Summary
Studies in both human and animal species have suggested that oxidative stress may be associated with health outcomes, including the risk of infertility in both males and females. Sex hormones have been shown to have antioxidant properties. The difficulty in studying the role of oxidative stress in females is partly due to fluctuation in these endogenous sex hormones across the menstrual cycle.
The aim of this study was to determine the association of oxidative stress levels with endogenous reproductive hormone levels and antioxidants, including vitamin levels, across the menstrual cycle in a prospective cohort of premenopausal women. The goal was to enrol 250 healthy, regularly menstruating premenopausal women for two menstrual cycles. Participants visited the clinic up to 8 times per cycle, at which time blood and urine were collected. The visits occurred at key hormonally defined phases of the menstrual cycle, with the help of an algorithm based on cycle length and data from a fertility monitor. In addition, participants were administered standardised questionnaires, had various physical measures taken, and had other pertinent data collected. A total of 259 women were enrolled in this study, with 250 completing two cycles, despite a demanding study protocol which participants were required to follow. This report describes the study design, baseline characteristics and visit completion rate for the BioCycle study.
Keywords: menstrual cycle, sex hormones, antioxidants, BioCycle study, study design, oxidative stress
Introduction
In 1954, Gerschman described how the combination of hyperbaric oxygen and X-irradiation caused formation of oxygen radicals and showed a correlation with decreased survival time.1 Her observation that females were significantly less sensitive to this oxidative stress was the first indication of a possible protection afforded to women that may be due at least in part to circulating hormones such as oestrogen. In the years that have followed, the antioxidant effect of oestrogen has been regarded as the main mechanism by which hormones protect tissues from oxidative damage and much research has been conducted in animals.2–4
In premenopausal women, the role of oxidative stress on health outcomes is not well understood, in part due to potential variation in measures of oxidative stress across the menstrual cycle. Oxygen free radicals have been studied for their association with spontaneous miscarriage and female infertility,5 endometriosis,6–8 male infertility and success of in-vitro fertilisation in females,9 and chronic diseases such as diabetes10 and cardiovascular disease.11,12 Several studies have attempted to characterise factors associated with variation in oxidative stress levels.13–15 However, these studies were not designed to take individual hormonal variation into account. Complicating these measurement issues is the fact that plasma lipoprotein levels fluctuate during the course of the menstrual cycle. This cyclic fluctuation in lipoproteins has been associated with unstable plasma antioxidant concentrations.16 A small well-controlled study showed that both lipoproteins and plasma antioxidant concentrations varied across the menstrual cycle.17 The human menstrual cycle includes cycle-related changes in oestrogen and other endogenous hormone concentrations, e.g. lutenising hormone (LH), follicle-stimulating hormone (FSH), progesterone, that may impact oxidative stress levels. There may be further influence by life style and environmental factors that may be related to hormone variation and influence oxidative stress levels.
Understanding the role of endogenous oestrogen and other hormones as potential modulators of oxidative stress level is necessary to clarify the effect of oxidative stress on premenopausal women’s health. The complex interrelation between oxidative stress and hormones requires carefully designed studies to assess the inter- and intra-individual variability across the menstrual cycle. The BioCycle study was designed to capture this variability without overburdening participants.
The purposes of this paper are to present the design of the BioCycle study, including the recruitment of the study cohort and the study methodology, and to present the baseline characteristics of the enrolled participants. This study was funded by a contract from the intramural branch of the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD) and was conducted at the University at Buffalo.
Methods
Study objectives
The overall objective of this study was to conduct a longitudinal assessment of the association of endogenous hormones with biomarkers of oxidative stress and antioxidant status during the menstrual cycle. There were four main objectives. The first was to study the intra-menstrual cycle variation of various measures of oxidative stress. This objective is intended to assess variation in several measures of oxidative stress during different phases of the menstrual cycle, including F2-8-isoprostanes in serum. Assessment of variation across individuals is planned. The second objective was to determine the relationship between specific reproductive hormone levels and oxidative stress during specific times in the menstrual cycle of premenopausal women. The panel of reproductive hormones in the blood that were of primary interest are oestradiol, progesterone, LH, FSH and sex hormone binding globulin (SHBG). The third objective was to examine the influence of external factors on both oxidative stress and hormone levels, and their interrelation. The study measured various biological factors that might influence oxidative stress, including serum concentration of certain antioxidant vitamin levels (retinoids, tocopherols, carotenoids and ascorbic acid). In addition, the study assessed other factors that might affect oxidative stress such as medication and supplement intake, cigarette smoking, alcohol consumption, dietary intake, physical activity and levels of stress. Lastly, the study was designed to evaluate the validity and reproducibility of the various biological markers included in the BioCycle study.
Study design
The study was designed to enrol 250 participants for two menstrual cycles and collect biological samples, conduct physical measurements and administer questionnaires across two complete menstrual cycles. In order to test the feasibility of the study and its measures, a pilot study of nine subjects was conducted for one cycle prior to finalising the study protocol. The full study involved biospecimen collection (blood and urine) at eight key times per cycle for two cycles per participant. The collection times were selected to include points in the menstrual cycle with the most hormonal variation. Sample collection was planned to occur during menstruation, at the middle of the follicular phase, at the time of the oestrogen peak, at the time of the LH and FSH surge, on the day of ovulation, at the time of progesterone elevation and peak and immediately before menstruation. Based on an approximate 28-day cycle length, these would represent approximately days 2, 7, 12, 13, 14, 18, 22 and 27.
Recruitment of subjects
Participants were recruited from regularly menstruating premenopausal female volunteers aged 18–44 years from the western New York state region. We used a variety of recruitment methods including: advertising in clinical practices from the region and the University at Buffalo student health service, placing paid advertisements in local newspapers and other print media, using articles about the study written by the University at Buffalo news bureau, doing radio and television interviews about the study, sending notices about the study to various list serves from the region, and posting flyers at the University and throughout western New York state (mainly in Erie and Niagara counties). These methods were supplemented by our participant web site, which was used as a recruitment tool, but also provided detail about the study that potential participants could access. As potential participants contacted the study, we recorded the methods by which they learned about the BioCycle study.
Initial telephone contact
Regardless of recruitment method, participants were instructed to call our clinical research centre for information and to schedule a screening visit appointment. On these initial calls, participants were given an overview of the study purpose, a description of the baseline enrolment procedures, and a description of the study cycle visit requirements. They also had an opportunity to ask questions about the study. If they were interested in learning more about the study and potentially participating, they were asked to provide contact information (name, address, telephone number) and were scheduled for a screening visit appointment at our clinical research centre. No eligibility screening questions were asked on the telephone call.
