]that stimulation of eNOS by acetylcholine or forearm exercise increases both forearm blood flow and plasma nitrite level. Similarly, inhibition of eNOS by L-NAME reduced the blood flow and serum nitrite levels in a dose dependent manner. A significant correlation has been observed between the changes in blood flow and serum nitrite concentration, but not with serum nitrate levels. Subsequent studies in humans, as well as animals (monkeys, dogs, rabbits, rats etc.) by the same group [6] concluded that two thirds of the basal plasma nitrite (100 nM to 600 nM) is derived from basal eNOS activity.
Recently, several reports suggest that nitrite plays an important role in vasodilation under hypoxic conditions by being converted back to NO by erythrocytic deoxyhemoglobin [10-14]. Reduction of nitrite to NO in vivo has also been attributed to xanthine oxidase [15], mitochondria, endoplasmic reticulum [16, 17]and under cellular acidosis that occurs during ischemic conditions [18]. The emerging roles of nitrite in biology were summarized in the recent report of a meeting on nitrite held at the National Institutes of Health [12].
Several methods are available in the literature to determine nitrite and nitrate accurately in human biological fluids (see review [19]). The most popular and simple method for the determination of nitrite is the colorimetric method using the Griess reagent [20]. This method is, however, not sensitive enough to measure the physiological level of nitrite that is expected to be < 1μM. The chemiluminescence method is one of the highly sensitive methods to determine NO in nanomolar concentrations. Initially, the KI reductive chemiluminescence assay under acidic conditions was employed to determine nitrite in biological fluids [21, 22]. Subsequent reports have shown that this reagent also releases NO from S-nitrosothiols to various extents depending on the type of nitrosothiol [23]. This method has been further modified by adding iodine crystals (I2) and/or Cu(I) to KI to efficiently measure both S-nitrosothiols and nitrite. Later studies have shown that KI/I2 reagent also releases NO from N-nitrosamines. In order to determine the concentration of an individual species, the samples are subjected, to various pretreatments such as acidified sulfanilamide to remove nitrite or mercuric chloride to degrade S-nitrosothiols (RNNOs are stable) before injection into the purge vessel. It is then necessary to subtract readings obtained after different pretreatments to obtain determinations of a single nitrite related species. No direct method to determine plasma nitrite has, however, been previously published.
In this paper, we present a direct reductive chemiluminescence method to determine the plasma nitrite without interference of nitrate, RSNOs, RNNOs and nitrated lipids or proteins.
Materials and methods
Ascorbic acid, sulfanilamide, sodium nitrite, diethylenetriaminepentaacetate (DTPA), albumin, glutathione, cysteine, N-ethyl maleimide (NEM), N-nitrosodimethylamine, N-nitroso-N-methylurea and nitrated-BSA were obtained from Sigma-Aldrich Chemicals. 10-nitrooleate and nitrotyrosine were obtained from Caymen Chemical Company. Minimum essential medium (MEM) and fetal calf serum was obtained from Sigma-Aldrich Chemical Company. Sulfanilamide (200 mM) was prepared in 87.5% glacial acetic acid. All reagents were prepared with fresh milli-Q water with a conductivity of <18.2 MΩ cm-1.
Blood was obtained from 8 healthy human volunteers (age: 35 to 65 years) by the approval of the Institutional Tissue Procurement Protocol (No.NIA-2003-071). Venous blood was drawn from overnight fasting subjects into heparin vacutainers and centrifuged in less than 30 sec at 5800 RPM for 3 min using a portable Labnet bench-top centrifuge type Z150A (Labnet International, Inc). 1 ml clear plasma that is free from hemolysis was transferred into dark colored microtubes that contained 0.1 mM DTPA and 6.25 mM NEM. The nitrite was determined either immediately or kept at -150°C for future analysis. We found that EDTA vacutainers contain 50 nM to 200 nM nitrite and only heparin vacutainers, which contain < 1 nM nitrite, were used. One needs to check the nitrite contamination in every reagent and glassware. The problems and pitfalls involved in measurement of nitrite in biological samples have been discussed in recent review [24].
Determination of nitrite by chemiluminescence method
The nitric oxide was determined by a gas phase chemiluminescence reaction of NO with ozone using a Sievers Nitric Oxide Analyzer (NOA) model 280i (GE Analytical Instruments). NO related species (nitrite, nitrate, RSNO etc) are converted to NO in the purge vessel of the analyzer. The released NO is carried by inert gas to the detector where it reacts with ozone to produce a chemiluminescence signal proportional to the concentration [25].
Conversion of nitrite to NO in the purge vessel
The injection of nitrite (20 pmoles) into the purge vessel that contained only 87.5% glacial acetic acid resulted in no chemiluminescence signal (Fig. 1). A chemiluminescence signal was readily produced when nitrite was injected into glacial acetic acid that contained 50 mM ascorbic acid. This signal was almost completely suppressed by sulfanilamide (200mM), a scavenger of nitrite under acidic conditions.
Fig.1.
