Abstract
Abnormal N-methyl-d-aspartate receptor (NMDAR) function has been implicated in the pathophysiology of schizophrenia. d-serine is an important NMDAR modulator, and to elucidate the role of the d-serine synthesis enzyme serine racemase (Srr) in schizophrenia, we identified and characterized mice with an ENU-induced mutation that results in a complete loss of Srr activity and dramatically reduced d-serine levels. Mutant mice displayed behaviors relevant to schizophrenia, including impairments in prepulse inhibition, sociability and spatial discrimination. Behavioral deficits were exacerbated by an NMDAR antagonist and ameliorated by d-serine or the atypical antipsychotic clozapine. Expression profiling revealed that the Srr mutation influenced several genes that have been linked to schizophrenia and cognitive ability. Transcript levels altered by the Srr mutation were also normalized by d-serine or clozapine treatment. Furthermore, analysis of SRR genetic variants in humans identified a robust association with schizophrenia. This study demonstrates that aberrant Srr function and diminished d-serine may contribute to schizophrenia pathogenesis.
INTRODUCTION
Schizophrenia is a severely debilitating psychiatric disorder that affects up to 1% of the population worldwide. In addition to the psychotic features of delusions, hallucinations and disorganized thought, schizophrenia is characterized by profound cognitive deficits, including impairments in attention, learning and working memory and by negative symptoms that involve social withdrawal and affective flattening (1,2). Current anti-psychotics have limited efficacy, particularly in ameliorating the cognitive and negative symptoms, and further understanding of the molecular mechanisms that underlie schizophrenia is imperative for the development of novel therapeutics (1,3).
Deficient glutamatergic neurotransmission mediated by the N-methyl-d-aspartate receptor (NMDAR) may be involved in the pathophysiology of schizophrenia (4). Non-competitive antagonists of the NMDAR, such as phencyclidine, elicit schizophrenic-like symptoms in healthy individuals and exacerbate such symptoms in patients (5,6). Schizophrenia is known to be influenced by heritable factors and genetic studies have identified a number of susceptibility genes that modulate NMDAR function (2,4). These include several genes that regulate the endogenous NMDAR glycine site agonist, d-serine (7–9). Growing evidence indicates that the d-serine catabolic enzyme, d-amino acid oxidase (DAO), and its activator, G72, are associated with an increased risk of schizophrenia (7,8,10), and DAO activity is augmented in the brains of schizophrenic patients (11). d-serine is synthesized from l-serine by the enzyme serine racemase (Srr) (12). In support of a Srr dysfunction in schizophrenia patients is the observation of reduced levels of d-serine in the CSF and serum, with a corresponding increase in its precursor, l-serine (13,14). Together, these studies indicate that an abnormality in available d-serine may be involved in schizophrenia pathogenesis and prompt a need to further investigate the role of d-serine modulators in schizophrenia.
Activation of the NMDAR requires the binding of glutamate to NR2 concomitant with the binding of glycine or d-serine to the NR1 subunit (15–17). d-serine is selective for the NR1 subunit and has been shown to be the predominant physiological co-agonist in several brain regions (18–20). Furthermore, d-serine and Srr are present in both astrocytes and neurons of the brain (12,21,22), with a regional distribution that closely resembles that of NMDARs (23). d-serine is capable of enhancing NMDAR signaling, as in vivo studies indicate that the glycine site is not saturated in many brain regions (18,24). Additionally, recent reports indicate that elimination of Srr function and lowered d-serine levels in mice reduces NMDAR-mediated neurotransmission and results in spatial memory impairments in mice (19,20,25). These findings support the capacity for d-serine and Srr to dynamically regulate NMDAR function, and disturbances in this pathway could conceivably contribute to psychopathologies associated with abnormal NMDAR activity.
To further establish the role of Srr in schizophrenia, we screened mice for a mutation in the Srr gene, and then examined biochemical markers, mRNA expression, behavioral phenotypes and pharmacological responses that are relevant to the abnormalities found in this disease. In addition, we conducted a human genetic association study to determine the involvement of SRR polymorphisms in schizophrenia risk. Human and mouse Srr have highly conserved enzymological characteristics (26), and the use of a mouse model permits an in-depth analysis of the effects specifically related to reduced Srr function. Here, we report that lack of Srr activity and decreased d-serine levels produce schizophrenia-related phenotypes in mice and that a genetic variant in the SRR gene is associated with schizophrenia in humans.