Each person scheduled for a visit was mailed a study packet prior to the appointment. The packet contained information describing the study, a copy of the consent form to review before their visit, a parking pass and directions to the clinic. The study was reviewed and approved by the Health Sciences Institutional Review Board (IRB) at the University at Buffalo and was approved by an IRB of the National Institutes of Health. The consent form included detailed information on the study requirements, including frequency and volume of blood and urine collection. Also included in the packet was an instruction sheet detailing what to expect at the screening visit and instructions on what they needed to do for the visit (e.g. fast; bring in all current medications). A reminder call was made the day before each scheduled screening visit.
Screening visit
The screening visit was the first clinic visit. Initial contact included review of the study procedures and review and signing of the informed consent. A copy of the consent was provided to all participants for later review. After a signed consent was obtained, a series of screening questions were used to determine initial eligibility based on the inclusion/exclusion criteria listed in Table 1. The full questionnaire was administered to all potential participants in order to determine all reasons for ineligibility. Detailed questions on menstrual and reproductive history were recorded both for determination of eligibility and for use in scheduling. Other questions were asked regarding demographic and life style factors, as well as medical history, for use in characterising the potential and actual participants. In addition, fasting blood (33 mL) and urine specimens were collected (Table 2). Blood samples were used as part of screening and eligibility [complete metabolic profile (CMP), total cholesterol, Chlamydia IgG], for detection of trace metals (lead, mercury, cadmium) and for storage for future testing (glass red top for environmental trace elements). A spot urine was collected in the clinic, spun and frozen for future use. After the blood sample and urine collection, subjects were provided a light breakfast.
Table 1.
BioCycle study inclusion/exclusion criteria
| Inclusion criteria: |
|
| Exclusion criteria: |
|
Table 2.
Screening visit blood and urine sample collection
| Collection tube (anticoagulant) (no. tubes × volume) |
Specimen type | No. of aliquots × volume |
Test to be performed | Method | Test lab |
|---|---|---|---|---|---|
| Red top (Glassa) (No anticoagulant) (2 × 10 mL) |
Serum | 2×5mL | Extra stored serum | ||
| Lavendar top (EDTA) (1×3 mL) |
Whole blood | 3 mL | Heavy metals | AAS and ICP-MS | CDC |
| Red top (no anticoagulant) (1 × 10 mL) |
Serum | 1 × 1.1 mL | CMP and total cholesterol | ACA | Kaleida |
| Serum | 1 × 1.1 mL | Chlamydia IgG screen | Serology | Kalieda | |
| Serum | 1 × 0.6 mL | Extra stored | |||
| Urine | Spot urine | 7 × 4.0 mL | Extra stored |
Glass tubes used for trace elements and/or environmental toxins.
AAS, atomic absorption spectroscopy; ACA, automated chemistry analysis; CMP, complete metabolic profile; ICP, inductively coupled plasma mass spectroscopy; CDC, Centers for Disease Control, Atlanta, Georgia; Kaleida Health Laboratories, Buffalo, New York State.
A negative blood test for history of Chlamydia infection (IgG) was required for eligibility. Chlamydia infection has been shown to be associated with oxidative stress in several clinical and experimental studies. We therefore excluded individuals with past infection with Chlamydia species. Positive Chlamydia tests and abnormal values of other screening blood tests were referred to the participant’s personal health care provider for evaluation. To further assess eligibility, participants were asked to bring all medications (prescription and over-the-counter) to the clinic where information on type and frequency of use was recorded. Finally, physical measures were obtained, including height and weight using standardised protocols, which were used to determine eligibility with respect to body mass index (BMI).
The screening visit took approximately 2 h to complete. At the end of the visit, an appointment for the baseline visit was made for those who remained interested. The baseline visit was scheduled 1–2 weeks prior to the participant’s next expected bleeding episode.
Baseline (enrolment) visit
At baseline, participants were informed of their final eligibility, including results of their Chlamydia IgG screen. All participants received a handout explaining the Chlamydia test and the meaning of the test results, regardless of whether the result was positive or negative. Those who were positive were advised to discuss these findings with their personal physician. Those who were ineligible for any reason were advised of this and had no other study activities completed.
Participants who were eligible and remained interested had physical/anthropometric measures taken, completed additional questionnaires and provided a urine sample. Physical and anthropometric measures were obtained according to standardised protocols and included blood pressure; pulse; sitting height; knee height; abdominal height; weight; waist, hip, abdominal, arm and thigh circumferences; and skinfolds of the upper arms, thighs, subscapular (shoulder blade) and suprailiac (hip) areas. The urine sample was used to rule out current pregnancy (Quick Vue, Quidel Corporation, San Diego, CA, USA). Excess urine was frozen and stored for future use. One pregnancy was found during the baseline visit screen and no pregnancies occurred during cycle visits.
Participants were asked to complete questionnaires on the following topics (Table 3): life style; health history; skin and body hair patterns;18,19 physical activity (IPAQ – The International Physical Activity Questionnaire); family medical history questionnaire; Perceived Stress Scale (14 items);20 Depression Scale (Center for Epidemiologic Studies Depression Scale, CES-D);21–23 and occupational history. Nutrient data were collected using a food frequency questionnaire (FFQ) developed by the Nutrition Assessment Shared Resource of the Fred Hutchinson Cancer Research Center.24
Table 3.
Questionnaires and physical measures by visit type
| Visita | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening questionnaire | × | |||||||||||||||||
| Physical measures (long) | × | |||||||||||||||||
| Physical measures (short) | × | × | × | × | × | × | × | × | × | × | ||||||||
| Lab form questionnaire | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| Family history | × | |||||||||||||||||
| Life style | × | |||||||||||||||||
| Occupation | × | |||||||||||||||||
| Food frequency (FFQ) | × | × | × | |||||||||||||||
| Health history | × | |||||||||||||||||
| Stress | × | |||||||||||||||||
| Depression | × | |||||||||||||||||
| Physical activity (IPAQ) | × | |||||||||||||||||
| Skin history | × | |||||||||||||||||
| Hair patterns | × | |||||||||||||||||
| Clinical visit questionnaire | × | × | × | × | × | × | × | × | ||||||||||
| 24-h food recall | × | × | × | × | × | × | × | × | ||||||||||
| DXA | × | |||||||||||||||||
| Daily diary | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | ||
Note visit days are approximate. Actual days were modified based on self-reported cycle length and fertility monitor results. DXA, dual energy X-ray absorptiometry.