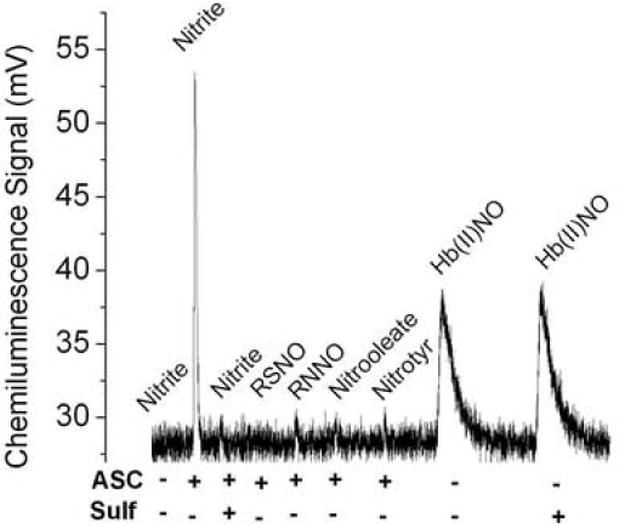
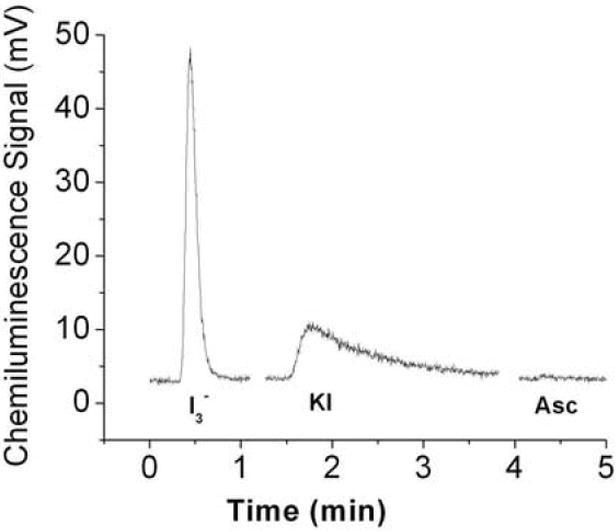
A) Chemiluminescence signals obtained under acidic conditions. The purge vessel contained 87.5% glacial acetic acid in absence and presence of 50 mM ascorbic acid and/or 200mM sulfanilamide in a total volume of 8 ml. Samples injected into the purge vessel were: 20 pmoles nitrite, 100 pmoles of each RSNO (GSNO or albumin-SNO), RNNO (N-nitrosodimethylamine or N-nitroso-N-methylurea), nitrooleate, nitrotyr (nitrotyrosine) or Hb(II)NO (iron nitrosylhemoglobin). ASC indicates the addition of ascorbic acid; Sulf indicates the addition of sulfanilamide. B) Release of NO from S- nitrosoalbumin by KI or I3- or ascorbate in glacial acetic acid. The reaction mixture for I3- chemiluminescence assay consisted of 7 ml glacial acetic acid, 0.5 ml (1M) KI solution and 0.5 ml (0.35 M) I2 solution. The reaction mixture for KI assay consisted of 7 ml glacial acetic acid and 1 ml (0.5 M) KI solution. The reaction mixture for ascorbate consisted of 7 ml glacial acetic acid and 1 ml (0.5 M) ascorbate solution. 50 pmoles S-nitrosoalbumin was injected into the purge vessel.
Detection of other NO related compounds by glacial acetic acid/ascorbate method (see above)
No chemiluminescence signal was produced when S-nitrosothiols (S-nitrosoglutathione (100 pmoles) and SNO-albumin (100 pmoles)), N-nitrosamines (N-nitrosodimethylamine (100 pmoles) and N-nitroso-N-methylurea (100 pmoles)), nitrated lipid (10-nitrooleate) and nitrated amino acid (3-nitro-L-tyrosine) were injected into the purge vessel that contained glacial acetic acid and ascorbic acid (Fig. 1A). Unpurified nitrated albumin gave a chemiluminescence signal when injected into the purge vessel (data not shown). This was, however, due to the presence of nitrite impurities, which were eliminated when the sample was passed through a Sephadex G-25 column or pre-treated with acidified sulfanilamide.
Nitric oxide was, however, slowly released from metal nitrosylated species (iron nitrosylhemoglobin) in glacial acetic acid. This release of NO did not require ascorbic acid. However, while sulfanilamide prevents the release of NO from nitrite, the presence of sulfanilamide in 87.5% glacial acetic acid did not affect the release of nitric oxide from iron nitrosylhemoglobin (Hb(II)NO) (Fig. 1A).
Nitric oxide release from SNO-albumin by different chemiluminescent assays
S-nitrosoalbumin, pretreated with acidified sulfanilamide to remove any nitrite contamination, was injected into the purge vessel that contained one of the following different reagent systems. (1) 7 ml glacial acetic acid and1 ml 0. 5 M KI; (2) 7 ml glacial acetic acid , 0.5 ml 1M KI and 0.5 ml 0.35 M I2; (3) 7 ml glacial acetic acid and 1 ml of 0.5 M ascorbate. The formation of chemiluminescence signals were recorded for each injection.
Ascorbate concentration dependent nitrite reduction in glacial acetic acid
25 pmoles nitrite in water or 25 pmoles nitrite in100 μl plasma was injected into glacial acetic acid (7 ml) that contained varying concentrations (0 to 60 mM) of ascorbic acid in a total volume of 8ml. The formation of chemiluminescence signals were monitored at each concentration of ascorbic acid.
Preparation of nitrite standard curve
A 10.24 mM stock solution of sodium nitrite was prepared in 1 mM sodium hydroxide (NaOH). A working standard was prepared by diluting the stock solution to 10.24 μM. This stock solution was serially diluted 2-fold (1:2, 1:4, 1:8… 1:512) with milli Q water to obtain series of concentrations down to 10 nM. The purge vessel contained 7.0 ml glacial acetic acid and 1.0 ml of 0.5M ascorbic acid. The temperature of the purge vessel was set at 37°C. Antifoam reagent is not needed in acetic acid. The NaOH trap (15 ml) was connected between the purge vessel and detector. 100 μl of diluted standard solutions were injected into the bottom of the purge vessel using a Hamilton syringe that is fitted with a 5 inches long needle and the chemiluminescence signals were recorded. The data were transferred to the Origin program 6.1 (OriginLab Corporation, One Roundhouse Plaza, Suite 303, and Northampton, MA 01060 USA) to determine the area under the curve for each signal. Nitrite standard curves were constructed by plotting the average area versus the concentration. The nitrite standard curve prepared by this method was also compared with the I3- reductive chemiluminescence method as described earlier [26] [27].
