Abstract
The de novo generation of Foxp3+ regulatory T (Treg) cells in the peripheral immune compartment and the differentiation of Th17 cells both require TGF-β, and IL-6 and IL-21 are switch factors that drive the development of Th17 cells at the expense of Treg generation. The major vitamin A metabolite all-trans retinoic acid (RA) not only enforces the generation of Treg but also inhibits the differentiation of Th17 cells. Here we show that RA enhances TGF-β signaling by increasing the expression and phosphorylation of Smad3 and this results in increased Foxp3 expression even in the presence of IL-6 or IL-21. RA also inhibits the expression of IL-6Rα, IRF-4 and IL-23R and thus inhibits Th17 development. In vitro, RA significantly promotes Treg conversion but in vivo during the development of experimental autoimmune encephalomyelitis (EAE) it does not increase the frequency of Treg cells in the face of an ongoing inflammation. However, RA suppresses the disease very efficiently by inhibiting proinflammatory T cell responses, especially pathogenic Th17 responses. These data not only identify the signaling mechanisms by which RA can affect both Treg and Th17 differentiation, but also highlight that in vivo during an autoimmune reaction, RA suppresses autoimmunity mainly by inhibiting the generation of effector Th17 cells.
Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the United States National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
Introduction
Proinflammatory T helper type 1 (Th1) cells have long been linked to the induction of organ specific autoimmune diseases (1). However the fact that loss of IFN-γ or lack of IFN-γ signaling results in increased susceptibility rather than resistance to autoimmunity has led to the hypothesis that a T cell subset other than Th1 cells might be crucial in the induction of autoimmunity (2). This observation provided the impetus for the identification of a new subset of effector T cells called Th17 cells, which produce the hallmark cytokine, IL-17A (also called IL-17). Th17 cells are highly proinflammatory cells, and emerging data suggest that they orchestrate tissue inflammation and organ-specific autoimmune diseases (3). On the other hand, CD4+ CD25+ regulatory T cells (Treg) are a subset of CD4+ T cells which express Foxp3 and are important in inhibiting inflammation and mediating self tolerance (4). Therefore, Treg and Th17 cells antagonize functions of each other, whereas Th17 cells are crucial for inducing tissue inflammation and autoimmunity, Treg cells prevent tissue inflammation, autoimmunity and mediate self tolerance (5).
Treg cells are normally generated in the thymus upon contact with self-antigen (natural Treg, nTreg) and seeded to the peripheral immune compartment, where they regulate immune responses through out the life span of the individual (4). Treg cells can also be induced in the peripheral immune compartment by conversion of naïve CD4+ T cells into Foxp3+ T cells by TGF-β (induced Treg, iTreg) (4). TGF-β also supports the maintenance of Foxp3 expression and suppressive function of nTreg cells (6). TGF-β signals by binding its cell surface serine/threonine kinase receptors, which in turn phosphorylate Smad2 and Smad3. Phosphorylated Smad2 and Smad3, together with Smad4, enter the nucleus to regulate the expression of target genes, either directly or in association with other transcription factors (7). A number of factors have been identified to be able to enhance or inhibit the TGF-β mediated conversion of Treg cells. Cytokines (like IL-6 and IL-21) and various metabolites, such as retinoic acid, have been shown to regulate the conversion of TGF-β mediated Foxp3+ Treg cells (8, 9). IL-6, an acute phase protein induced early during inflammation, has been shown to suppress TGF-β mediated Foxp3 induction but in turn induce the generation of Th17 cells from differentiating naïve T cells (3). IL-6 binds its cell surface receptors, IL6Rα and a shared common receptor gp130, to recruit and phosphorylate STAT3 (9). IL-6 or IL-21 in combination with TGF-β, induces the expression of retinoic acid receptor-related orphan receptor (ROR)γt in T cells, a transcription factor important for the development of Th17 cells (9). RORγt also upregulates the expression of IL-23R, and engagement of IL-23R by IL-23R further induces RORγt expression and reinforces and stabilizes the development and function of Th17 cells (3, 9, 10).
In addition to RORγt, interferon-regulatory factor 4 (IRF4), a transcription factor induced by TcR/CD3 signaling, has recently been reported to be also required for Th17 differentiation (11). Lack of IRF4 in CD4+ T cells led to decreased RORγt expression and IL-17 production with an associated defect in Foxp3 downregulation in Th17 cultures, due to a reduced IL-6 response. However, IL-6-induced phosphorylation of STAT3 is normal in IRF4-/- cells, suggesting that the downstream events of STAT3 phosphorylation in the IL-6 signaling pathway are impaired in the absence of IRF4.
Retinoic acid (RA) is an active metabolite of vitamin A that regulates various cellular functions such as proliferation, differentiation and cell death in a variety of cell types through two specific families of nuclear receptors, the retinoic acid receptors (RAR, including α, β and γ subtypes) and the retinoic X receptors (RXR, also including α, β and γ subtypes), which function as ligand-inducible transcription factors (12-15). All-trans-RA binds preferentially to RAR, whereas 9-cis-RA binds to both RAR and RXR (12, 13). In the immune system, RA plays important roles in regulating the functions of various types of immune cells (15). For example, RA prevents activation-induced cell death of T cells and inhibits Th1 but enhances Th2 responses (16, 17). RA in vivo suppresses inflammatory responses and tissue damage and ameliorates a variety of autoimmune diseases in animal models, including experimental autoimmune encephalomyelitis (EAE), rheumatoid arthritis, type I diabetes, inflammatory bowel disease, and lupus (18-22). However, the beneficial effect of RA in these diseases was so far thought to be due to its inhibition of Th1 responses. A number of recent reports have demonstrated that RA produced by CD103+ DC in the gut preferentially induces iTreg cells (23-26) but inhibits Th17 differentiation (26) and this may be one of the mechanisms by which RA suppresses autoimmune reactions in vivo. However, the biochemical and molecular mechanism by which RA promotes iTreg conversion and suppresses Th17 cell generation has not been addressed.
Here we report that RA indeed affects both Treg conversion and Th17 differentiation by influencing the components of TGF-β, IL-6/IL-21, and TcR/CD3 signaling pathways, which are involved in the development of Th17 and Treg cells. Interestingly, although RA in vitro dramatically enhances the development of Treg cells, RA in vivo does not increase the frequency of Treg cells significantly in an autoimmune disease setting but mainly inhibits the generation of effector T cells. We conclude that the beneficial effect of RA in autoimmunity is mainly due to its inhibition of pathogenic Th17 responses.
Materials and Methods
Mice and reagents
C57BL/6 mice were purchased from The Jackson Laboratory. Foxp3gfp.KI mice were generated and maintained as described previously (32). Mice were maintained and all animal experiments were done according to the animal protocol guidelines of Harvard Medical School. MOG35-55 peptide was synthesized by Quality Controlled Biochemicals and was > 80% pure, as determined by HPLC. ATRA and DMSO were purchased from Sigma-Aldrich. ATRA (100 mM) was solved in DMSO (Vehicle) and stored at -80 °C as aliquots before use. All of the fluorescence-conjugated antibodies were obtained from eBioscience and BD Biosciences. All of the antibody pairs for ELISA were purchased from BD Biosciences. All cytokines were purchased from eBiosciences and R&D Systems.