Participants received both verbal and written instructions in the use of a fertility monitor (Clearblue® Easy Fertility Monitor, Inverness Medical, Waltham, MA, USA). These monitors were used to assist in scheduling visits to correspond with specific phases of the menstrual cycle.25 The monitors assessed fertility based on levels of oestrone-3-glucuronide (E3G, an oestrone metabolite) and LH in urine. Testing began on approximately the sixth day following the start of the woman’s menstrual cycle and continued for 10 or 20 days, depending on the timing of the LH surge and cycle length. For the participants, monitor indications of low, high and peak fertility were used to time mid-cycle visits, with peak day and the following 2 days being those that would approximately represent late follicular, LH surge and ovulation dates. Participants were instructed to bring the monitors to every clinic visit, where we downloaded information using software provided by the manufacturer. Testing history and the corresponding values of E3G and LH were accessed. This information was helpful in assessing participant compliance with daily monitor use, as well as assessing patterns and levels of E3G and LH in participants during the study. Further information about the fertility monitors can be found on the company web site.26
Participants were also provided with a daily diary where they were instructed to record the dates of their bleeding segments, symptoms during these bleeding segments, physical activity, life style habits (alcohol, smoking and caffeine use), sexual activity, stress, hours of sleep, medication intake and findings related to use of their fertility monitor. The participants were instructed to begin their diaries on the first day of their next bleeding segment and to continue entries daily for two complete menstrual cycles. Women were instructed not to start taking any supplements or medications or have X-rays taken during the study, unless medically indicated. Any use was recorded on the daily diaries. Only four women reported taking any medication during the study. The women brought their daily diaries to each clinic visit for review and for recording of information. A calendar of scheduled clinic visits was provided with dates initially based on self-reported cycle length. These calendars were adjusted in an ongoing fashion using information from the fertility monitor.
Cycle-specific visits (16 visits: 8 visits per cycle for 2 cycles)
Participants were asked to contact the clinic at the first sign of bleeding. The first cycle visit was scheduled on the next day (day 2 of their menstrual cycle). Study staff began reminder calls on a daily basis several days before the expected date of their next bleeding episode (based on history) in order to remind participants to call and schedule their first visit as soon as they began menstruating. For subsequent visits, participants received a reminder call 1 day before each scheduled visit to reduce missed appointments. During the call, the women were reminded of their appointment time, of the need to fast, and of the need to bring study forms with them to the clinic. During the first cycle, the visits were scheduled using an algorithm accounting for cycle length, but the mid-cycle visits were adjusted based on the monitor data. If the monitor indicated ‘peak fertility’ on a day without a scheduled visit, the women were asked to come in that morning and the following two mornings. Those not reaching peak by mid-cycle were scheduled according to an algorithm related to their cycle length. A visit calendar for the second cycle was created based on the length of the first cycle and the monitor data from the first cycle. The visit schedule was modified as necessary based on the second cycle monitor readings.
The study included 16 cycle visits (eight per cycle) over two cycles. The timing of these visits was scheduled to correspond with specific times of the menstrual cycle where the most hormonal variation would be expected, approximately days 2, 7, 12, 13, 14, 18, 22 and 27 of a 28-day cycle, adjusted for cycle length (Table 4).
Table 4.
Cycle visit schedule by cycle day (based on a 28-day cycle)
| Approximate day of the cycle |
Phase of the cycle | Expected hormonal variation |
|---|---|---|
| 2 | Menstrual | Low E2, low P, low LH/FSH |
| 7 | Mid-follicular | Low E2, low P, low LH/FSH |
| 12 | Late follicular | Peak E2, low P, low/rising LH/FSH |
| 13 | LH/FSH surge | Low E2, low P, peak LH/FSH |
| 14 | Ovulation | Low E2, rising P, decline LH/FSH |
| 18 | Early luteal | Moderate E2, high P, low FSH/LH |
| 22 | Mid-luteal | Moderate E2, peak P, low FSH/LH |
| 27 | Late luteal | Decline E2, decline P, low FSH/LH |
E2, oestradiol; P, progesterone; LH, lutinising hormone; FSH, follicle-stimulating hormone.
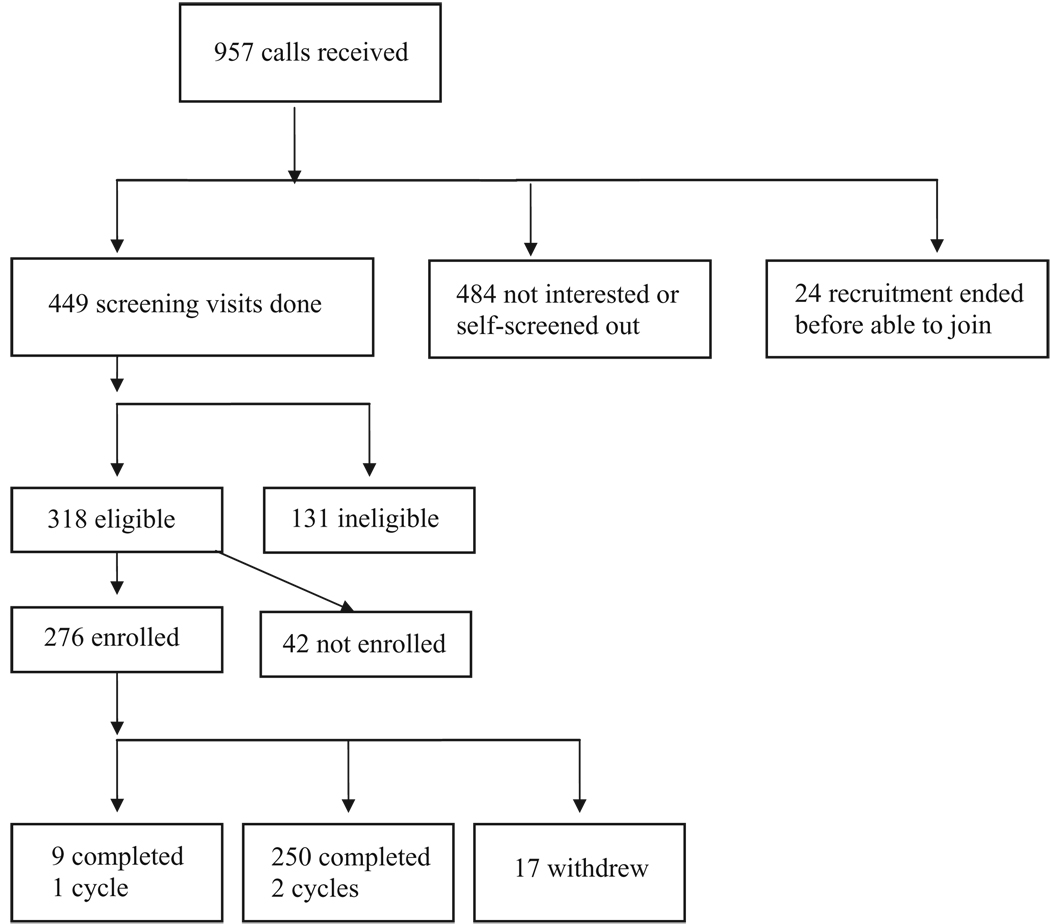
Cycle visits were routinely scheduled between 7:00 am and 8:30 am to allow for collection of fasting samples and to reduce diurnal variation. The study clinic was open 7 days per week 363 days per year (no visits Christmas or New Year’s Day). The clinical centre had a ‘no wait’ policy in effect to aid in participant retention. This was accomplished by staggered visit scheduling, adequate staffing and blocking of visit activities. Visits lasted approximately 30 min. At peak capacity, 25 new subjects were enrolled per month, with about 50 subjects seen in any 1 month. Enrolment lasted 12 months, with completion of all visits taking 16 months. Figure 1 provides an overview of the number of subjects screened and enrolled in the BioCycle study.