Measurement of Plasma nitrite
Plasma was thawed just prior to the determination, while being protected from light. 50 μl to 100 μl fresh or thawed plasma aliquots were injected into the NOA purge vessel that contained 8 ml of 87.5% glacial acetic acid or 7 ml glacial acetic acid and 1 ml of 0.5 M ascorbic acid at 37°C. Even with plasma, antifoam reagent is not required to prevent foaming in glacial acetic acid. For each sample an average of four determinations was used. To verify that the signal is from nitrite, 7 ml of 200 mM sulfanilamide (nitrite chelator under acidic conditions) in glacial acetic acid was used in the purge vessel instead of 7 ml glacial acetic acid. Data were transferred to the Origin program and smoothened using adjacent averaging of 20 points. The chemiluminescence signals were regenerated using the smoothened data. The area under the curve was determined using the method provided with the software.
The following precautions need to be taken for reproducible results. 1) Maintain constant cell pressure by adjusting the inert gas flow. 2) Make sure that there is no gas leak in the purge system. 3) Inject the sample at the bottom of the purge vessel using a syringe with a 5” long needle. 4) When plasma samples are injecting, provide a gap between the duplicates until the gas bubbles are regenerated in the purge vessel.
Time course of nitrite consumption in whole blood
Nitrite (1μM) was added to freshly collected venous blood in a vacutainer and kept on ice. One ml aliquots of this blood were centrifuged at 5000 RPM for 3 min at various time points followed by the determination of plasma nitrite. The initial value of plasma nitrite was assumed to be 100%.
Recovery and precision of assays
Clear plasma was incubated with NEM (6.25 mM) and DTPA (0.1 mM) for 30 min at room temperature to block the free sulfhydryl groups. The basal level of nitrite was determined. Then 100, 250 or 500 nM nitrite was added to the plasma. Plasma was stored at -150°C in different aliquots. The recovery of plasma nitrite was measured 6 different times over a three month period. 100 μl of plasma was used to determine the nitrite as mentioned above.
The recovery of nitrite from MEM medium with 20% fetal calf serum was also tested. 500 nM nitrite and 0.1mM DTPA were incubated with MEM cell culture medium for 60 min followed by the determination of the nitrite
The recovery was calculated using the formula.
%R = percent recovery, Y= measured value X = true value.
Precision is quantified using the coefficient of variation (C.V). Percent coefficient variation for the nitrite assay was calculated using the following equation.
Results
Reduction of nitrite to NO
Nitrite is known to be protonated under acidic conditions to form HNO2. The pKa of HNO2 is 3.4. At pH < 3, nitrite is expected to form NO through a disproportionation reaction of N2O3 [28]. Although the pH of 87.5% glacial acetic acid is less than 1, nitrite did not show any chemiluminescence signal in glacial acetic acid (Fig 1A). The reducing agent, ascorbic acid, is required, even in glacial acetic acid, for the reduction of nitrite to NO (Fig.1A). Interestingly, other nitric oxide species such as RSNOs, RNNOs, nitrated lipid and nitrated protein did not show any signals in the glacial acetic and ascorbic acid reagent system. However, NO is slowly released from iron nitrosylhemoglobin in glacial acetic acid. This release did not require the reducing action of ascorbic acid with the same results obtained with or without the addition of ascorbic acid. This is explained by the fact that nitrosylhemoglobin has NO bound and reduction is not necessary. The acidic condition in this case reduces the half-life of heme bound NO. The release of NO from iron nitrosylhemoglobin was also not suppressed by sulfanilamide (Fig.1A).
Comparison of S-nitrosylated albumin reduction to NO by different chemiliminescence assays
S-nitrosated albumin is a predominant RSNO in plasma. Figure 1B compares the release of NO from S-nitrosoalbumin by various reductive chemiluminescence assays. As reported KI/I2 reagent readily releases NO from S-nitrosoalbumin. KI/acetic acid also releases NO from S-nitrosoalbumin, although much less efficiently than in the KI/I2/acetic acid reagent system, whereas ascorbate/acetic acid does not release any NO from S-nitrosoalbumin.
Ascorbate concentration dependent reduction of nitrite to NO
As shown in figure 2, the reduction of nitrite to nitric oxide in glacial acetic acid depends on the concentration of ascorbic acid. Nitrite (25 pmoles) was completely reduced to NO with 10 mM ascorbic acid. Reduction of the same amount of nitrite in plasma was also investigated (data not shown), indicating that more ascorbic acid is needed for plasma samples containing a high concentration of protein. Consistent reproducible sharp peaks were obtained at 60 mM ascorbic acid, which was subsequently used for the plasma nitrite determination.
Fig. 2.
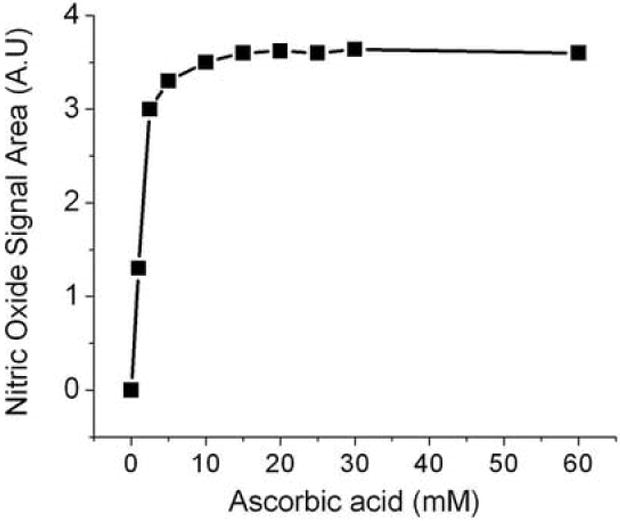
Ascorbate concentration dependent nitrite reduction to NO under acidic conditions. The reaction mixture contained 7 ml of glacial acetic acid and varying concentration of ascorbic acid in a total volume of 8 ml. 25 pmoles nitrite was injected into the purge vessel. A.U, Arbitrary Units.
Nitrite standards
Figure 3A shows a concentration dependent increase in the intensity of the NO chemiluminescence signals when standard nitrite was injected into the glacial acetic acid-ascorbic acid reaction mixture. The inset in figure 3A shows a linear standard curve was obtained over the range of 0.01 μM to 5 μM. In figure 3B is shown the calibration curve that had a slope of 0.0152 with a correlation coefficient of 0.999, N=6, p< 0.001 for the <1000 nM nitrite concentration range present in plasma. The sensitivity of the assay is typically less than 10 nM or 1 pmole.