Naïve CD4+ T cell purification and stimulation
Naïve CD4+ T cells (CD4+ CD62Lhi CD25-) were purified by FACS-sorting following a MACS bead-isolation of CD4+ cells. Naïve CD4+ cells were activated with plate-bound anti-CD3 (2 μg/ml) and soluble anti-CD28 (2 μg/ml) in 96-well plates. ATRA (10 nM) was included in the cultures. Cultures were also supplemented with IL-6 (20 ng/ml), IL-21 (200 ng/ml), IL-6 (20 ng/ml) or IL-21 (200 ng/ml) plus TGF-β (2 ng/ml) for Th17 cell differentiation, and TGF-β (2 ng/ml) for Treg conversion.
Flow cytometry
For intracellular cytokine staining, cells were stimulated in culture medium containing PMA (30 ng/ml, Sigma-Aldrich), ionomycin (500 ng/ml, Sigma-Aldrich), and monensin (GolgiStop, 1 μl/ml, BD Biosciences) in a cell incubator with 10% CO2 at 37 °C for 4 h. After staining surface markers, cells were fixed and permeabilized using Cytofix/Cytoperm and Perm/Wash buffer (BD Biosciences) according to the manufacturer's instructions. Then, cells were stained with fluorescence-conjugated cytokine antibodies at 25 °C for 30 min before analysis. In most of the staining experiments, if possible, 7-AAD (BD biosciences) was also included to gate out the dead cells. MOG tetramer staining has been described previously (32, 42). All data were collected on a FACSCalibur (BD Biosciences) and analyzed with FlowJo software (TreeStar).
RNA isolation, real-time PCR and Western Blot
Total RNA isolation, Taqman Quantitative PCR and Western blot were performed as described previously (42, 43). All primers and probes for real-time PCR were purchased from Applied Biosystems.
Immunization and EAE evaluation
Female B6 mice (8-12-wk old) were immunized subcutaneously in the flanks with an emulsion containing MOG35-55 (100 μg/mouse) and Mycobacterium tuberculosis H37Ra extract (3 mg/ml, DIFCO) in CFA (100 μl/mouse). Pertussis toxin (100 ng/mouse, List Biological Laboratories) was administered intraperitoneally on days 0 and 2. RA was intraperitoneally injected every other day (450 μg/mouse/time point). Mice were monitored and assigned grades for clinical signs of EAE using the following scoring system: 0, healthy; 1, limp tail; 2, impaired righting reflex or waddled gait; 3, hind limb paralysis; 4, total limb paralysis; 5, moribund or death.
Proliferation assay and ELISA
Draining lymph node cells were isolated from treated mice and plated in round-bottomed 96-well plates (BD Biosciences) in culture medium with various concentrations of antigen. After 48 h, plates were pulsed for 16 h with 1 μCi [3H]-thymidine per well. Proliferation was measured as counts per minute by using a Wallac Liquid Scintillation Counter (Perkin Elmer). Cytokine production in the supernatant of cell cultures was measured by ELISA as described previously (42, 43).
Statistics
The clinical score and incidence of EAE were analyzed by Fisher's exact test, and other comparisons were analyzed by Student's t test. P < 0.05 was considered significant.
Results
Differential effects of RA on the development of Th17 and Foxp3+ Treg cells
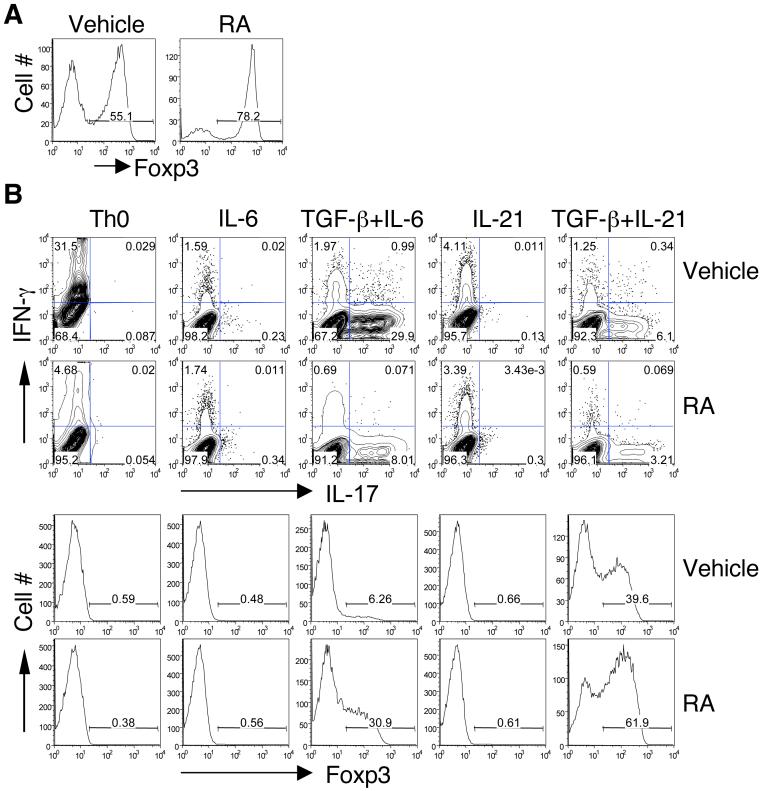
TGF-β plays an important role in Foxp3+ Treg conversion and together with IL-6 or IL-21, in Th17 development (27, 28). Several recent studies have shown that RA synergizes with TGF-β to induce iTreg cells (23-26) and reciprocally inhibit development of Th17 cells induced by TGF-β plus IL-6 (26). To understand the mechanism by which RA enhances iTreg conversion and inhibits Th17 differentiation, we first studied whether RA would affect the TGF-β-induced development of Foxp3+ Treg and Th17 cells. Naive CD4+ T cells were activated with anti-CD3 and anti-CD28 plus TGF-β, under these conditions 55% of T cells became Treg cells, as measured by Foxp3 expression (Figure 1A). Addition of RA further increased the frequency of Foxp3+ Treg cells (78%). In the absence of TGF-β, RA did not induce any Foxp3 expression (Figure 1B). Similarly, whereas TGF-β plus IL-6 or IL-21 induced IL-17, RA alone or in combination with IL-6 or IL-21 had no effect on IL-17 production. However, RA inhibited both TGF-β plus IL-6- and TGF-β plus IL-21-induced IL-17 production (Figure 1B). In both cases, the inhibition of IL-17 by RA was associated with an increased expression of Foxp3 suggesting that RA inhibited the capacity of both IL-6 and IL-21 to suppress the induction of Foxp3 by TGF-β. As reported previously (18), RA also strongly inhibited IFN-γ production in the neutral T cell differentiation conditions (Th0). These data indicate that although RA alone does not affect either IL-17 or Foxp3 expression, RA can enhance TGF-β-induced Foxp3+ Treg conversion and reciprocally inhibit IL-6- and IL-21-induced Th17 differentiation in a TGF-β dependent manner.
Figure 1. Differential effect of RA in the development of Foxp3+ Treg and Th17 cells.
Naïve CD4+ T cells were activated with anti-CD3 and anti-CD28 for 4 days under indicated conditions in the absence or presence of RA or Vehicle (DMSO). Aliquots of cells cultured with TGF-β (A) or different combinations of cytokines (B) were fixed, permeabilized, and stained intracellularly with PEFoxp3 mAb and analyzed by flow cytometry. Cells were also reactivated with PMA and ionomycin for 4 h in the presence of GolgiStop, and then stained with APC-IFN-g and PE-IL-17 mAb and analyzed by flow cytometry. Numbers in lower right quadrants indicate the percentage of IL-17-producing cells (B). Data are representative of 3-5 experiments.