Figure 1.
BioCycle recruitment flow diagram.
Every cycle visit included collection of fasting (12 h overnight) blood and urine, diary review and questions on medication consumption. After specimen collection, participants were given a light breakfast and asked to complete brief questionnaires on recent activity. In addition, data were retrieved from their monitors. On approximately days 2, 7, 14 and 22, participants had longer visits, during which a 24 h dietary recall and an additional clinic visit questionnaire were administered. The questionnaire included questions on physical activity, stress, menstrual symptoms, X-ray exposure, emergency contraception use and illness in the past 7 days. On approximately day 27 of each cycle, participants were asked to complete a 1-month FFQ and a urine-based pregnancy test. During the final visit, after all samples were collected, participants had a dual energy X-ray absorptiometry (DXA) scan (Hologic Discovery Elite, Hologic, Waltham, MA, USA) to determine body composition (fat, lean, bone) and bone mineral density (spine, hip, wrist, whole body).
For the purpose of this study, a completed cycle included a minimum of five visits per cycle, with at least one visit during mid-cycle. Those with less than eight visits per cycle were generally the result of having shorter cycles (e.g. 21 days) or having unexpected events occur (travel, illness). Most women (n = 223) completed the study in two consecutive months, but 27 (10.8%) completed the study in non-consecutive cycles, mostly due to travel or illness. At the end of their participation, monetary reimbursement forms were completed. Participants were provided a modest compensation for their time and travel. Based on the number of visits completed, a participant could receive up to $500 (approximately $25 per visit). Participants were also asked to sign a release if they wanted copies of clinically pertinent tests to be sent to their personal physician (optional).
Specimen collection, processing, testing and storage for cycle visits
Both blood and urine were collected at every cycle visit. A bar code system was developed for labelling all collection tubes, storage vials and data collection sheets. This computer-generated labelling system used a unique participant ID along with the date of collection and specimen type. A database with additional information on the specimen type, time of collection, timing of specimen processing and storage, as well as the location of the stored sample within the biological specimen bank, was developed and used for all biological samples.
Samples were collected at the beginning of each clinic visit Participants were provided a 50mL sterile cup for collecting urine. Capped specimen cups were placed in a cooler and delivered to the processing laboratory within 30 min of collection. At the laboratory, urine was transferred to a 50mL conical tube and cen-trifuged (1500 × g) for 10 min in a 4°C centrifuge. Urine was decanted into 5mL polypropylene storage tubes, being careful not to disturb the sediment. A maximum of seven vials per participant visit were then placed in a −80°C freezer in 7 × 7 storage boxes.
Cycle visit blood samples were collected in a standardised fashion. Each participant was seated in the phlebotomy area by clinic personnel and remained seated for 10 min to allow fluid shifts to equilibrate. A total of up to 50mL of blood was taken at each cycle visit. At the time of collection, blood specimens were immediately wrapped in aluminum foil to protect them from light exposure. Blood tubes with anticoagulant were inverted a minimum of five times after collection. Heparin and EDTA tubes were placed in biohazard bags and put into a cooler with a chiller pack. Serum tubes remained at room temperature for no less than 20 min and no more than 30 min before being delivered to the specimen processing laboratory. At the processing laboratory, specimens were placed in a 4°C centrifuge at 1500 × g for 10 min. Samples for analyses that were processed using fresh blood on a daily basis were placed in a cooler and sent to a local laboratory (Kaleida Center for Laboratory Medicine) for testing (CMP, lipid profile, uric acid, high sensitivity C-Reactive Protein). All other specimens were placed in 9 × 9 boxes according to test and stored frozen at −80°C. The total time between phlebotomy and freezing specimens was recorded and was to be completed within 90 min.
Table 5 lists the tests that were performed on the samples and the methodology used. Samples not used immediately for tests were frozen and stored for later analysis. Once a complete cycle of samples was obtained from a participant, her frozen samples were sent as a complete cycle set for testing at the specific study laboratories. This helped to reduce batch-to-batch variation within an individual. The storage plan allowed for appropriately sized aliquots for planned tests, which eliminated repeated freeze-thaw cycles for the pre-specified analytes. A mapping system and database were used to track samples.
Table 5.