Fig.3.
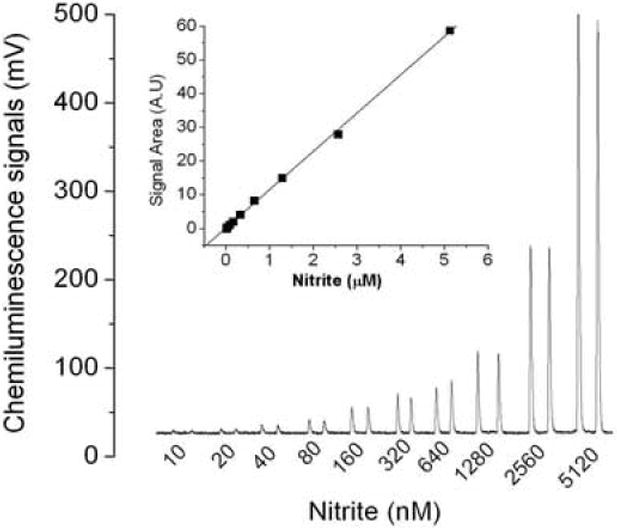
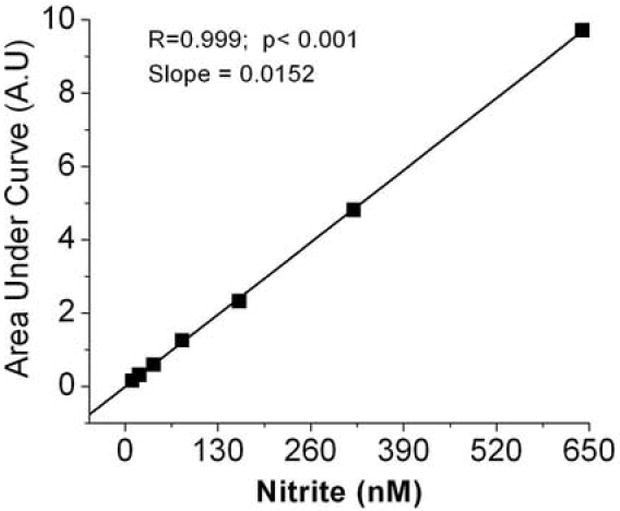
Chemiluminescence signals of nitrite and standard curve. (A) 100 μl of 10 nM to 5120 nM nitrite was injected into reaction mixture contained 7 ml glacial acetic acid and 1 ml (0.5 M) ascorbic acid solution (inset shows standard curve obtained by integrating the area under the curve using the Origin program) (B) Standard curve for nitrite from 10nM to 640 nM. The values are the mean of 3 experiments with duplicate determinations for each experiment.
Comparison of ascorbate/chemiluminescence method with I3- chemiluminescence method for determination of nitrite
The I3- reductive chemiluminescence assay is well validated to determine NO species in various biological fluids [26] [27]. As shown in figure 4, the reduction of standard nitrite to NO by our method was comparable with the I3- chemiluminescence method. The difference in the mean values between these two methods was less than 2%.
Fig.4.
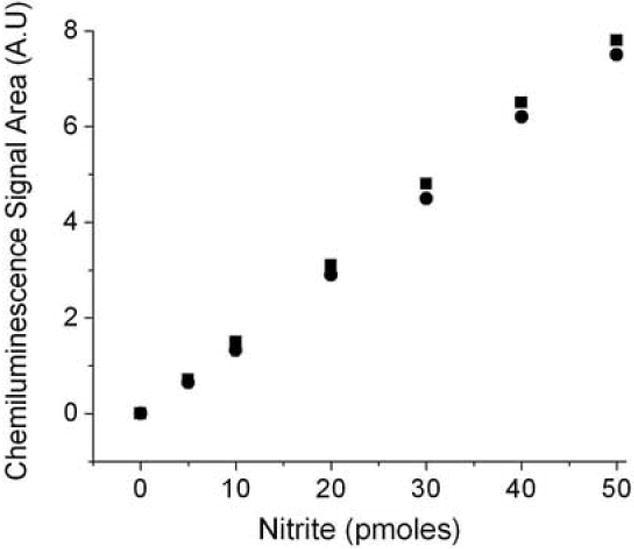
The reduction of nitrite to NO by the ASC reductive chemiluminescence assay is comparable with I3- reductive chemiluminescence assay. The reaction mixture for I3- (square) and ASC chemiluminescence assay (circle) was as mentioned in figure 1B. 10 μl to 250 μl of 0.2 μM nitrite that corresponds to 10 pmoles, 20 pmoles, 30 pmoles, 40 pmoles and 50 pmoles were injected into the purge vessel. Values are the mean of 3 experiments.
Chemiluminescence signals of plasma Nitrite
Standard nitrite did not show any signal in 87.5% acetic acid (Fig.1A). However, the direct injection of plasma into 87.5% glacial acetic acid showed a small NO signal (Fig.5). This signal was completely suppressed by sulfanilamide, which is known to form a complex with nitrite under acidic conditions. The signal observed without ascorbic acid in plasma can, therefore, be attributed to reducing agents in plasma that reduce the nitrite to NO. The higher intensity of the plasma signal in the presence of ascorbic acid is due to the quantitative reduction of nitrite to NO. About 95% of this signal was suppressed by sulfanilamide suggesting that this signal was formed from nitrite. The small signal still seen with sulfanilamide in the purge vessel can be attributed to competition between sulfanilamide and ascorbic acid both of which react with the same species (HNO2 or NO+) to form a stable complex (sulfanilamide) or to reduce the nitrite to NO (ascorbic acid). Pre-treatment of plasma with acidified sulfanilamide, prior to being exposed to ascorbic acid, completely suppressed the peak (data not shown). Our method detects the nitrite in plasma directly without applying any pretreatments and subtraction procedures unlike the I3- method (Fig.6).
Fig.5.