RA enhances the expression and phosphorylation of Smad3 but not Smad2 in the presence of TGF-β
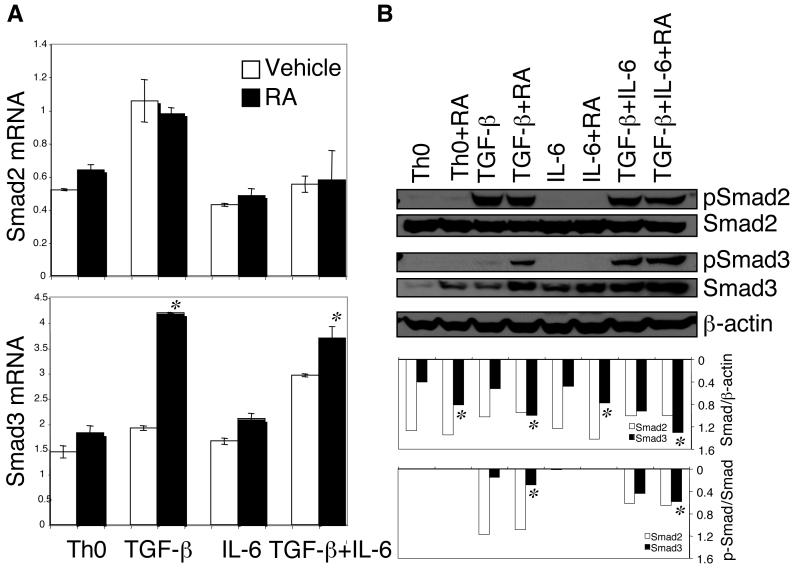
TGF-β signals through Smad2 and Smad3 to regulate various target genes including Foxp3 (7). Therefore, we examined whether RA affected Smad2 and Smad3 transcription during Treg conversion. As shown in Figure 2A, RA did not affect the TGF-β-induced expression of Smad2 mRNA. In contrast, RA clearly increased the TGF-β-induced expression of Smad3 mRNA.
Figure 2. RA enhanced TGF-β-induced expression and phosphorylation of Smad3 but not Smad2.
Naïve CD4+ T cells were activated with anti-CD3 and anti-CD28 for 24 h under indicated conditions with or without RA. (A) Total RNA was isolated and expression of Smad2 and Smad3 mRNA was determined by Taqman quantitative PCR. (B) Cell lysates from indicated conditions were separated by SDS-PAGE gel, and total Smad and p-Smad were detected by immunoblotting. For quantification, the ratio of total Smad/β-actin and the ratio of p-Smad/total Smad were determined. Data are representative of three experiments. *, P <; 0.05 when RA treatment was compared to vehicle treatment.
We then determined the levels of total and phosphorylated Smad (p-Smad) proteins by Western blot. Neither expression nor phosphorylation of Smad2 was affected by RA in any condition, whereas the TGF-β-induced expression of Smad3 and its phosphorylation (p-Smad3) were markedly increased in the presence of RA (Figure 2B). RA alone apparently increased the expression of Smad3 but had no effect on its phosphorylation (Figure 2B). These data suggest that RA in the presence of TGF-β specifically enhances the expression and phosphorylation of Smad3, which then upregulates the expression of target genes including Foxp3
RA inhibits TGF-β-induced expression of IL-6Rα
Besides inducing Smad3 and Foxp3, RA also inhibited the generation of Th17 cells by TGF-β plus IL-6. We wanted to determine whether the increase in TGF-β-induced Foxp3 expression was sufficient to explain Th17 inhibition. Thus, we analyzed IL-6 signaling in the presence of RA. First, we determined whether RA affected the expression of IL-6 receptors.
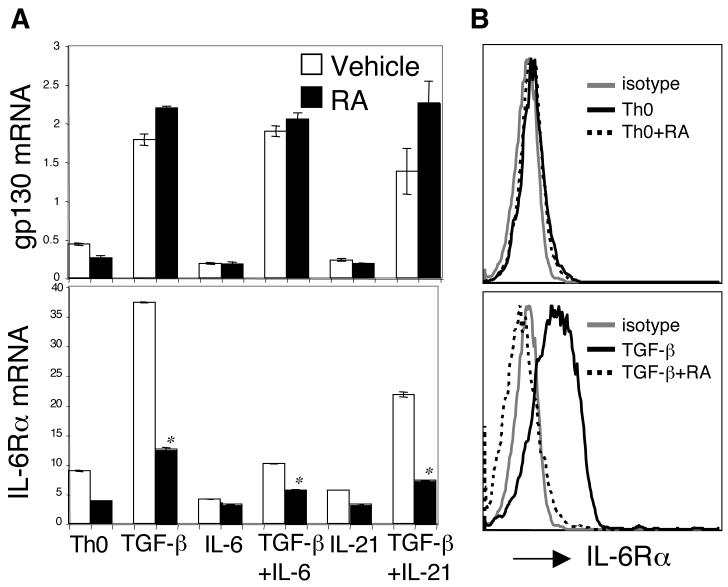
IL-6 receptors consist of the IL-6Rα and gp130 subunits. Interestingly, TGF-β but not IL-6 or IL-21 upregulated the expression of both IL-6Rα and gp130 mRNA in CD4+ T cells (Figure 3A). TGF-β-induced gp130 upregulation was not affected by RA. However, RA strongly inhibited the upregulation of IL-6Rα mRNA by TGF-β (Figure 3A). Consistent with the quantitative PCR data, surface expression of IL-6Rα on CD4+ T cells was upregulated by TGF-β, which was blocked by RA (Figure 3B). Similar results were observed in the condition where RA was included together with TGF-β plus IL-21 (data not shown). Collectively, these data suggest that the RA-mediated inhibition of Th17 differentiation not only relates to an increased Foxp3 expression, but also to blocking of the TGF-β-induced upregulation of the IL-6Rα and thus prevention of IL-6 signaling.
Figure 3. RA blocked TGF-β-induced upregulation of IL-6Rα expression.
Naïve CD4+ T cells were activated under indicated conditions with or without RA. After 48 h, cells were collected for the determination of gp130 and IL-6Rα mRNA by Taqman quantitative PCR (A) and surface expression of IL-6Ra protein by flow cytometry (B). Data are representative of three experiments. *, P < 0.05 when RA treatment compared to vehicle treatment.
Effect of RA on STAT3 phosphorylation, IL-21 production, and IL-23R expression during Th17 differentiation
Naive CD4+ T cells activated by IL-6 secrete IL-21 through STAT3. In concert with TGF-β, IL-21 induces RORγt and IL-17 expression. IL-21, which amplifies its own production by an autocrine feedback loop, also acts upstream of RORγt mainly through STAT3 to induce the expression of IL-23R. Engagement of IL-23R by IL-23 induces further RORγt expression and stabilizes the Th17 phenotype (3, 9, 10). Given that RA inhibited TGF-β-induced upregulation of IL-6Rα, we then examined whether RA could directly influence downstream components of the IL-6 signaling pathway.