Cycle visit blood and urine specimen collection
| Collection tube (anticoagulant) (no. tubes × volume) |
Specimen type | No. of aliquots × volumea |
Test to be performedb | Methodb | Testlabc |
|---|---|---|---|---|---|
| Lavendar top (EDTA) (2 × 10 mL) |
Whole blood | 1 × 0.15 | Total haemoglobin (Hgb) | Cyan met haemoglobin method |
UB |
| Whole blood | 0.15 mL stabilised into 1.35 mL PCA |
Thiols (e.g. glutathione, glutathione disulphide) |
Stored future use | UB | |
| EDTA plasma | 1 × 1.25 mL | F2-8-isoprostanes | GC-MS | U Minn | |
| EDTA plasma | 1 × 1.1 mL | Lipid peroxidation profile | HPLC | UB | |
| EDTA plasma | 1 × 0.75 mL | TBARS | Spectrofluorometry | UB | |
| EDTA plasma | 1 × 0.75 mL | NMR lipoproteins (1 per cycle) |
NMR | Liposcience | |
| EDTA plasma | 1 × 1.1 mL | Homocysteine (3 per cycle) |
CL-EIA | Kalieda | |
| EDTA plasma | 1 × 1.25 mL | Extra stored | |||
| EDTA plasma | 8 × 0.6 mL | Extra stored | |||
| Buffy coat | Stored buffy coat | Future DNA extraction | |||
| Green top (sodium heparin) (1×5 mL) |
Heparin plasma | 0.5 stabilised into 2.0 mL MPA |
Vitamin C | DNPH Colorimetric assay | UB |
| Heparin plasma | 1 × 0.3 mL | Plasma glutathione peroxidase |
Kinetic enzyme assay | UB | |
| Heparin plasma | 3 × 0.6 mL | Extra stored | |||
| Buffy coat | 1 × 0.5 mL | Stored for DNA extraction | |||
| Washed erythrocytes |
1 × 0.5 mL | Antioxidant enzymes (SOD, GPx, GSHR) |
Kinetic enzyme assay | UB | |
| Red top (no anticoagulant) (1 × 10 mL; 1 × 15 mL) |
Serum | 1 × 0.3 mL | PON | Kinetic enzyme assay | UB |
| Serum | 1 × 1.1 mL | Fat soluable vitamins | HPLC | UB | |
| Serum | 1 × 1.25 mL | CMP | Auto chemistry analyser | Kalieda | |
| Lipid profile | Auto chemistry analyser | Kalieda | |||
| Uric acid | Colorimetry | Kalieda | |||
| Bilirubin | Colorimetry | Kalieda | |||
| hsCRP | CL-EIA | Kalieda | |||
| Serum | 1 × 0.6 mL | Oestradiol | Radio immunoassay | Kalieda | |
| Serum | 1 × 2.0 mL | Insulin, progesterone, LH, FSH, SHBG |
CL-EIA | Kalieda | |
| Serum | 8 × 0.6 mL | Extra stored | |||
| Urine | Spot urine | 7 × 4.0 mL | Extra stored | ||
| Cytokines | Luminex flow cytometry | Gainesville |
PCA, perchloric acid stabilising solution; MPA, meta-phosphoric acid stabilising solution.
CL-EIA, DPC Inc., Immunlite 2000 Solid phase competitive Chemiluminescent Enzymatic Immunoassay; DNPH, dinitrophenyl hydrazine; EDTA, ethylenediamine tetraacetic acid; FSH, follicle-stimulating hormone; GC-MS, gas chromatograph-mass spectrometry; GPx, glutathione peroxidase; GSHR, glutathione reductase; hsCRP, high sensitivity C-reactive protein; LH, lutinising hormone; NMR, nuclear magnetic resonance spectroscopy; PON, paraoxinase; SHBG, sex hormone binding globulin; SOD, superoxide dismutase; TBARS, thiobarbituric acid reactive substances.
UB, Dr Richard Browne, University at Buffalo, Buffalo NY; Kaleida Health Laboratories, Buffalo NY; U Minn, Dr Myron Gross, University of Minnesota, Minneapolis, MN; Liposicence, Raleigh, NC; Gainesville, Dr Chegini, U Florida, Gainesville, FL. Note blood draw order was: 15 mL red, 10 mL lavender, 4 mL green, 10 mL lavender, 10 mL red.
Plasma free F2-8-isoprostanes were measured with a gas chromatography–mass spectrometry-based method27 by the Molecular Epidemiology and Bio-marker Research Laboratory at the University of Minnesota (Minneapolis, MN).
Data management procedures
Databases for information collected were developed in Microsoft® Access. Data entry screens were created to mimic the study forms in order to create a flow for data entry personnel. Tight validation rules and referential integrity were applied to our entry forms to ensure further accurate data entry. Coding of variables was contained in the questionnaire in most instances. In addition, the Access database included variable tables and numeric codes. For all physical measures, questionnaires and laboratory results initially recorded on paper, 100% double entry was performed, and macros were created that produced discrepancy reports in Access. If discrepancies were found, data entry personnel reviewed hard copy records and made corrections. Personal identifiers were maintained in a separate database from the study data, and all data collection was carried out using a unique study ID. A second unique study ID was used for laboratory samples. The laboratory ID and study ID could be linked for data analysis purposes. Data were stored on a secure network server. Data from all sources were converted to SAS® data sets (SAS version 9.1, SAS Institute, Cary, NC, USA).
Results
A total of 969 women inquired about the study. Sources of recruitment were recorded. A total of 24.6% of our callers reported that they learned about the study through flyers placed throughout the region. Other sources included electronic mailing lists (8.6%) and advertisements (10.5%). By far the largest source of referrals (47.5%) came from friends or family members who knew about the study or were current study participants. Only three sets of first degree relatives were enrolled (one set of sisters; two mothers/daughters). As a result, we frequently reminded participants to tell their friends and families about the study. Women recruited early in the study (8.4%) were not asked how they heard about it; however, during this time advertisements, electronic mailing lists and word of mouth were the most used methods. Of the 259 who completed at least one cycle in the study, the source of recruitment was through friend or family referral (46.8%), flyer (31.2%), news advertisements (11.6%), email (8.4%) or hearing about the study at a talk (0.4%). Source of referral was unknown for 1.6% of the participants.
A total of 969 women telephoned our clinical centre to enquire about the study. During these telephone calls, we provided a brief summary of the study, including information on general inclusion/exclusion criteria. Many women opted not to join based on the information provided in the call. No screening questions were asked in the call. Of the 969 who called, 449 women expressed interest, were scheduled and completed a screening visit. Of these, 318 met eligibility criteria and 131 were ineligible; 276 of the 318 who were eligible enrolled. Table 6 presents information on those eligible compared with those ineligible and the total group screened. Although not markedly different, those eligible were slightly younger and more likely to report being of white race.
Table 6.
Summary of participant accrual for the BioCycle study
| Study totals of those screened until end of study | |||
|---|---|---|---|
| Eligible | Ineligible | Screened | |
| Total number | 318 | 131 | 449a |
| Age | |||
| Mean number of years ± SD | 27.6 ± 8.0 | 31.3 ± 8.6 | 29.7 ± 8.4 |
| Min, max years | 18.1, 44.9 | 18.1, 44.9 | 18.1, 44.9 |
| Race/ethnicity | |||
| White | 186 (58.5%) | 68 (51.9%) | 254 (56.6%) |
| African-American | 63 (19.8%) | 49 (37.4%) | 112 (24.9%) |
| Asian | 50 (15.7%) | 2 (1.6%) | 52 (11.6%) |
| Other raceb | 18 (5.7%) | 11 (8.3%) | 29 (6.4%) |
| Unknown | 1 (0.3%) | 1 (0.8%) | 2 (0.4%) |
| Education | |||
| Not high school graduate | 2 (0.6%) | 2 (1.5%) | 4 (0.9%) |
| High school graduate/GED | 37 (11.6%) | 19 (14.6%) | 56 (12.5%) |
| Any college | 240 (75.5%) | 93 (71.0%) | 333 (74.2%) |
| Graduate school or higher | 38 (12.0%) | 16 (12.2%) | 54 (12.0%) |
| Unknown | 1 (0.3%) | 1 (0.8%) | 2 (0.4%) |
| Self-reported cycle length | |||
| Mean number of days ± SD | 28.4 ± 2.0 | 28.4 ± 2.4 | 28.5 ± 2.1 |
| Min, max days | 21, 35 | 20, 35 | 20, 35 |
One participant fainted and did not complete the screening questionnaire at the blood draw.