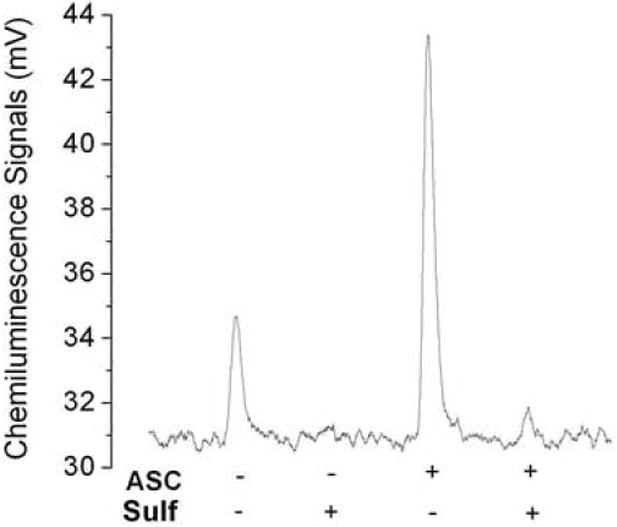
Chemiluminescence signals of plasma nitrite: Peak 1, 100 μl plasma was injected into 8 ml of 87.5% glacial acetic acid reagent. This peak was completely suppressed in the presence of 200mM sulfanilamide (sulfa) in 87.5% glacial acetic acid. Peak 2, 100 μl plasma was injected into7 ml glacial acetic acid and 1 ml (0.5M) ascorbic acid. This peak was almost completely suppressed in the presence of 200 mM sulfa in 87.5% glacial acetic acid and 1 ml (0.5M) ascorbic acid.
Fig.6.
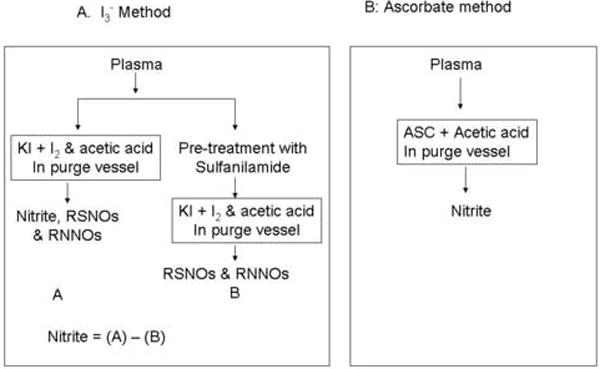
Flow diagram for determination of plasma nitrite using (A) I3- reductive chemiluminescence method and (B) ascorbate reductive chemiluminescence method.
Time course of nitrite consumption in whole blood
Nitrite is not stable in blood due to the reaction with erythrocytic hemoglobin. In order to know how fast the blood sample needs to be processed, the rate of nitrite consumption in whole blood was investigated (Fig. 7). The nitrite was consumed with a half-life of 40 min. In 30 sec only ~ 2% of the nitrite was consumed. The loss of nitrite within 30 sec was within the assay error. To obtain a reliable measure of in vivo nitrite the sample must be centrifuged and removed from erythrocytes within 30 sec after being collected.
Fig.7.
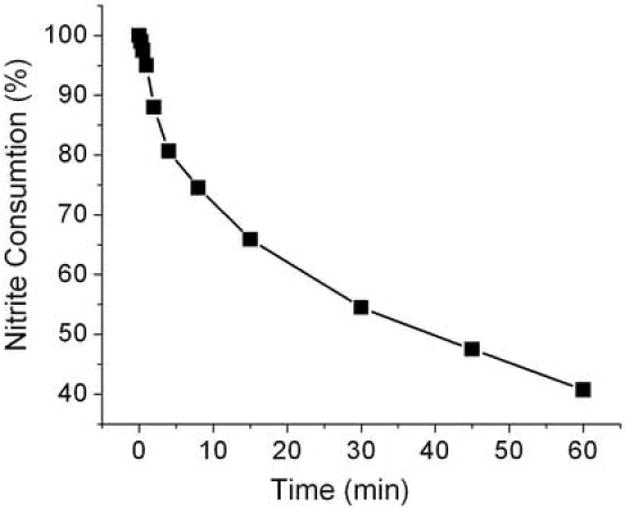
Nitrite consumption in blood: Nitrite (1μM) was added to venous blood and kept on ice. The first aliquot was centrifuged within 10 sec to separate plasma. This plasma nitrite value was considered as 100%. Additional aliquots were centrifuged at different times followed by a determination of the nitrite in the supernatant.
Plasma nitrite in human subjects
Figure 8 shows the plasma nitrite levels of fasting healthy human subjects. The nitrite content for all subjects was in the range of 55 nM to 210 nM with an average value of 110 nM ± 36. There was no difference between the values of plasma nitrite determined immediately after collection of blood and samples frozen (-85 °C) for a 3 months period.
Fig.8.
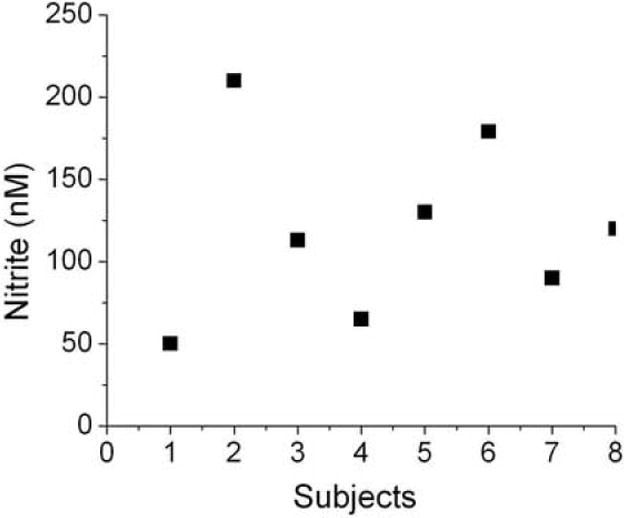
Nitrite levels in human plasma: 100μl plasma was injected into the purge vessel that contained 7 ml of glacial acetic acid and 1 ml of 0.5 M ascorbic acid at 37 °C. Values are the mean of quadruplicates for each sample.