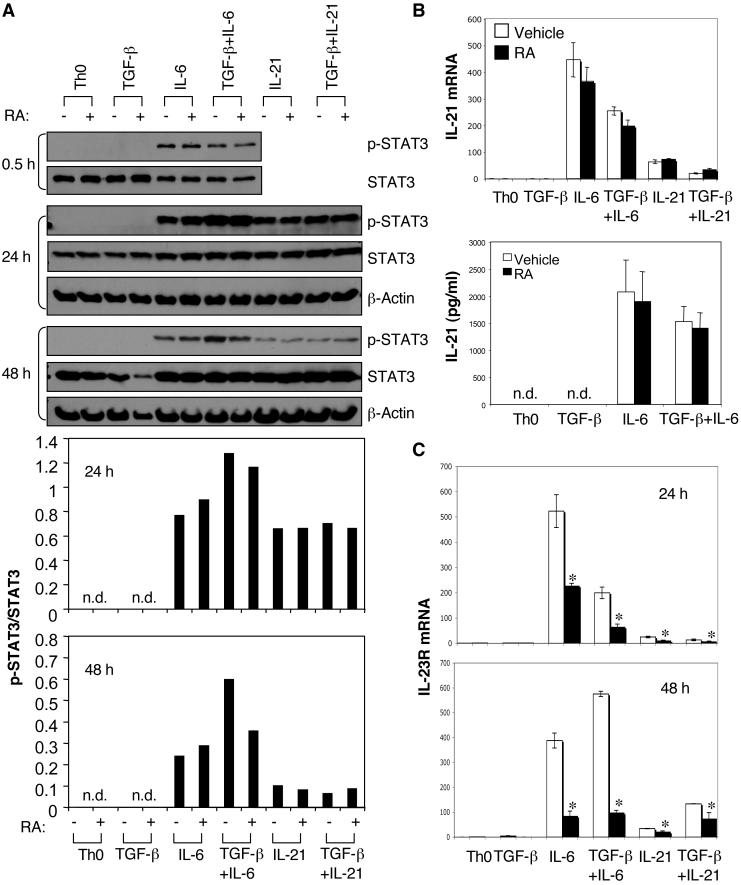
We first examined STAT3 phosphorylation (p-STAT3) induced by IL-6 in the presence of RA. After 30 min, neither TGF-β nor RA affected IL-6-induced STAT3 phosphorylation (Figure 4A). Because the effect of RA may not be achieved as early as 30 min, we therefore determined STAT3 phosphorylation after longer treatments. After 24h-culture, a sustained level of p-STAT3 was observed by the treatment with IL-6 alone, this level was further increased in the presence TGF-β (Figure 4A), which is in agreement with the observation that TGF-β could upregulate the expression of IL-6 receptors. After 24h, RA still did not affect IL-6 or IL-21-induced STAT3 phosphorylation irrespective of whether or not TGF-β was present. Notably, unlike in the condition with IL-6, addition of TGF-β did not increase IL-21-induced STAT3 phosphorylation, indicating that TGF-β may not affect the expression of the IL-21 receptor. However after 48h culture, RA decreased the IL-6 plus TGF-β-driven enhancement of p-STAT3 to the level obtained with IL-6 alone (Figure 4A). These data demonstrate that while TGF-β enhances IL-6-induced p-STAT3 by upregulating the expression of IL-6 receptor subunits, this enhancement is inhibited by RA. The data also illustrate that although RA decreases the level of p-STAT3 induced by IL-6 plus TGF-β, it does not inhibit STAT3 phosphorylation induced by IL-6 alone, IL-21 alone or IL-21 plus TGF-β (Figure 4A). Taken together, these data suggest that RA is unlikely to directly affect IL-6- or IL-21-mediated STAT3 phosphorylation. Instead, RA can indirectly decrease the level of IL-6-induced p-STAT3 in the presence of TGF-β by inhibiting the TGF-β-induced upregulation of IL-6Rα.
Figure 4. Effect of RA on STAT3 phosphorylation, IL-21 production, and IL-23R expression in CD4+ cells cultured with different combinations of TGF-β, IL-6, and IL-21.
Naïve CD4+ T cells were activated under indicated conditions with or without RA. Cells were collected for the determination of STAT3 phosphorylation by immunoblotting at various time points, and the ratio of p-STAT3/total STAT3 was analyzed (A). IL-21 mRNA expression at 24 h by Taqman quantitative PCR and IL-21 production in 96-h culture supernatants by ELISA (B). Expression of IL-23R mRNA at 24 h and 48 h by Taqman quantitative PCR (C). Data are representative of 3-5 experiments. *, P < 0.05 when RA treatment was compared to vehicle treatment.
We next examined the IL-6- and IL-21-induced IL-21 expression in the presence of RA. As shown in Figure 4B, both IL-6 and IL-21 enhanced expression of IL-21 mRNA. However, IL-6 is a stronger inducer of IL-21 than IL-21 itself. Addition of RA did not affect the expression of IL-21 mRNA or IL-21 protein (Figure 4B).
Both IL-6 and IL-21 induce IL-23R expression and both IL-6-induced and IL-21-induced expression of IL-23R is independent on TGF-β (29). However, irrespective of the stimulation condition, the expression of IL-23R is dependent on the transcription factor RORγt. As the expression of RORγt has been shown to be decreased in the presence of RA (26, 30), we predicted that the expression of IL-23R would also be decreased by RA treatment. Indeed, the expression of IL-23R was strongly suppressed by RA irrespective of whether T cells were activated in the presence of IL-6 alone or IL-6 plus TGF-β. RA similarly inhibited the IL-23R expression when incubated with IL-21 alone or TGF-β plus IL-21, although the IL-23R upregulation with IL-21 was much lower than that with lL-6 (Figure 4C).
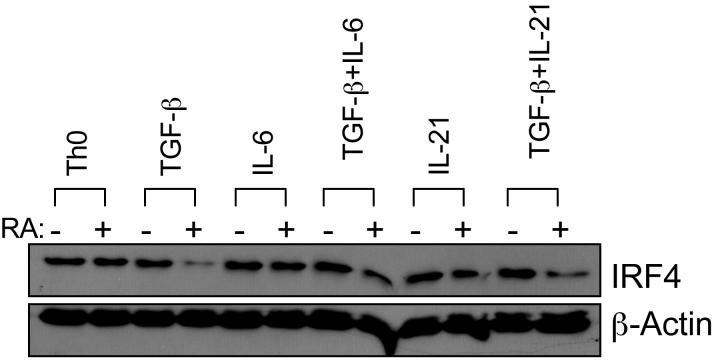
RA decreased IRF4 expression in both Th17 and Treg cell cultures
Given that IRF4, a transcription factor induced by TcR/CD3 signaling, is also required for Th17 differentiation (11), we studied the effect of RA on IRF4 expression during the development of Th17 and Treg cells. When RA was added to the stimulation cocktail, a significant decrease of IRF4 expression could only be observed in conditions containing TGF-β. RA did not affect IRF4 expression under Th0 condition or conditions containing IL-6 or IL-21 alone (Figure 5). These data suggest that RA downregulates IRF4 expression through cooperating with TGF-β signaling, which is TGF-β dependent.
Figure 5. RA decreased expression of IRF4 protein in the presence of TGF-β, IL-6 or IL-21.
Naïve CD4+ T cells were activated under indicated conditions with or without RA. Cells were collected after 24 h or 48 h, lyzed, and detected for IRF4 protein expression by immunoblotting. Data are representative of three experiments.