RESULTS
Identification of a mutation in the Srr gene
To establish the Srr mutation in mice, we employed N-nitroso-N-ethylurea (ENU) mutagenesis, an effective means of introducing point mutations into the genome (27). All Srr isoforms contain coding exons 3, 4, 8 and 9, and these were screened in the F1 progeny of ENU-mutagenized C57BL/6JJcl male mice and untreated DBA/2JJcl female mice (mutation discovery rate of 7.22 × 10−7/bp/gamete). At the start of exon 9, a T to A transversion resulting in a nonsense mutation (Tyr269 converted to a stop codon) was identified (Supplementary Material, Fig. S1). Exon 9 is the last coding exon of Srr. The C57BL/6JJcl and DBA/2JJcl parental mouse strains have identical exon 9 sequences (data not shown), indicating that the mutation was induced by ENU treatment. The F1 founder carrying the SrrY269* mutation was bred with C57BL/6JJcl female mice for one generation (N2), before heterozygous N2 mice were backcrossed for six generations onto the C57BL/6J strain (N3–N8) using male and female animals. Experimental animals were then bred from heterozygous intercrosses of N8 SrrY269* mice, which contained >99.6% of the C57BL/6J genome. Mutant mice are available from the RIKEN BioResource Center (Rgsc1872; www.brc.riken.jp/lab/animal/en/gscmouse.shtml).
Lack of Srr activity and diminished d-serine in mutant mice
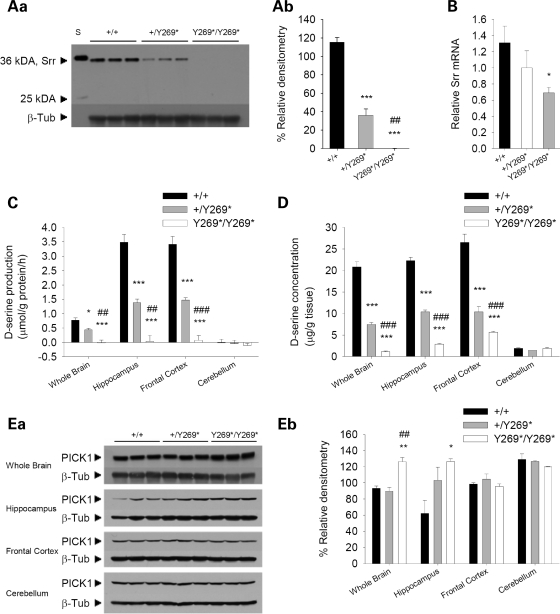
The effects of the SrrY269* mutation on the protein levels and function of Srr were explored in the mouse brain. Western blot analysis showed a loss of Srr protein in the mutant mice using either a monoclonal (Fig. 1A) or a polyclonal (Supplementary Material, Fig. S2) antibody directed to N-terminal epitopes. No truncated versions of Srr were observed, based on the predicted size of a putative truncated Srr protein of 29 kDA. Real-time RT–PCR indicated a ∼50% reduction in Srr mRNA levels in mutant animals (Fig. 1B). When Srr activity was measured in an assay that examines the production of d-serine above basal levels, no additional d-serine was synthesized in whole brain, hippocampus and frontal cortex preparations of mutant mice (Fig. 1C), demonstrating a complete lack of Srr function. To a lesser extent Srr activity was also decreased in the whole brain, hippocampus and frontal cortex of heterozygous mice (Fig. 1C). No Srr activity was detected in the cerebellum, consistent with reports of minimal Srr protein in the cerebellum of adult animals (28). Furthermore, d-serine concentrations were greatly reduced in the whole brain, hippocampus, frontal cortex, but not the cerebellum of adult SrrY269* mutant animals (Fig. 1D). Additionally, in the frontal cortex of mutant mice, an elevation in l-serine levels was observed (Supplementary Material, Table S1). No changes were detected when the whole brain, hippocampus, frontal cortex and cerebellum were assayed for the concentrations of other amino acids, including glutamate, glutamine, glycine, arginine, alanine and GABA (Supplementary Material, Table S1).
Figure 1.