Recovery of nitrite in human plasma
Table 1 shows the accuracy and precision of the nitrite assay. Accuracy is defined as the closeness of agreement between true values to reference values. The average mean recovery that represents the accuracy was 96.4% for 100 nM, 95.7% for 250 nM and 99% for 500 nM. Precision is usually expressed in terms of the deviation of results from the arithmetic mean of the set. Precision is quantified using the coefficient of variation (C.V). The lowest percent C.V is 8.1 and the highest is 10.4. 100 % of nitrite added was recovered from cell culture media that contains phenol red and 20% fetal calf serum (Data not shown).
Table 1.
Recovery and coefficient variation of ascorbate reductive chemiluminescence assay for plasma nitrite determination.
| S.NO | Nitrite added (nM) | Recovery of Nitrite (nM) Mean± SD | Accuracy (%) | Coefficient Variation (%) |
|---|---|---|---|---|
| 1 | 100 | 96.4+ 8.5 | 96.4 | 8.8 |
| 2 | 250 | 239.4 + 25 | 95.7 | 10.4 |
| 3 | 500 | 495 + 40 | 99.0 | 8.1 |
Plasma samples were spiked with 100, 250 and 500 nM nitrite and stored at -150 °C in different aliquots. Nitrite was analyzed in six different times over the period of three months.
Discussion
Nitric oxide that diffuses into blood from the endothelium has a short half-life <2milliseconds [3], and is converted to various species such as nitrite, nitrate, RSNOs, RNNOs, iron-nitrosyls, nitrated proteins and nitrated lipids. Endothelial nitric oxide synthase (eNOS) activity was originally estimated based on determinations of plasma nitrite and nitrate. However, recent studies suggest that plasma nitrite alone provides a more reliable measure of eNOS activity [5-8, 29]. There has recently been a great deal of interest directed at reliable determinations for plasma nitrite, because it not only reflects eNOS activity but also possess a vasorelaxant activity independent of eNOS [11] [30]. Nitrite is present in plasma in nanomolar concentrations making reliable measurements very challenging. This is further complicated by the reactions of nitrite with erythrocytic hemoglobin requiring rapid centrifugation to separate plasma from the erythrocytes.
The chemiluminescence method is one of the most sensitive methods to measure low nanomolar concentrations of NO. Several chemiluminescence methods have been developed to determine nitrite by converting the nitrite into NO in the chemiluminescence purge vessel. The released NO is then carried into the detector by inert gas to react with ozone. The original chemiluminescence method for the determination of nitrite was developed by Cox [21] and Garside [22] using glacial acetic acid and KI to reduce nitrite to nitric oxide. This method reduces nitrite, but not nitrate, providing a method to determine nitrite in the presence of the large excess of nitrate found in plasma. Studies by others [23] and confirmed by us (Fig.1B) show that KI under acidic conditions also slowly releases NO from RSNOs. This method was in fact modified to efficiently release NO from nitrosothiols by adding iodine crystals to KI to form the stronger reducing agent I3- [23]. The disadvantage of this method for the determination of nitrite is that it measures both nitrite and S-nitrosothiols. Marley et al [31] measured plasma RSNOs using Cu(1)/ KI/I2 reagents after removing the nitrite with acidified sulfanilamide, which forms a diazonium complex that does not generate a chemiluminescence signal. Subsequent studies [32] have shown that the I3- reductive chemiluminescence assay also releases NO from RNNOs of plasma. It is not known whether the I3- reagent releases NO from plasma nitrated lipids and proteins.
To determine nitrite by the I3- reductive chemiluminescence assay, one aliquot of plasma is injected directly into the I3- reagent measuring nitrite, RSNOs and RNNOs and another aliquot is pretreated with acidified sulfanilamide and then injected into the I3- reagent measuring RSNO and RNNOs. The nitrite value is obtained by subtraction of the sample value pretreated with sulfanilamide from the non-treated sample [26] [27] (Fig. 6). These methods for the determination are not direct requiring separate processing and subtractions of results obtained by different determinations.
In this investigation, we have developed a direct chemiluminescence method without processing and without interference of other nitric oxide species using ascorbic acid and glacial acetic acid reagent in the purge vessel (Fig 6). In glacial acetic acid with a pH <1 nitrite becomes free nitrous acid. In this highly acidic solution the nitrous acid can be further protonated producing H2NO2+, which is in equilibrium with the nitrosonium cation (NO+). Cox [33] had shown that even in acetic acid, NO is not
| (1) |
| (2) |
released unless a reducing agent is present. We also find no nitric oxide detected by chemiluminescence when nitrite was injected into acetic acid (Fig.1A). Nitrite is, therefore, determined by the use of a reducing agent, which will specifically reduce the acidified nitrite and not other nitric oxide related species. Ascorbic acid readily reduces nitrite to NO under acidic conditions, while nitrate, RSNOs, RNNOs, nitrated lipids and nitrated proteins remain intact in this reagent system (Fig.1A). The following reactions may be responsible for the formation of NO in the presence of ascorbic acid (equations 3 to 5).
| (3) |
| (4) |
| (5) |
The formation of NO is dependent on the concentration of ascorbic acid. Sulfanilamide is also known to react with nitrous acid and/or NO+ to produce a diazonium salt [20]. It, therefore, competes with ascorbic acid inhibiting the reduction to NO by ascorbic acid (Figs.1&5). The inhibition by sulfanilamide further confirms that ascorbic acid is specifically reducing nitrous acid and NO+ to NO.
Although the acetic acid /ascorbate system does not release NO from nitrosothiols, Cu+2 present in the reagents used and/or in the samples can releases NO from S- nitrosothiols. The glacial acetic acid and ascorbic acid in our studies did not have a sufficient amount of Cu+2 to release NO from S- nitrosothiols (Fig.1A&B). However, plasma contains trace levels of copper that can release NO from some of the nitrosothiols present. Thus DTPA was included in the plasma samples to chelate any Cu+2 ions present.