Other race self-described by participant.
GED, General Educational Development.
Reasons for ineligibility in the 449 women who came to the screening visit included Chlamydia screen positive (n = 46), outside BMI range (n = 26), history of a sexually transmitted disease (n = 23), current use of an excluded medication (n = 15) and self-reported fibroids (n = 14) (Table 7).
Table 7.
Reasons for participant ineligibility (n = 131)
| Reasons for ineligibility | No. (%) of total ineligiblea |
|---|---|
| Chlamydia screening positive | 46 (35.1) |
| Body mass index out of range | 26 (19.8) |
| Sexually transmitted disease | 23 (17.6) |
| Medication use | 15 (11.5) |
| Fibroids | 14 (10.7) |
| Infection (recent) | 7 (5.3) |
| Infertility treatment (ever) | 6 (4.6) |
| Endometriosis history | 5 (3.8) |
| Surgery in past 6 months | 4 (3.1) |
| Fainted at screening blood draw | 4 (3.1) |
| Self-selected out, not otherwise described | 3 (2.3) |
| Pelvic inflammatory disease | 2 (1.5) |
| Pregnant | 2 (1.5) |
| Irritable bowel syndrome | 2 (1.5) |
| Recent miscarriage | 2 (1.5) |
| Recent surgical procedure | 2 (1.5) |
| Hormone use | 1 (0.8) |
| Cancer history | 1 (0.8) |
| Trying to currently conceive | 1 (0.8) |
| Dietary restriction | 1 (0.8) |
| Irregular menstrual period | 1 (0.8) |
| Abnormal pap smear | 1 (0.8) |
| Abnormal lab value | 1 (0.8) |
Participants may have had more than one reason for ineligibility. Total ineligible participants: 131(= 100%).
Of the 276 who enrolled, 250 completed two cycles, nine completed one cycle and 17 women dropped out of the study prior to completing at least one cycle. Among the 259 who completed at least one cycle, the number of study cycle visits ranged from 5 to 8 per cycle, with shorter menstrual cycle length being the primary reason for fewer than eight visits. A total of 94% of all participants completed seven or eight visits per cycle (Table 8).
Table 8.
Number of visits per cycle for BioCycle participants
| Cycle 1 (n = 259) | Cycle 2 (n = 250) | |
|---|---|---|
| Number of visits | n (%) | n (%) |
| 5 | 3 (1.2) | 2 (0.8) |
| 6 | 14 (5.4) | 9 (3.6) |
| 7 | 57 (22.0) | 50 (20.0) |
| 8 | 185 (71.4) | 189 (75.6) |
Table 9 presents summary data for the participants enrolled in the BioCycle study. The data are presented for the 259 women who completed at least one cycle (column 2), and then separately for the 250 participants who completed two cycles (column 3), and the nine subjects that completed one cycle (column 4). For most variables, there were minimal differences between those who completed one and two cycles. Overall, the study participants ranged in age from 18 to 44 years (mean = 27.3), with 60% white race. Nearly 90% had some college education, with income levels ranging evenly across categories. The majority (68%) were of single marital status. BMI ranged from 18 to 35 with a mean (SD) BMI of 24.1 (3.9) kg/m2. Bone mineral density was within normal range for most participants. The mean (SD) per cent body fat by DXA was 29.5 (6.0) (range 15.1–45.2). A small percentage of participants reported a past history of anorexia (6.9%) or bulimia (4.2%). The majority of women had had a Pap test done at least once (76.2%) but few had had a mammogram (25.1%), not surprisingly given the age range of this group. Only 12.4% of participants were current smokers, with 78.4% reporting never smoking. Most reported drinking at least one drink per month in the past 6 months (66.4%), with 36.6% reporting drinking at least one drink per week. Although current illicit drug use was an exclusion criterion, 12% reported at least some intake in the past year. Average hours of sleep per night ranged from 4.4 to 11.8 h. Some form of acne was reported in 90% of participants; however, only 5.8% reported the acne as deep inflammatory type.
Table 9.
BioCycle study participant selected characteristics
| Mean ± SD (min, max) n (column %)a | |||
|---|---|---|---|
| Total participants enrolled | Participants completed | Participants completed | |
| (n = 259) | 2 cycles (n = 250) | 1 cycle (n = 9) | |
| Demographics | |||
| Age (years) | 27.3 ± 8.2 (18, 44) | 27.5 ± 8.3 (18, 44) | 21.9 ± 3.7 (18, 29) |
| Race/ethnicity | |||
| White | 154 (59.5) | 148 (59.2) | 6 (66.7) |
| African American | 51 (19.7) | 50 (20.0) | 1 (11.1) |
| American Indian | 1 (0.4) | 1 (0.4) | 0 (0.0) |
| Asian Indian | 13 (5.0) | 12 (4.8) | 1 (11.1) |
| Chinese/other Asian | 26 (10.2) | 25 (10.0) | 1 (11.1) |
| Any Pacific Islander | 1 (0.4) | 1 (0.4) | 0 (0.0) |
| Other (not specified) | 13 (5.0) | 13 (5.2) | 0 (0.0) |
| Education (highest grade completed) | |||
| ≤High school | 33 (12.7) | 32 (12.8) | 1 (11.1) |
| Post-secondary | 226 (87.3) | 218 (87.2) | 8 (88.9) |
| Estimated median family income | |||
| <$19 999 | 55 (21.2) | 53 (21.2) | 2 (22.2) |
| $20 000–39 999 | 61 (23.6) | 59 (23.6) | 2 (22.2) |
| $40 000–74 999 | 72 (27.8) | 70 (28.0) | 2 (22.2) |
| $75 000–99 999 | 45 (17.4) | 42 (16.8) | 3 (33.3) |
| $100 000+ | 24 (9.3) | 24 (9.6) | 0 (0.0) |
| Marital status | |||
| Married/living as married | 66 (25.5) | 65 (26.0) | 1 (11.1) |
| Separated/divorced | 16 (6.2) | 16 (6.4) | 0 (0.0) |
| Single | 177 (68.3) | 169 (67.6) | 8 (88.9) |
| Physical measures | |||
| Height (cm) | 163.9 ± 6.3 (148.1, 183.0) | 164.1 ± 6.3 (148.1, 183.0) | 159.8 ± 4.9 (154.0, 165.8) |