Biochemical changes in mice with a nonsense mutation in exon 9 of Srr. (Aa) Western blot examining Srr levels using a monoclonal Srr antibody to probe protein extracts from whole brain of wild-type (+/+), heterozygous (+/Y269*) and mutant (Y269*/Y269*) mice. A loss of Srr protein was observed in mutant mice. β-Tubulin III was used as a loading control. The molecular weights in kiloDaltons (kDa) of Srr and a protein standard (S) are marked. (Ab) Densitometric quantification of western blot for Srr. The mean densitometry (± SEM) is an indicator of Srr protein levels in wild-type, heterozygote and mutant animals (genotype, F2,6 = 140.9, P < 0.001). ***P < 0.001 compared with wild-type mice; ##P < 0.01 compared with heterozygous mice. (B) Real-time RT–PCR analysis of Srr mRNA. Mean levels of Srr mRNA (± SEM) assessed in whole brain of wild-type (n = 6), heterozygous (n = 7) and mutant mice (n = 6) revealed a significantly reduced abundance of Srr mRNA in mutant animals. *P < 0.05 compared with wild-type mice. (C) In an Srr activity assay, the mean d-serine production (± SEM) by endogenous Srr was examined in the whole brain, hippocampus, frontal cortex and cerebellum of wild-type (n = 7–8), heterozygous (n = 4–6) and mutant mice (n = 5–7). An absence of Srr activity was found in mutant animals. *P < 0.05, ***P < 0.001 compared with wild-type mice, within the same brain region; ##P < 0.01, ###P < 0.001 compared with heterozygous mice, within the same brain region. (D) Mean concentration (± SEM) of d-serine was examined by HPLC in the whole brain, hippocampus, frontal cortex and cerebellum of wild-type (n = 5–8), heterozygous (n = 6–9) and mutant mice (n = 6–8). Lower d-serine concentrations were found in the whole brain, hippocampus, frontal cortex, but not the cerebellum of mutant mice. ***P < 0.001 compared with wild-type mice, within the same brain region; ###P < 0.001 compared with heterozygous mice, within the same brain region. (Ea) Western blot assessing PICK1 protein levels in whole brain, hippocampus, frontal cortex and cerebellum of wild-type, heterozygous and mutant mice. PICK1 was found to be elevated in the whole brain and hippocampus of mutant animals. β-Tubulin III was used as a loading control. (Eb) Densitometric quantification of western blot for PICK1. The mean densitometry (± SEM) indicates PICK1 protein levels in wild-type, heterozygote and mutant animals (genotype, F2,6 = 10.7, P < 0.05; genotype×brain region interaction, F6,18 = 5.6, P < 0.01). *P < 0.05, **P < 0.01 compared with wild-type mice, within the same brain region; ##P < 0.01 compared with heterozygous mice, within the same brain region.
The expression of other proteins involved in the d-serine pathway and in NMDAR-mediated neurotransmission were also evaluated in the whole brain, hippocampus, frontal cortex and cerebellum. Protein-interacting with kinase C (PICK1) is a scaffolding protein that regulates the subcellular localization and surface expression of a number of binding partners (29), and the PDZ domain of PICK1 has been shown to bind to a consensus sequence on the C-terminus of Srr (30). PICK1 protein levels were elevated in SrrY269* mutant mice in the whole brain and hippocampus (Fig. 1E), though whole brain mRNA abundance was not significantly increased (Supplementary Material, Fig. S2). Protein levels of the NMDAR-NR1 subunit, AMPA receptor GluR1 and GluR2 subunits, DAO and glycine transporter-1 (GlyT-1) remained unchanged in the whole brain, hippocampus and frontal cortex (Supplementary Material, Fig. S3). In the cerebellum of mutant mice, a decreased amount of GlyT-1 was detected and there was also a trend indicating lower levels of DAO, suggesting compensatory changes in glycine and d-serine availability in this brain region (Supplementary Material, Fig. S3).
Mutant mice display behavioral phenotypes relevant to schizophrenia
Mice with the SrrY269* mutation were physically indistinguishable from their wild-type littermates. Examination of reflexes, vision, fur condition and weight did not reveal any differences between wild-type and mutant mice (Supplementary Material, Fig. S4). Performance in a 3-day accelerating rotarod task, an indicator of motor coordination, balance, and motor learning, did not significantly differ between the genotypes, although there was a trend towards the mutant mice displaying a reduced latency to fall from the rotating axle (Supplementary Material, Fig. S4). In an open field arena, locomotor activity throughout the 30-min testing period was comparable in wild-type and mutant animals (Supplementary Material, Fig. S4). Additionally, no genotype differences were observed in an assessment of depression-like behavior in the forced swim test or anxiety-like behavior in the elevated plus-maze (Supplementary Material, Fig. S4).