In vivo treatment with RA decreased antigen-specific Th17 and Th1 responses but had only minor effects on the Foxp3+ Treg population
RA enhanced TGF-β-induced Foxp3+ Treg cell conversion but reciprocally inhibited TGF-β plus IL-6/IL-21-induced Th17 cell differentiation in vitro. Therefore, we next examined in vivo effects of RA on T cell responses and the development of experimental autoimmune encephalomyelitis (EAE), which serves as an animal model for multiple sclerosis (31). EAE is mediated by myelin-reactive Th17 and Th1 cells, and Treg and Th2 cells have been shown to inhibit EAE (5, 31). From our in vitro data we predicted that RA would increase the Treg population but decrease encephalitogenic Th17 responses and thus inhibit development of EAE.
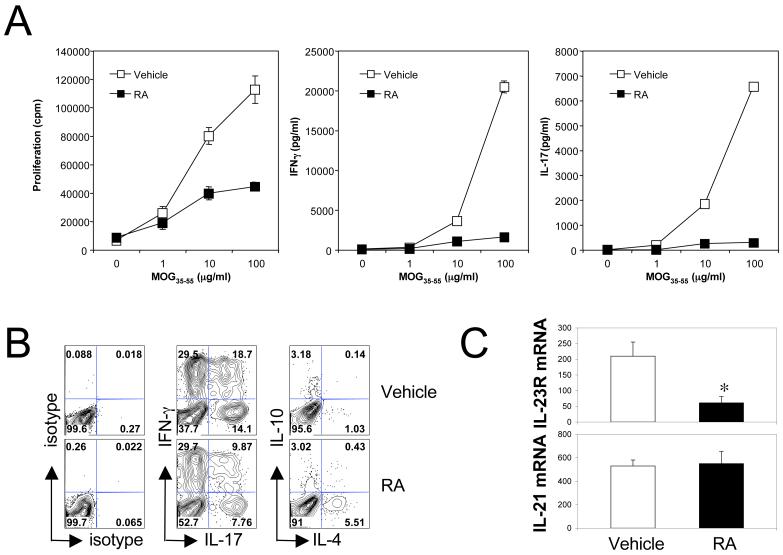
We first determined the effect of RA on the development of antigen-specific effector T cell responses. Foxp3gfp.KI mice were immunized with MOG35-55/CFA and intraperitoneally treated with RA in corn oil every other day starting from day 0. On day 10, draining lymph node (LN) cells from these MOG35-55-immunized Foxp3gfp.KI mice treated with RA were tested for T cell proliferation and cytokine production. Compared to vehicle treated animals, cells from RA-treated mice showed decreased proliferation upon antigen restimulation in vitro (Figure 6A). In line with the decreased proliferation, RA dramatically inhibited the production of IL-17 as well as IFN-γ upon antigen specific restimulation, suggesting that RA strongly inhibited the priming/expansion of pathogenic Th17 and Th1 responses in vivo. However, the decrease of IFN-γ and IL-17 in the culture supernatants may reflect a decrease in the production of these effector cytokines or a decrease in the frequency of IL-17- and IFN-γ-producing T cells. By intracellular cytokine staining we could clearly see a decreased frequency of IL-17- and IL-17/IFN-γ-producing CD4+ T cells in RA treated mice (Figure 6B). Furthermore, we found that CD4+ T cells from RA-treated mice showed a strong reduction of IL-23R mRNA expression but no change in IL-21 mRNA expression (Figure 6C) thus corroborating the in vitro data. These data suggest that the reduced Th17 response was also partly due to the decreased expression of IL-23R after RA treatment in vivo.
Figure 6. Treatment with RA decreased pathogenic Th17 and Th1 responses and IL-23R expression in CD4+ T cells.
C57BL/6 mice were immunized with MOG35-55/CFA, and RA was intraperitoneally injected every other day from day 0 to day 8. On day 10, (A) draining lymph node cells were isolated and restimulated with MOG35-55. Proliferation and cytokine production in 48-h culture supernatants were measured by 3[H]-thymidine incorporation and ELISA assays, respectively; (B) isolated spleen cells were cultured with 5 μg/ml of MOG35-55 for 5 days. Cytokine production from CD4+ cells was determined by intracellular cytokine staining following 4-h activation with PMA and ionomycin; (C) expression of IL-23R and IL-21 mRNA was measured in CD4+ T cells purified from spleen cells by Taqman quantitative PCR. *, P < 0.05 when RA treatment was compared to vehicle treatment. Data are the average or representative of 4-6 mice in each group.
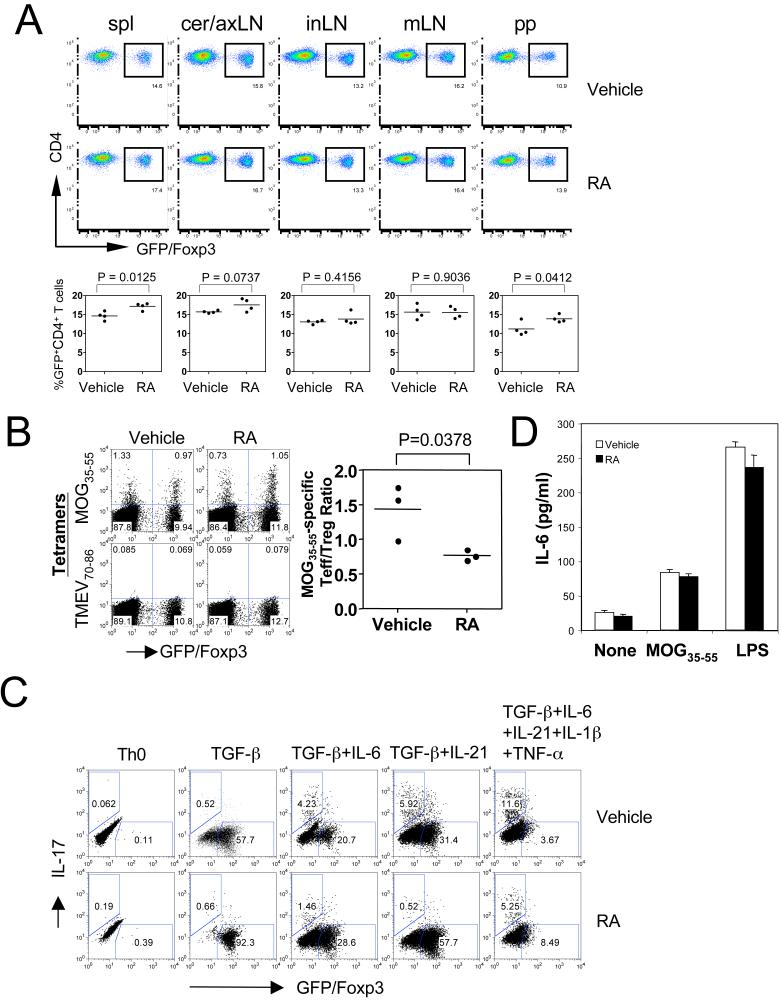
We then analyzed the in vivo effect of RA on Treg cells in these immunized Foxp3gfp.KI mice, which allow us to track Foxp3+ Treg cells based on GFP expression (32). We determined the frequency of Foxp3/GFP+ cells among CD4+ T cells in different lymphoid compartments on day 10 after immunization. Surprisingly, RA treatment only slightly increased the frequency of Foxp3+ Treg cells among CD4+ T cells in the spleen and peyer's patches but not in cervical and axillary, inguinal or mesenteric lymph nodes from MOG35-55-immunized mice (Figure 7A). Furthermore, the MOG35-55/IAb tetramer staining demonstrated that RA treatment did not increase the frequency of tetramer-reactive MOG35-55-specific Foxp3+ Treg cells. However, RA treatment inhibited dramatically the frequency of antigen-specific effector T cells and therefore reduced the ratio of MOG35-55-specific effector T versus Treg cells (Figure 7B).