| Weight (kg) | 64.8 ± 11.0 (40.8, 97.6) | 65.0 ± 11.1 (40.8, 97.6) | 60.0 ± 7.8 (46.2, 70.7) |
| Body mass index (kg/m2) | 24.1 ± 3.9 (18, 35) | 24.1 ± 4.0 (18, 35) | 23.5 ± 3.1 (18, 29) |
| Health and life style | |||
| Ever had Pap test | 189 (73.0) | 184 (73.6) | 5 (55.6) |
| Ever had mammogram | 65 (25.1) | 64 (25.6) | 1 (11.1) |
| Smoking | |||
| Never | 203 (78.4) | 197 (78.8) | 6 (66.7) |
| Former smoker | 21 (8.1) | 20 (8.0) | 1 (11.1) |
| Current smoker | 32 (12.4) | 30 (12.0) | 2 (22.2) |
| Alcohol consumption in past 12 months | |||
| <12 drinks | 85 (32.8) | 83 (33.2) | 2 (22.2) |
| ≥12 drinks | 172 (66.4) | 165 (66.0) | 7 (77.8) |
| Physical activity – total MET minutes | 5555.9 ± 6907.6 (0, 47772) | 5534.6 ± 6878.9 (0, 47772) | 6148.1 ± 8098.8 (0, 25788) |
| per week | |||
| Energy (kcal) from FFQ | 1504.7 ± 728.6 (305.8, 5269.7) | 1505.6 ± 727.6 (305.8, 5269.7) | 1472.9 ± 824.6 (305.9, 2731.0) |
| Reproductive measures | |||
| Bleeding segment length | 28.4 ± 2.2 (21, 35) | 28.4 ± 2.2 (21, 35) | 26.8 ± 3.3 (21, 29) |
| (self-reported at enrolment) | |||
| Bleeding segment length – cycle 1 | 28.9 ± 4.6 (13, 58) | 28.9 ± 4.6 (13, 58) | 34 ± 0.0 (34, 34) |
| (actual cycle 1) (n = 250) | |||
| Bleeding segment length – cycle 2 | 28.7 ± 3.5 (20, 44) | 28.7 ± 3.5 (20, 44) | NA |
| (actual cycle 2) (n = 226) | |||
| Usual no. of days of bleeding | 5.1 ± 1.1 (2, 8) | 5.1 ± 0.07 (2, 8) | 5.7 ± 1.1 (4, 7) |
| (self-reported) | |||
| Age at menarche | 12.5 ± 1.2 (9, 16) | 12.4 ± 1.3 (9, 16) | 13.1 ± 1.1 (12, 15) |
| Age menstrual cycles became regular | 14.8 ± 2.9 (10, 30) | 14.8 ± 3.0 (10, 30) | 15.8 ± 1.3 (15, 18) |
| Ever sexually active | 191 (73.7) | 186 (74.4) | 5 (55.6) |
| Currently sexually active (n = 191) | 135 (70.7) | 132 (71.0) | 3 (60.0) |
| Birth control (BC) use | |||
| Never used any birth control method | 78 (30.1) | 74 (29.6) | 4 (44.4) |
| Some BC used | 181 (69.9) | 176 (70.4) | 5 (55.6) |
| Method reported by 5%+ (of 191 | |||
| sexually active)b: | |||
| Male condom | 155 (85.6) | 151 (85.8) | 4 (80.0) |
| Pill | 133 (73.5) | 128 (72.7) | 5 (100.0) |
| Withdrawal | 77 (42.5) | 76 (43.2) | 1 (20.0) |
| Rhythm method | 27 (14.9) | 27 (15.3) | 0 (0.0) |
| Spermicidal foam | 24 (13.3) | 24 (13.6) | 1 (20.0) |
| Morning after pill | 20 (11.0) | 19 (10.8) | 0 (0.0) |
| Patch | 18 (9.9) | 17 (9.7) | 1 (20.0) |
| Injectables | 12 (6.6) | 12 (6.8) | 0 (0.0) |
| Diaphragm | 10 (5.5) | 10 (5.7) | 0 (0.0) |
| Ever been pregnant (n = 191) | 78 (40.8) | 76 (40.9) | 2 (40.0) |
| Gravidity (n = 191) | 2.6 ± 1.4 (0, 7) | 2.6 ± 1.4 (0, 7) | 1.5 ± 0.7 (1, 2) |
| Parity (n = 191) | 2.0 ± 1.3 (0, 7) | 2.0 ± 1.3 (0, 7) | NA |
| Age at first birth (n = 52) | 25.3 ± 5.1 (14, 33) | 25.3 ± 5.1 (14, 33) | NA |
Row totals that do not add to 259 reflect missing information reported for that variable. Other N’s noted elsewhere.
Multiple methods could be reported.
FFQ, food frequency questionnaire; MET, metabolic equivalent.
Self-reported bleeding segment length ranged from 21 to 35 days with a mean (SD) length of 28.4 (2.2) days. Actual cycle length in the study ranged from 13 to 58 days in cycle 1 and from 20 to 44 days in cycle 2. In cycle 1 three participants had lengths <21 days and 17 had lengths >35 days. In cycle 2, only one participant had a cycle length <21 days and nine had cycle lengths >35 days (data not shown). Participants reported a mean (SD) usual bleeding length of 5.1 (1.1) days with heavy bleeding reported by 61.0%. About half of the women reported taking some medication for symptoms at some point during their bleeding episodes. Age at menarche ranged from 9 to 16 years with a mean (SD) age of 12.5 (1.2) years and regular cycles at 14.8 (2.9) years. A total of 70.1% reported being currently sexually active, with most women reporting being heterosexual (93.7%) and 92% reporting some form of birth control use when having sexual relations with a male partner. With respect to family history, cardiovascular disease (49.4%), cancer (73%) and/or gynaecological disorders (73.0%) were reported in at least one first degree relative. The mean (SD) kilocalo-rie intake ranged from 305.8 to 5269.7 [mean (SD) of 1504.7 (729)] by FFQ with specific nutrient intake ranging across all categories.
Discussion
Studies in both humans and animal species have suggested that oxidative stress may be associated with health outcomes. In females there is limited understanding of the role of oxidative stress in many disorders, including infertility, in part due to variation of endogenous hormones across the menstrual cycle. The BioCycle study was designed to provide an opportunity to understand further the relation of measures of oxidative stress and reproductive hormones and other antioxidants across the menstrual cycle in a group of well-characterised reproductive aged women. Caveats regarding the design should be acknowledged. This is a select group of healthy, educated young women and findings may not be generalisable to all women. Nonetheless, this study provides a unique opportunity to explore variation of oxidative stress in these well-characterised women. In summary, we demonstrated the ability to enrol a large cohort of women into a demanding protocol for the purpose of better understanding these complex relationships in reproductive-age women.