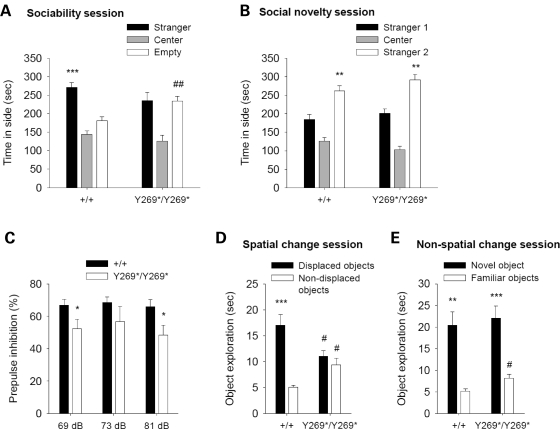
Social dysfunction is an important component of schizophrenia negative symptoms, often present in the prodromal stages and persisting throughout life (2,31). Social behaviors were assessed in SrrY269* mice, since these behaviors have served as a heuristic model for social deficits in schizophrenia (31,32). During the sociability phase of the social affiliations task, wild-type animals preferred the chamber with the unfamiliar conspecific mouse over the empty chamber, whereas mutant mice did not (Fig. 2A). The social approach deficit observed in mutant mice could not be attributed to alterations in exploratory activity, as wild-type and mutant animals displayed a similar number of chamber entries (Supplementary Material, Fig. S5). In the social novelty phase, both wild-type and mutant animals favored the chamber containing a newly introduced mouse (stranger 2) over the chamber containing a now familiar mouse (stranger 1) (Fig. 2B). Wild-type and mutant mice had similar olfactory acuity, as both genotypes displayed comparable latencies to find a hidden food pellet (Supplementary Material, Fig. S5).
Figure 2.
Schizophrenia-like behaviors in SrrY269* mutant mice. (A) Sociability session in social affiliations task. Mean time spent in a chamber containing a stranger mouse, a central chamber and a chamber with an empty cage. In contrast to wild-type mice (+/+; n = 13), mutant animals (Y269*/Y269*; n = 12) did not favor the chamber with the stranger mouse (genotype×apparatus side, F1,23 = 6.7, P < 0.05). ***P < 0.001 compared with the chamber with empty cage, within genotype; ##P < 0.01 compared with wild-type mice in the same chamber. (B) Social novelty session in social affiliations task. Mean time spent in a chamber containing a newly introduced mouse (stranger 2), a central chamber and a chamber with a familiar mouse (stranger 1). Both wild-type and mutant mice preferred the chamber with the novel stranger (apparatus side, F1,23=26.2, P < 0.001). **P < 0.01 compared with the chamber with stranger 1, within each genotype. (C) Prepulse inhibition (PPI) of the acoustic startle response. Mean % inhibition of the startle response at prepulse intensities of 69, 73 and 81 dB. Compared with wild-type animals (n = 13), mutant mice (n = 12) demonstrated a PPI deficit (genotype, F1,23 = 6.9, P < 0.05; genotype with collapsed prepulse intensities P < 0.02). *P < 0.05 compared with wild-type mice, within the same prepulse intensity. (D) Spatial change session in the object recognition task. Mean time spent exploring the displaced and non-displaced objects. Wild-type (n = 8), but not mutant mice (n = 11), showed greater exploration of the objects that underwent a spatial change (object category, F1,17 = 65.6, P < 0.001; genotype×object category, F1,17 = 36.7, P < 0.001). ***P < 0.001 compared with the non-displaced objects, within genotype; #P < 0.05 compared with wild-type mice exploring the same object category. (E) Non-spatial change session in the object recognition task. Mean time spent exploring the novel item and three familiar objects. Both wild-type and mutant mice preferentially explored the novel object (object category, F1,17 = 52.4, P < 0.001). **P < 0.01, ***P < 0.001 compared with the familiar objects, within each genotype; #P < 0.05 compared with wild-type mice exploring the same object category. Data are shown as mean ± SEM.