The reduction of nitrite to nitric oxide is stoichiometric and linear over the range of 10nM to 5μM. This method has a high sensitivity to be able to detect concentrations of nitrite in plasma as low as10 nM with a high recovery (for three concentrations a mean of 96.7%) and good precision (CV <10%) in inter assays. This method can also be used to determine nitrite in cell culture media even in presence of phenol red, which interferes in the colorimetric Griess method.
Although nitrite does not give any signal (Fig 1), without added ascorbic acid, plasma shows a small signal in glacial acetic acid without ascorbic acid (Fig. 5). As shown in figure 1, NO is released from iron nitrosylhemoglobin even without ascorbic acid. Recent in vitro and in vivo (NO gas inhalation) studies suggests that the haptoglobin-hemoglobin complex can scavenge NO in blood to form a heme nitrosylated complex [34]. The presence of this complex in plasma can account for the glacial acetic acid signal in plasma without added ascorbate (Fig. 5). However, the inhibition of the plasma signal (Fig. 5) by sulfanilamide, which does not affect the release of NO from iron nitrosylhemoglobin (Fig.1A), rules out this possibility. This signal is, therefore, presumably due to the presence in plasma of reducing agents such as ascorbic acid protein sulfhydryl groups, uric acid, and dietary phenolic acids that can partially reduce the plasma nitrite under acidic conditions.
Plasma nitrite is completely reduced to NO in glacial acetic acid in the presence of excess ascorbic acid (Fig 5). This signal was almost completely attenuated by sulfanilamide conforming that the chemiluminescence signal originates from nitrite.
The plasma concentration of nitrite has been found to be in the range of 56 nM to 210 nM with the average value of 110 ± 36 nM, which is less than that of earlier reported values in the range of 150 nm to 1000 nM [8] [6]. Our values of plasma nitrite are, however, comparable with the most recently reported values [24]. A recent report suggests that red blood cells also contain a significant amount of nitrite [35]. Our method is, however, not suitable to determine nitrite in red cells.
The plasma NO pool that consists of nitrite, S-nitrosothiols and iron-nitrosyls levels has been shown to be less in individuals with cardiovascular risk factors than corresponding control individuals [36]. It has also been reported that the plasma nitrite increase in response to hyperemia of the forearm is attenuated in patients with cardiovascular dysfunction [30]. Similarly, the increase in plasma nitrite levels in response to exercise is less in healthy older subjects than in younger individuals [37]. These findings are attributed to endothelial dysfunction which impairs eNOS production of NO. The measurement of plasma nitrite may, therefore, represent a valuable parameter to assess endothelial dysfunction.
In summary, our method determines plasma nitrite directly without processing the sample and without interference of other nitric oxide species such as RSNOs, RNNOs, nitrated lipids and nitrated proteins that are present in plasma. Moreover, this method is very simple, minimizes the contamination of nitrite that comes from filtrations, column chromatography and pretreatment of samples with various reagents to remove the proteins or to discriminate individual species. The sample must, however, be collected in nitrite free tubes (heparin tubes) and centrifuged immediately after collection. This assay would be suitable in basic research and clinical research to use in evaluating endothelium dysfunction during aging and under pathological conditions.
Acknowledgments
Grant Support: This research was supported by the Intramural Research Program of the NIH, National Institute on Aging
Abbreviations
- ASC
Ascorbic acid
- A.U
Arbitrary Units
- C.V
Coefficient variation
- eNOS
Endothelial nitric oxide synthase
- NO
Nitric oxide
- HNO2
Nitrous acid
- NO+
Nitrosonium cation
- KI
Potassium iodide
- I2
Iodine
- I3-
Triiodide
- RNNOs
N-nitrosamines
- RSNOs
S-nitrosothiols
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marin J, Rodriguez-Martinez MA. Role of vascular nitric oxide in physiological and pathological conditions. Pharmacol Ther. 1997;75:111–134. doi: 10.1016/s0163-7258(97)00051-x. [DOI] [PubMed] [Google Scholar]
- 2.Vanhoutte PM. Endothelial dysfunction and atherosclerosis. Eur Heart J. 1997;18(Suppl E):E19–29. doi: 10.1016/s0195-668x(97)90005-1. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Miller MJ, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR., Jr Diffusion-limited reaction of free nitric oxide with erythrocytes. J Biol Chem. 1998;273:18709–18713. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 4.Babaei S, Stewart DJ. Overexpression of endothelial NO synthase induces angiogenesis in a co-culture model. Cardiovasc Res. 2002;55:190–200. doi: 10.1016/s0008-6363(02)00287-0. [DOI] [PubMed] [Google Scholar]
- 5.Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci U S A. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 7.Allen JD, Cobb FR, Gow AJ. Regional and whole-body markers of nitric oxide production following hyperemic stimuli. Free Radic Biol Med. 2005;38:1164–1169. doi: 10.1016/j.freeradbiomed.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Kelm M, Preik-Steinhoff H, Preik M, Strauer BE. Serum nitrite sensitively reflects endothelial NO formation in human forearm vasculature: evidence for biochemical assessment of the endothelial L-arginine-NO pathway. Cardiovasc Res. 1999;41:765–772. doi: 10.1016/s0008-6363(98)00259-4. [DOI] [PubMed] [Google Scholar]