Figure 7. RA in vivo did not measurably increase the population of Foxp3+ Treg cells.
Foxp3gfp.KI mice were immunized with MOG35-55/CFA, and RA was intraperitoneally injected every other day from day 0 to day 8. On day 10, (A) the frequency of GFP+ Treg cells was determined in different lymphoid compartments by flow cytometry. “Spl”, spleen; “cer/ax”, cervical/axillary; “in”, inguinal; “m”, mesenteric; “pp”, peyer's patch; (B) spleen cells were restimulated with 2 μg/ml of MOG35-55 for 5 days, and then stained with PE-conjugated IAb tetramer, APC-anti-CD4, and 7-AAD. The MOG35-55/IAb tetramer-positive cells were determined in the live CD4+ cell population (CD4+7-AAD-). TMEV70-86/IAS tetramers were used as a negative control. (C) Inflammatory cytokines IL-6, TNF-α, and IL-1β inhibited RA-enhanced Foxp3 expression but could not rescue RA-inhibited IL-17 production. Naïve CD4+ T cells (CD4+ CD62Lhi Foxp3/GFP-) purified from Foxp3gfp.KI mice were activated with anti-CD3 and anti-CD28 under indicated conditions in the absence or presence of RA for 5 days. Cells were reactivated with PMA and ionomycin for 4 h in the presence of GolgiStop. Then, cells were stained with APC-anti-CD4, PE-anti-IL-17 and 7-AAD, and analyzed by flow cytometry for IL-17 production and Foxp3/GFP expression in gated CD4+7-AAD- cells. (D) Isolated spleen cells from immunized mice on day 10 were cultured with 5 μmg/ml of MOG35-55 peptide or 500ng/ml LPS. IL-6 production was determined in 48-h cultures by ELISA. None, no treatment.
We have shown that IL-6 inhibits the generation of iTreg cells and in MOG/CFA immunized mice or under strongly inflammatory conditions (in the presence of IL-6, TNF-α, and IL-1β), antigen specific Treg cells are not generated de novo but are derived from nTreg cells (32). Therefore, we first determined the effect of IL-6, TNF-α, and IL-1β on the generation of Foxp3+ iTreg cells induced by TGF-β. As an inflammatory cytokine, IL-6 showed a stronger inhibitory capacity in TGF-β-induced Foxp3 expression than IL-21, although both cytokines could induce the production of IL-17, which was dramatically inhibited by RA treatment (Figure 7C). However, addition of all the inflammatory cytokines (IL-6, TNF-α, and IL-1β) into Th17 differentiation cultures strongly inhibited the enhancement of Foxp3 by RA, but could not abrogate the inhibitory effects of RA on IL-17 production (Figure 7C). Also, spleen cells from RA-treated mice produced similar amounts of IL-6 upon treatment with antigen or LPS (Figure 7D), suggesting that RA treatment did not decrease IL-6 production.
RA treatment inhibits EAE both during the induction and the effector phase of the disease
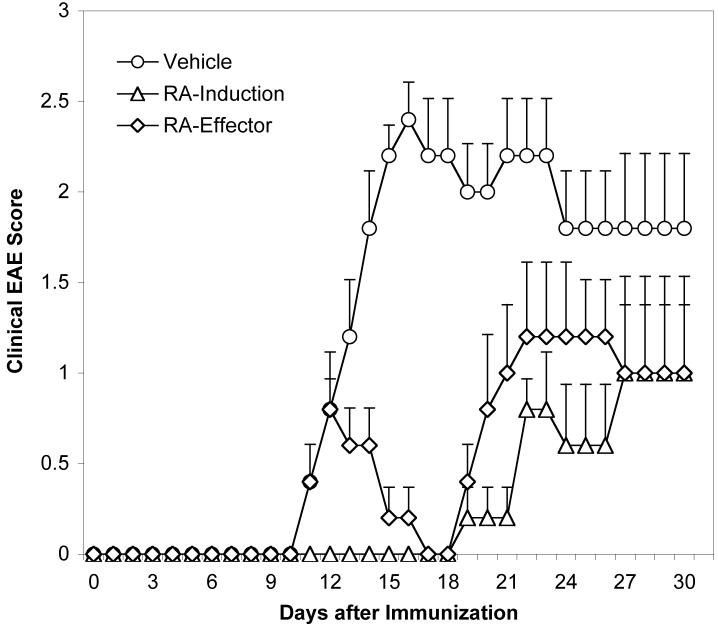
Although RA treatment in vivo under inflammatory conditions (i. e., immunization with CFA) has only minor effects on the Foxp3+ Treg population, pathogenic Th17 and Th1 responses nevertheless were strongly inhibited by RA. Therefore, based on the shift in the ratio of MOG35-55-reactive effector and regulatory T cells, RA would inhibit development of EAE. Indeed, when mice were immunized with MOG35-55/CFA to induce EAE, treatment with RA from day 0 not only dramatically delayed the disease onset but also strongly decreased the disease severity, compared to the control group (Figure 8 and Table I). Furthermore, when administrated therapeutically after the onset of clinical signs of EAE, RA significantly ameliorated the disease course (Figure 8 and Table I). These results confirm that RA treatment in vivo not only inhibits proinflammatory Th17 and Th1 responses, but also has functional consequences in that it dramatically suppresses the progression and severity of autoimmune inflammation.
Figure 8. Treatment with RA inhibited EAE both during the induction and after disease onset.
C57BL/6 mice were immunized with MOG35-55/CFA, and RA was intraperitoneally injected every other day starting from day 0 (RA-induction) or after all mice in the group showed signs of clinical EAE (RA-effector). Mice were evaluated daily for the signs of EAE. EAE was significantly (P < 0.05) reduced in mice in the “RA-induction” group after day 10 and in the “RA-effector” group after day12 compared to the vehicle treated mice.
Table I.
EAE in RA- and vehicle-treated mice
| Treatment | Incidence | Mean day of onset (mean ± SEM) | Mean Maximum score (mean ± SEM) |
|---|---|---|---|
| Vehicle | 10 of 10 (100%) | 12.20 ± 1.23 | 2.40 ± 0.52 |
| RA-Induction | 8 of 10 (80%) | 21.25 ± 1.39a,b | 1.75 ± 0.89a,b |
| RA-Effector | 10 of 10 (100%) | 11.60 ± 0.52c | 1.00 ± 0.94c |
Represents the mice that showed clinical signs of EAE (8 of 10 mice)
P<0.05, RA-treated groups compared with vehicle-treated group
P<0.05, RA-treated groups compared with vehicle-treated group
Discussion
RA, the physiological vitamin A metabolite, has recently been shown to enhance TGF-β-induced Foxp3 expression and Treg conversion (23-26) and to decrease Th17 differentiation and IL-17 production (26). In this study, we confirmed these results and determined the mechanism by which RA increased Foxp3 expression but reciprocally decreased IL-17 production. Furthermore, we analyzed whether RA exerted similar effects in vivo during an autoimmune reaction. We found that RA affected components in the TGF-β, IL-6/IL-21, and TcR/CD3 signaling pathways that are involved in the development of Treg and Th17 cells. Moreover, treatment with RA prevented autoimmune disease mainly by decreasing pathogenic Th17 and Th1 responses, however, under this in vivo setting RA had no significant effect on Treg cells possibly due to ongoing inflammation.