An impaired ability to filter or ‘gate out’ sensory and cognitive information is a prominent clinical feature of schizophrenia (33). A well-established operational measure of sensorimotor gating is prepulse inhibition (PPI) of the startle response (34). PPI refers to the attenuation of the startle response by a weak stimulus (prepulse) appearing shortly before a startle stimulus. PPI deficits are observed in schizophrenic patients, and PPI is considered to be a model with reasonable face, predictive and construct validity (33). SrrY269* mutant mice demonstrated diminished PPI compared with wild-type animals (Fig. 2C). In the absence of the prepulse, responses to the startle stimulus and to a range of stimulus intensities did not differ between genotypes (Supplementary Material, Fig. S5). Also, startle responses were increased at greater stimulus intensities (Supplementary Material, Fig. S5), suggesting normal hearing.
Cognitive deficits are a central characteristic of schizophrenia and include impairments in spatial recognition (35,36). In mice, visuospatial cognition can be investigated using a spatial object recognition procedure (37,38). Performance in the spatial discrimination task has previously been demonstrated to be sensitive to pharmacological manipulation with dopaminergic and serotonergic compounds (37,38), and is impaired by lesions to areas such as the nucleus accumbens, hippocampus and prefrontal cortex, that are recognized to be important for cognitive function and part of the corticolimbic network implicated in schizophrenia (39,40). During the spatial change session of this object discrimination task, wild-type mice spent more time investigating the objects with a novel spatial configuration (displaced objects) than the objects that remained stationary (non-displaced objects) (Fig. 2D). In contrast, the mutant mice did not react differently to the displaced objects (Fig. 2D). Prior to the spatial displacement, wild-type and mutant animals acclimatized to the objects similarly in the habituation sessions (Supplementary Material, Fig. S5), and no preference for either object category (to be displaced or non-displaced) was demonstrated in the last habituation session by either genotype (Supplementary Material, Fig. S5). When a novel object was introduced, both wild-type and mutant animals spent more time exploring the novel object than the three familiar objects (Fig. 2E). Locomotor activity did not differ between genotypes during this task (Supplementary Material, Fig. S5).
Cognitive disturbances in SrrY269* mice were also observed in the Morris water maze (MWM), a classic test of spatial learning and memory. Performance in a visible platform session and in the 7-day acquisition training phase when the platform was hidden was similar in wild-type and mutant mice (Supplementary Material, Fig. S5). However, spatial memory measured in a probe trial conducted after 5 days of acquisition training was considerably deficient in SrrY269* mutant mice. Compared with wild-type animals, mutant mice spent less time in an area 3× the platform diameter centered over its former location (Supplementary Material, Fig. S5). Furthermore, mutant mice did not display a greater amount of time over the target location compared with the averaged analogous non-target areas, indicating a lack of preference for the target location. A second probe trial performed after seven acquisition days demonstrated that with greater training the spatial memory deficit in mutant mice could be partially ameliorated; however, mutant animals in this probe trial continued to lack a significant preference for the target location over the averaged analogous non-target areas (Supplementary Material, Fig. S5).
Pharmacological responses in mutant mice
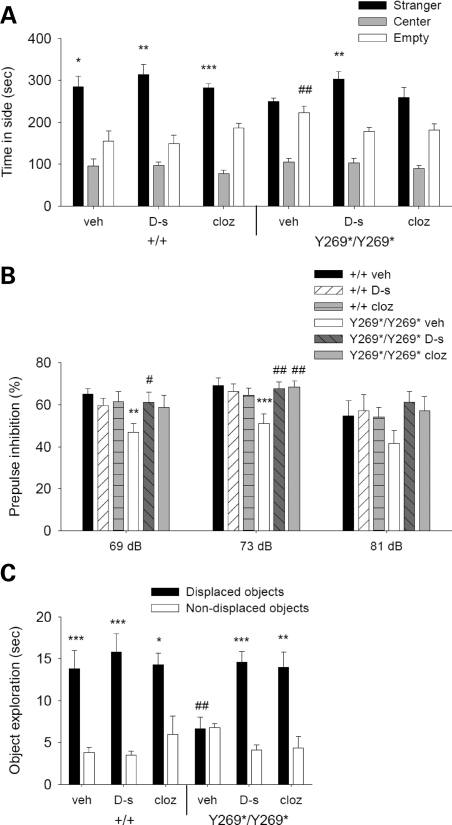
The efficacy of d-serine to reverse the behavioral impairments in SrrY269* mutant mice was assessed and compared with clozapine. Clozapine is a widely used atypical anti-psychotic with a pharmacological profile that includes affinity for D1- and D2-like dopamine receptors and 5-HT2 receptors (2). In the social affiliations task, d-serine fully reversed the social impairment observed in mutant mice (Fig. 3A). Administration of clozapine to mutant animals, on the other hand, did not significantly induce a preference for the chamber containing the unfamiliar mouse (Fig. 3A). The number of chamber entries did not differ significantly between genotype and drug treatment groups, indicating normal exploratory activity (Supplementary Material, Fig. S6). Additionally, pharmacological treatments did not affect olfactory function, as similar latencies to find a buried food pellet were observed (Supplementary Material, Fig. S6).