- 9.Gladwin MT, Shelhamer JH, Schechter AN, Pease-Fye ME, Waclawiw MA, Panza JA, Ognibene FP, Cannon RO., 3rd Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Natl Acad Sci U S A. 2000;97:11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagababu E, Ramasamy S, Abernethy DR, Rifkind JM. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J Biol Chem. 2003;278:46349–46356. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 11.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 12.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, Cannon RO, 3rd, Kelm M, Wink DA, Espey MG, Oldfield EH, Pluta RM, Freeman BA, Lancaster JR, Jr, Feelisch M, Lundberg JO. The emerging biology of the nitrite anion. Nat Chem Biol. 2005;1:308–314. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 13.Luchsinger BP, Rich EN, Gow AJ, Williams EM, Stamler JS, Singel DJ. Routes to S-nitroso-hemoglobin formation with heme redox and preferential reactivity in the beta subunits. Proc Natl Acad Sci U S A. 2003;100:461–466. doi: 10.1073/pnas.0233287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagababu E, Ramasamy S, Rifkind JM. S-nitrosohemoglobin: a mechanism for its formation in conjunction with nitrite reduction by deoxyhemoglobin. Nitric Oxide. 2006;15:20–29. doi: 10.1016/j.niox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 16.Reutov VP, Sorokina EG. NO-synthase and nitrite-reductase components of nitric oxide cycle. Biochemistry (Mosc) 1998;63:874–884. [PubMed] [Google Scholar]
- 17.Nohl H, Staniek K, Kozlov AV. The existence and significance of a mitochondrial nitrite reductase. Redox Rep. 2005;10:281–286. doi: 10.1179/135100005X83707. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 2001;276:24482–24489. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 19.Tsikas D. Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic Res. 2005;39:797–815. doi: 10.1080/10715760500053651. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt HHHW, Kelm M. Determination of nitrite and nitrate by the Griess Reaction. Methods in Nitric Oxide Research. 1996:491–497. [Google Scholar]
- 21.Cox RD, Frank CW. Determination of nitrate and nitrite in blood and urine by chemiluminescence. J Anal Toxicol. 1982;6:148–152. doi: 10.1093/jat/6.3.148. [DOI] [PubMed] [Google Scholar]
- 22.Garside C. A chemiluminescent technique for the determination of nanomolar concentrations of nitrate and nitrite in seawater. Marine Chem. 1982;11:159–167. [Google Scholar]
- 23.Samouilov A, Zweier JL. Development of chemiluminescence-based methods for specific quantitation of nitrosylated thiols. Anal Biochem. 1998;258:322–330. doi: 10.1006/abio.1998.2609. [DOI] [PubMed] [Google Scholar]
- 24.Pelletier MM, Kleinbongard P, Ringwood L, Hito R, Hunter CJ, Schechter AN, Gladwin MT, Dejam A. The measurement of blood and plasma nitrite by chemiluminescence: Pitfalls and solutions. Free Radic Biol Med. 2006;41:541–548. doi: 10.1016/j.freeradbiomed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Hampl V, Waters CL, Archer SL. Determination of nitric oxide by the chemiluminescence reaction with ozone. Methods in Nitric Oxide Research. 1996:309–318. [Google Scholar]
- 26.Feelisch M, Rassaf T, Mnaimneh S, Singh N, Bryan NS, Jourd’Heuil D, Kelm M. Concomitant S-, N-, and heme-nitros(yl)ation in biological tissues and fluids: implications for the fate of NO in vivo. Faseb J. 2002;16:1775–1785. doi: 10.1096/fj.02-0363com. [DOI] [PubMed] [Google Scholar]
- 27.Yang BK, Vivas EX, Reiter CD, Gladwin MT. Methodologies for the sensitive and specific measurement of S-nitrosothiols, iron-nitrosyls, and nitrite in biological samples. Free Radic Res. 2003;37:1–10. doi: 10.1080/1071576021000033112. [DOI] [PubMed] [Google Scholar]
- 28.Feelisch M, Stamler JS. Donors of Nitrogen Oxides. In: Feelisch M, Stamler JS, editors. Methods in Nitric Oxide Research. 1996. pp. 71–115. [Google Scholar]
- 29.Rhodes P, Leone AM, Francis PL, Struthers AD, Moncada S, Rhodes PM. The L-arginine:nitric oxide pathway is the major source of plasma nitrite in fasted humans. Biochem Biophys Res Commun. 1995;209:590–596. doi: 10.1006/bbrc.1995.1541. [DOI] [PubMed] [Google Scholar]
- 30.Rassaf T, Heiss C, Hendgen-Cotta U, Balzer J, Matern S, Kleinbongard P, Lee A, Lauer T, Kelm M. Plasma nitrite reserve and endothelial function in the human forearm circulation. Free Radic Biol Med. 2006;41:295–301. doi: 10.1016/j.freeradbiomed.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Marley R, Feelisch M, Holt S, Moore K. A chemiluminescense-based assay for S-nitrosoalbumin and other plasma S-nitrosothiols. Free Radic Res. 2000;32:1–9. doi: 10.1080/10715760000300011. [DOI] [PubMed] [Google Scholar]
- 32.Rassaf T, Bryan NS, Kelm M, Feelisch M. Concomitant presence of N-nitroso and S-nitroso proteins in human plasma. Free Radic Biol Med. 2002;33:1590–1596. doi: 10.1016/s0891-5849(02)01183-8. [DOI] [PubMed] [Google Scholar]
- 33.Cox RD. DETERMINATION OF NITRATE AND NITRITE AT THE PARTS PER BILLION LEVEL BY CHEMI-LUMINESCENCE. Analytical Chemistry. 1980;52:332–335. [Google Scholar]
- 34.Wang X, Tanus-Santos JE, Reiter CD, Dejam A, Shiva S, Smith RD, Hogg N, Gladwin MT. Biological activity of nitric oxide in the plasmatic compartment. Proc Natl Acad Sci U S A. 2004;101:11477–11482. doi: 10.1073/pnas.0402201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dejam A, Hunter CJ, Pelletier MM, Hsu LL, Machado RF, Shiva S, Power GG, Kelm M, Gladwin MT, Schechter AN. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106:734–739. doi: 10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heiss C, Lauer T, Dejam A, Kleinbongard P, Hamada S, Rassaf T, Matern S, Feelisch M, Kelm M. Plasma nitroso compounds are decreased in patients with endothelial dysfunction. J Am Coll Cardiol. 2006;47:573–579. doi: 10.1016/j.jacc.2005.06.089. [DOI] [PubMed] [Google Scholar]
- 37.Rassaf T, Lauer T, Heiss C, Balzar J, Kleinbongard P, Mangold S, Leyendecker T, Rottler J, Kelm M. Plasma nitrite reserves with exercise predicts age-related endothelial dysfunction in humans. Free Radic Biol Med. 2006;14:A69–74. Abstract NO P168. [Google Scholar]