RA strongly increased TGF-β-induced Foxp3 expression in vitro and thus enhanced Treg conversion. Under these conditions, RA enhanced TGF-β signaling by increasing the expression and phosphorylation of Smad3, which is one of the key elements responsible for the induction Foxp3. RA exerts its regulatory effects by binding to its receptor RAR, which is a transcription factor by itself. RA alone in the absence of TGF-β could increase the expression of Smad3, however, the increased Smad3 phosphorylation and Foxp3 expression by RA is completely dependent on TGF-β. On the other hand, it has been reported that the expression of RAR can be increased by TGF-β signaling (33). Therefore, RA and TGF-β could cooperatively augment their mutual signaling to further enhance Foxp3 expression. Moreover, RA may also enhance Smad3-driven transactivation by promoting a direct RAR-Smad3 interaction since a direct RAR/Smad3 interaction has been demonstrated previously (34). RA has also been reported to increase histone acetylation of the Foxp3 promoter regions (35). Since histone acetylation is associated with increased gene activation and expression (36), this may contribute to the RA-mediated increase in TGF-βinduced Foxp3 transcription.
In addition to the positive effect on TGF-β-induced Foxp3 expression and Treg conversion, RA also inhibited IL-6/IL-21-mediated Th17 differentiation. Inhibition of Th17 differentiation by RA appears to be achieved by multiple mechanisms: 1) The enhanced TGF-β-induced Foxp3 expression by RA counteracts Th17 differentiation; 2) Inhibition of IL-6 receptor upregulation by RA decreases Th17 differentiation; 3) RA downregulates IRF4 expression and thus impairs IL-6-induced inhibition of Foxp3 expression and finally 4) RA inhibits IL-23R expression and thus impairs the stabilization and further maturation of the Th17 phenotype. The inhibition of IL-6 receptor expression not only decreases IL-6-initiated Th17 differentiation directly but also counteracts the inhibitory effect of IL-6 on Foxp3 induction and results in increased Foxp3 levels that further inhibit the induction of IL-17. RA seems to have only minor effects on proximal IL-6 or IL-21 signaling events. RA did not considerably affect either STAT3 phosphorylation or IL-21 expression induced by IL-6 or IL-21. However, RA reduced the expression of IL-23R induced by either IL-6 or IL-21 alone or in combination with TGF-β. This might be due to the fact that the RA/RAR complex binds to the promoter of the IL-23R gene and transcriptionally represses IL-23R expression. Alternatively, RA binding to RAR might exert a direct inhibitory effect on IL-6/IL-21 signaling downstream of STAT3 since the expression of IL-23R is a downstream event of RORγt function and inhibition of RORγt expression by RA has recently been reported (26, 30). Both RAR and RORγt belong to a large superfamily of ligand-inducible nuclear receptors that includes RXR and ROR, steroid, vitamin D, and thyroid hormone receptors, estrogen receptor, peroxisome proliferator-activated receptors (PPAR), and a number of orphan receptors whose ligands are unknown as yet (15). Direct interactions among the family members (such as RAR/RXR heterodimers and RXR/vitamin D3 receptor heterodimers) and between the transcription factors from other families (such as vitamin D3 receptor/c-Jun and RAR/STAT3 interactions) have been reported (15, 37). Therefore, it is possible that RA interferes with the transactivation function of STAT3 and/or RORγt by inducing direct RAR/STAT3 and/or RAR/RORγt interactions and thus inhibiting the IL-6/IL-21-induced expression of IL-23R, in addition to RA caused decrease of RORγt expression. Conversely, it will be interesting to determine whether RAR also binds to Foxp3, similar to NFAT (38), and enhances Foxp3 function.
Although downregulation of IL-23R by RA may not play a role for Th17 differentiation in our in vitro APC-free systems, in which there is no source of IL-23 cytokine, IL-23/IL-23R signaling is required for stabilizing the development and pathogenic function of Th17 cells in vivo (10). Indeed, RA-treated MOG35-55-immunized mice have a decrease in the generation of pathogenic Th17 responses, which is associated with a decreased expression of IL-23R. In addition to its direct inhibition of pathogenic Th17 and Th1 responses, RA also exerts this effect indirectly by decreasing the ability of APCs to generate Th1 and Th17 cells (data not shown), although RA does not affect the production of IL-6 in these cells (Figure 7D). It has previously been demonstrated that RA impairs the secretion of IL-12 from APCs and therefore decreases Th1 differentiation (39, 40). Whether RA-treated dendritic cells have an impaired IL-23 production remains to be determined. Taken together, we propose that in vivo, the inhibitory effects of RA on the generation of Th17 cells are due to direct inhibitory effects on Th17 cells and modulation of APC function.
The decreased ratio of antigen-specific effector versus regulatory T cells upon treatment with RA attenuates autoimmunity. Here, we provide evidence that this change in Teff/Treg ratio is mainly due to a decrease of effector T cells in RA-treated mice. Unlike the in vitro observation that RA strongly enhances Treg conversion, RA treatment in vivo only slightly increases the population of Treg cells. Interestingly, Mucida et al also observed a remarkable reduction of Th17 cells but not measurable enhancement of the differentiation of Foxp3+ Treg cells in mice infected with Listeria monocytogenes and treated with RA, and they suggested that TGF-β might be a limiting factor in the lack of Treg cell differentiation induced by exogenous RA in vivo (26). Here we show that this minor expansion of Treg cells by RA in vivo appears to be due to the strong induction of IL-6 as well as TNF-α and IL-1 during the inflammatory process induced by MOG35-55/CFA immunization as addition of all these inflammatory cytokines into Th17 differentiation cultures in vitro strongly inhibited the enhancement of Foxp3 by RA, but could not abrogate the inhibitory effects of RA on Th17 cells (Figure 7C). This is also consistent with our previous data showing that IL-6 inhibits the generation of iTreg cells and in MOG/CFA immunized mice or under strongly inflammatory conditions, antigen specific Treg cells are not generated de novo but are derived from nTreg cells (32).
The significant reduction of EAE by RA is associated with a decrease in Th17 and Th1 responses. However, the observation that IFN-g signaling may not be absolutely required for the induction of EAE (2, 3) suggests that the decreased Th17 response is most likely responsible for the reduction of EAE by RA. Also, the decreased IL-23R expression by RA impairs IL-23 signaling which further dampens the encephalitogenicity of Th17 cells, since IL-23 signaling has been shown to be crucial for full expression of the pathogenic phenotype in Th17 cells (10). RA treatment after disease onset still attenuates the severity of EAE, suggesting that RA also inhibits established disease by regulating not just the expansion but most likely the effector functions of pathogenic Th17 and Th1 cells. Accordingly, we observed that addition of RA into in vitro cultures of LN or spleen cells from mice with EAE also strongly inhibited CD4+ T cell proliferation and their production of IL-17 and IFN-g upon antigenic restimulation (data not shown).