Figure 3.
d-serine (600 mg/kg) and clozapine (0.75 mg/kg) improve schizophrenia-like behaviors in mutant mice. (A) Mean time in a chamber containing a stranger mouse, a central chamber and a chamber with an empty cage during a test of sociability. Wild-type animals (+/+) given vehicle (veh; n = 10), d-serine (d-s; n = 8) or clozapine (cloz; n = 10) and mutant mice (Y269*/Y269*) treated with d-serine (n = 9) significantly preferred the chamber with the unfamiliar mouse, whereas mutant mice injected with vehicle (n = 10) or clozapine (n = 11) did not (genotype×apparatus side, F1,52 = 4.3, P < 0.05). *P < 0.05, **P < 0.01, ***P < 0.001 compared with the chamber with empty cage, within genotype and drug treatment group; ##P < 0.01 compared with vehicle-treated wild-type mice in the same chamber. (B) Mean % reduction of startle amplitude at prepulse intensities of 69, 73 and 81 dB. The PPI deficit in vehicle-treated mutant mice (n = 11) was improved by d-serine (n = 12) and clozapine (n = 8) to a level comparable to wild-type animals treated with vehicle (n = 9), d-serine (n = 9) or clozapine (n = 9) (genotype×drug treatment, F2,52 = 4.3, P < 0.05). **P < 0.01, ***P < 0.001 compared with vehicle-treated wild-type mice, within the same prepulse intensity; #P < 0.05, ##P < 0.01 compared with vehicle-treated mutant mice, within the same prepulse intensity. (C) Mean time spent investigating displaced and non-displaced objects in the spatial recognition task. Rescue of the impaired spatial reactivity in vehicle-treated mutant mice (n = 10) was apparent in mutant animals treated with d-serine (n = 8) and clozapine (n = 9), which demonstrated similar responses to wild-type mice given vehicle (n = 9), d-serine (n = 9) or clozapine (n = 10) (genotype×drug treatment×object category, F2,49 = 4.3, P < 0.05). *P < 0.05, **P < 0.01, ***P < 0.001 compared with the non-displaced objects, within genotype and drug treatment group; ##P < 0.01 compared with vehicle-treated wild-type mice exploring the same object category. Data are presented as mean ± SEM.
Deficient PPI in mutant animals was rescued by both d-serine and clozapine administration (Fig. 3B). These compounds did not affect responses to the acoustic startle stimulus (Supplementary Material, Fig. S6). In the spatial recognition task, impaired reactivity to a spatial change in mutant animals was substantially improved by both d-serine and clozapine (Fig. 3C). These effects were not related to alterations in object exploration during the habituation phase or to an innate preference for an object category (Supplementary Material, Fig. S6). d-serine-treated mutant mice demonstrated reduced locomotor activity in the initial empty open-field session; however, no differences in locomotor function were observed in any genotype or drug treatment group during the subsequent object habituation and spatial change sessions (Supplementary Material, Fig. S6). d-serine was also able to reverse the spatial memory deficits of mutant animals in the MWM. Vehicle- and d-serine-treated wild-type and mutant mice demonstrated comparable performances during the visible platform session and acquisition training phase (Supplementary Material, Fig. S6). In the probe trials, d-serine-injected mutant mice spent more time in a target area 3× the platform diameter than vehicle-treated mutant animals (Supplementary Material, Fig. S6). d-serine-treated mutant mice also significantly favored the target location over the averaged analogous non-target areas, whereas the vehicle-treated mutant mice did not.