Acknowledgements
This investigation was supported by the Intramural Research Program of the NIH, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) contract #HHSN275200403394C to the University at Buffalo. We are indebted to all the investigators and staff at the University at Buffalo and NICHD for their respective roles in the study, their dedication and effort; to Jennifer Reschke, Andrea Hughes and Christopher Bole for assistance in preparing tables for this manuscript; to Karen Falkner, Michael Bloom, Alan Wu and Leila Jackson for their assistance in study implementation; to Myron Gross for guidance on measures of oxidative stress and completion of the isoprostane samples; and to the Bio-Cycle participants for their extraordinary commitment to the study.
References
- 1.Gerschman R, Gilbert DL, Nye SW, Dwyer P, Fenn WO. Oxygen poisoning and x-irradiation: a mechanism in common. Science. 1954;119:623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- 2.Yagi K. Female hormones act as natural antioxidants – a survey of our research. Acta Biochimica Polonica. 1977;44:701–710. [PubMed] [Google Scholar]
- 3.Yagi K, Komura S. Inhibitory effect of female hormones on lipid peroxidation. Biochemistry International. 1986;13:1051–1055. [PubMed] [Google Scholar]
- 4.Yoshino K, Komura S, Watanabe I, Nakagawa Y, Yagi K. Effects of estrogens on serum and liver lipid peroxide levels in mice. Journal of Clinical Biochemistry and Nutrition. 1987;3:233–240. [Google Scholar]
- 5.Jenkins C, Wilson R, Roberts J, Miller H, McKillop JH, Walker JJ. Antioxidants: their role in pregnancy and miscarriage. Antioxidants and Redox Signaling. 2000;2:623–628. doi: 10.1089/15230860050192369. [DOI] [PubMed] [Google Scholar]
- 6.Murphy AA, Santanam N, Parthasarathy S. Endometriosis: a disease of oxidative stress? Seminars in Reproductive Endocrinology. 1998;16:263–273. doi: 10.1055/s-2007-1016286. [DOI] [PubMed] [Google Scholar]
- 7.Santanam N, Murphy AA, Parthasarsthy S. Macrophages, oxidation and endometriosis. Annals of the New York Academy of Sciences. 2002;955:183–198. doi: 10.1111/j.1749-6632.2002.tb02779.x. [DOI] [PubMed] [Google Scholar]
- 8.Jackson LW, Schisterman EF, Dey-Rao R, Browne R, Armstrong D. Oxidative stress and endometriosis. Human Reproduction. 2005;20:2014–2020. doi: 10.1093/humrep/dei001. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal A, Saleh RA, Bedaiwy MA. The role of reactive oxygen species in the pathophysiology of human reproduction. Fertility and Sterility. 2003;79:829–843. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 10.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocrine Reviews. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 11.Schisterman E, Farragi D, Browne R, Freudenheim J, Dorn J, Muti P, et al. Minimal and best linear combination of oxidative stress and antioxidant biomarkers to discriminate cardiovascular disease. Nutrition, Metabolism, and Cardiovascular Diseases. 2002;12:259–266. [PubMed] [Google Scholar]
- 12.Antoniades C, Tousoulis D, Tentolouris C, Toutouzas P, Stefanadis C. Oxidative stress, antioxidant vitamins, and atherosclerosis. From basic research to clinical practice. Herz. 2003;28:628–638. doi: 10.1007/s00059-003-2417-8. [DOI] [PubMed] [Google Scholar]
- 13.Trevisan M, Browne R, Ram M, Muti P, Freudenheim J, Carosella AM, et al. Correlates of markers of oxidative status in the general population. American Journal of Epidemiology. 2001;154:348–356. doi: 10.1093/aje/154.4.348. [DOI] [PubMed] [Google Scholar]
- 14.Block G, Dietrich M, Norkus E, Jensen C, Benowitz NL, Morrow JD, et al. Intraindividual variability of plasma antioxidants, markers of oxidative stress, C-reactive protein, cotinine, and other biomarkers. Epidemiology. 2006;17:404–412. doi: 10.1097/01.ede.0000220655.53323.e9. [DOI] [PubMed] [Google Scholar]
- 15.Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, et al. Factors associated with oxidative stress in human populations. American Journal of Epidemiology. 2002;156:274–285. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 16.Forman MR, Johnson EJ, Lanza E, Graubard BI, Beecher GR, Muesing R. Effect of menstrual cycle phase on the concentration of individual carotenoids in lipoproteins of premenopausal women: a controlled dietary study. American Journal of Clinical Nutrition. 1998;67:81–87. doi: 10.1093/ajcn/67.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Forman MR, Beecher GR, Muesing R, Lanza E, Olson B, Campbell WS, et al. The fluctuation of plasma carotenoid concentrations by phase of the menstrual cycle: a controlled diet study. The American Journal of Clinical Nutrition. 1996;64:559–565. doi: 10.1093/ajcn/64.4.559. [DOI] [PubMed] [Google Scholar]
- 18.Ferriman D, Gallwey JD. Clincial assessment of body hair growth in women. Journal of Clinical Endocrinology and Metabolism. 1961;21:1440–1447. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 19.Moncada Lorenzo E. Familial study of hirsutism. Journal of Clinical Endocrinology. 1970;31:556–564. doi: 10.1210/jcem-31-5-556. [DOI] [PubMed] [Google Scholar]
- 20.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 22.Burnam M, Wells K, Leake B, Landsverk J. Development of a brief screening instrument for detecting depressive disorders. Medical Care. 1988;26:775–789. doi: 10.1097/00005650-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Weissman M, Sholomskas D, Pottenger M, Prusoff B, Locke B. Assessing depressive symptoms in five psychiatric populations: a validation study. American Journal of Epidemiology. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 24. [last accessed September 2008]; http://www.fhcrc.org/science/shared_resources/nutrition/ffq.
- 25.Behre HM, Kuhlage J, Gassner C, Sonntag B, Schem C, Schneider HP, et al. Prediction of ovulation by urinary hormone measurements with the home use Clear Plan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Human Reproduction. 2000;15:2478–2482. doi: 10.1093/humrep/15.12.2478. [DOI] [PubMed] [Google Scholar]
- [last accessed September 2008]; http://www.clearplan.com.
- 27.Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Progress in Lipid Research. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]