Although under inflammatory conditions in vivo, RA has minor effects on increasing the frequency of Treg cells, it is likely that the blockade of TGF-β-induced upregulation of IL-6Rα and the decrease of IRF4 expression by RA would reduce IL-6-mediated Treg inhibition (41) and thus enhance their suppressive function, which may also play a role in the strong inhibition of pathogenic Th1/Th17 responses in RA treated mice during EAE.
In summary, we demonstrated that RA both in vitro and in vivo inhibits the development of Th17 cells by interfering with signaling events in the TGF-β and IL-6/IL-21/IL-23 pathways that are involved in the development and function of these cells. Although RA strongly promotes Treg conversion in vitro, RA treatment in vivo does not considerably increase the population of Treg cells in the face of ongoing inflammation. However, in the absence of strong inflammatory conditions, RA may be able to increase Treg cells in vivo as well. Therefore, RA regulates the balance between pathogenic Th17 and protective Treg cells by enhancing TGF-β signaling and by suppressing IL-6/IL-21/IL-23-driven signaling.
Acknowledgments
We thank D. Lee for animal care and D. Kozoriz for cell sorting.
This work is supported by research grants from the National Multiple Sclerosis Society (M. Oukka and V.K. Kuchroo) and the National Institutes of Health (M. Oukka, B. Lim, and V.K. Kuchroo). S. Xiao is a recipient of the National Multiple Sclerosis Society Postdoctoral Fellowship. T. Korn is supported by the Deutsche Forschungsgemeinschaft. S. Liu is supported by the Australian NH&MRC. B. Lim is a recipient of translational grant from Leukemia & Lymphoma Society of America. V.K. Kuchroo is a recipient of the Javits Neuroscience Investigator Award from the National Institutes of Health.
References
- 1.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 4.Korn T, Oukka M. Dynamics of antigen-specific regulatory T-cells in the context of autoimmunity. Semin Immunol. 2007;19:272. doi: 10.1016/j.smim.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Oukka M. Interplay between pathogenic Th17 and regulatory T cells. Ann Rheum Dis. 2007;66(Suppl 3):iii87. doi: 10.1136/ard.2007.078527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 7.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 8.von Boehmer H. Oral tolerance: is it all retinoic acid? J Exp Med. 2007;204:1737. doi: 10.1084/jem.20071251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007 doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 11.Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 12.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 13.Chambon P. A decade of molecular biology of retinoic acid receptors. Faseb J. 1996;10:940. [PubMed] [Google Scholar]
- 14.Collins SJ. The role of retinoids and retinoic acid receptors in normal hematopoiesis. Leukemia. 2002;16:1896. doi: 10.1038/sj.leu.2402718. [DOI] [PubMed] [Google Scholar]
- 15.Winoto A, Littman DR. Nuclear hormone receptors in T lymphocytes. Cell. 2002;109(Suppl):S57. doi: 10.1016/s0092-8674(02)00710-9. [DOI] [PubMed] [Google Scholar]
- 16.Iwata M, Mukai M, Nakai Y, Iseki R. Retinoic acids inhibit activation-induced apoptosis in T cell hybridomas and thymocytes. J Immunol. 1992;149:3302. [PubMed] [Google Scholar]
- 17.Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol. 2003;15:1017. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- 18.Racke MK, Burnett D, Pak SH, Albert PS, Cannella B, Raine CS, McFarlin DE, Scott DE. Retinoid treatment of experimental allergic encephalomyelitis. IL-4 production correlates with improved disease course. J Immunol. 1995;154:450. [PubMed] [Google Scholar]
- 19.Nozaki Y, Yamagata T, Sugiyama M, Ikoma S, Kinoshita K, Funauchi M. Anti-inflammatory effect of all-trans-retinoic acid in inflammatory arthritis. Clin Immunol. 2006;119:272. doi: 10.1016/j.clim.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Zunino SJ, Storms DH, Stephensen CB. Diets rich in polyphenols and vitamin A inhibit the development of type I autoimmune diabetes in nonobese diabetic mice. J Nutr. 2007;137:1216. doi: 10.1093/jn/137.5.1216. [DOI] [PubMed] [Google Scholar]
- 21.Osanai M, Nishikiori N, Murata M, Chiba H, Kojima T, Sawada N. Cellular retinoic acid bioavailability determines epithelial integrity: Role of retinoic acid receptor alpha agonists in colitis. Mol Pharmacol. 2007;71:250. doi: 10.1124/mol.106.029579. [DOI] [PubMed] [Google Scholar]
- 22.Perez de Lema G, Lucio-Cazana FJ, Molina A, Luckow B, Schmid H, de Wit C, Moreno-Manzano V, Banas B, Mampaso F, Schlondorff D. Retinoic acid treatment protects MRL/lpr lupus mice from the development of glomerular disease. Kidney Int. 2004;66:1018. doi: 10.1111/j.1523-1755.2004.00850.x. [DOI] [PubMed] [Google Scholar]
- 23.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 27.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 28.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 30.Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O'Shea JJ. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuchroo VK, Anderson AC, Waldner H, Munder M, Bettelli E, Nicholson LB. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu Rev Immunol. 2002;20:101. doi: 10.1146/annurev.immunol.20.081701.141316. [DOI] [PubMed] [Google Scholar]
- 32.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol. 2007;37:2396. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- 34.Pendaries V, Verrecchia F, Michel S, Mauviel A. Retinoic acid receptors interfere with the TGF-beta/Smad signaling pathway in a ligand-specific manner. Oncogene. 2003;22:8212. doi: 10.1038/sj.onc.1206913. [DOI] [PubMed] [Google Scholar]
- 35.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179:3724. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 36.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 37.Dong S, Chen SJ, Tweardy DJ. Cross-talk between retinoic acid and STAT3 signaling pathways in acute promyelocytic leukemia. Leuk Lymphoma. 2003;44:2023. doi: 10.1080/1042819031000116670. [DOI] [PubMed] [Google Scholar]
- 38.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 39.Kang BY, Chung SW, Kim SH, Kang SN, Choe YK, Kim TS. Retinoid-mediated inhibition of interleukin-12 production in mouse macrophages suppresses Th1 cytokine profile in CD4(+) T cells. Br J Pharmacol. 2000;130:581. doi: 10.1038/sj.bjp.0703345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoag KA, Nashold FE, Goverman J, Hayes CE. Retinoic acid enhances the T helper 2 cell development that is essential for robust antibody responses through its action on antigen-presenting cells. J Nutr. 2002;132:3736. doi: 10.1093/jn/132.12.3736. [DOI] [PubMed] [Google Scholar]
- 41.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 42.Xiao S, Najafian N, Reddy J, Albin M, Zhu C, Jensen E, Imitola J, Korn T, Anderson AC, Zhang Z, Gutierrez C, Moll T, Sobel RA, Umetsu DT, Yagita H, Akiba H, Strom T, Sayegh MH, DeKruyff RH, Khoury SJ, Kuchroo VK. Differential engagement of Tim-1 during activation can positively or negatively costimulate T cell expansion and effector function. J Exp Med. 2007;204:1691. doi: 10.1084/jem.20062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakravarti S, Sabatos CA, Xiao S, Illes Z, Cha EK, Sobel RA, Zheng XX, Strom TB, Kuchroo VK. Tim-2 regulates T helper type 2 responses and autoimmunity. J Exp Med. 2005;202:437. doi: 10.1084/jem.20050308. [DOI] [PMC free article] [PubMed] [Google Scholar]