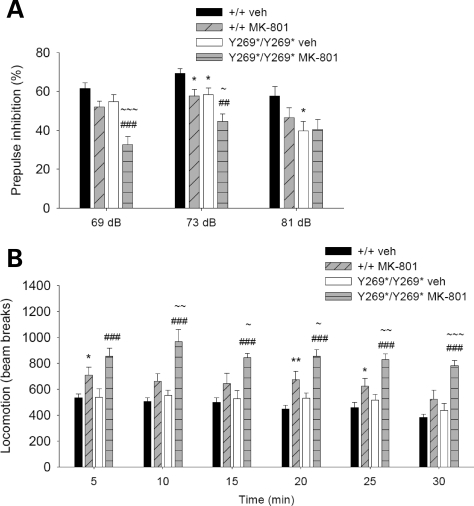
The capacity for NMDAR antagonists to induce psychotomimetic effects in humans prompted their application in pharmacological animal models of schizophrenia (41). In animals, NMDAR antagonists such as MK-801 potentiate a spectrum of behavioral abnormalities relevant to schizophrenia, including locomotor hyperactivity, stereotypy, impaired PPI, and perseveration (41,42). Locomotor hyperactivity is an animal model for the positive symptoms of schizophrenia that is proposed to correspond to psychomotor agitation (41). To examine the effects of the SrrY269* mutation in a pharmacological model relevant to schizophrenia, we assessed the effects of MK-801 treatment on PPI and locomotor activity. A low dose of MK-801 was chosen to avoid exceeding the range of the behavioral tests measured. Administration of MK-801 was found to produce greater impairments in SrrY269* mutant mice than in wild-type animals. PPI in mutant mice given MK-801 was more severely disrupted, as demonstrated by a significant reduction in comparison to MK-801-treated wild-type mice and vehicle-treated mutant animals at the prepulse intensities of 69 and 73 dB (Fig. 4A). The acoustic startle response in MK-801-treated mutant mice was also elevated (Supplementary Material, Fig. S7); however, no correlation was found between startle response and PPI in the mutant mice (r = −0.06 for 69 dB, r = 0.23 for 73 dB and r = 0.09 for 81 dB; P > 0.05). MK-801-induced locomotor hyperactivity was apparent in both wild-type and mutant mice, but was substantially greater in mutant animals throughout the 30-min testing period (Fig. 4B).
Figure 4.
Potentiation of behavioral responses relevant to schizophrenia following NMDAR inhibition. (A) Mean % inhibition of the acoustic startle response with prepulse intensities of 69, 73 and 81 dB. Wild-type (+/+) and mutant animals (Y269*/Y269*) were treated with vehicle (n = 17, 24) or MK-801 (0.1 mg/kg; n = 16, 17). PPI in mutant mice given the NMDAR antagonist was most severely affected (genotype, F1,70 = 18.8, P<0.001; drug treatment, F1,70 = 15.6, P < 0.001; genotype×drug treatment×prepulse intensity, F2,140 = 3.0, P ≤ 0.05). *P < 0.05 compared with vehicle-treated wild-type mice, within the same prepulse intensity; ##P < 0.01, ###P < 0.001 compared with vehicle-treated mutant mice, within the same prepulse intensity; ∼P < 0.05, ∼∼∼P < 0.001 compared with MK-801-treated wild-type mice, within the same prepulse intensity. (B) Mean number of beam breaks in an empty open field during a 30-min assessment of locomotor activity (5-min bins). Wild-type and mutant animals were given vehicle (n = 9, 9) or MK-801 (0.1 mg/kg; n = 10, 9), and locomotor activity was most enhanced in mutant mice administered the NMDAR antagonist (genotype, F1,33 = 9.5, P < 0.01; drug treatment, F1,33 = 35.2, P < 0.001; genotype×drug treatment, F1,33 = 4.0, P ≤ 0.05). *P < 0.05, **P < 0.01 compared with vehicle-treated wild-type mice, within the same time bin; ###P < 0.001 compared with vehicle-treated mutant mice, within the same time bin; ∼P < 0.05, ∼∼P < 0.01, ∼∼∼P < 0.001 compared with MK-801-treated wild-type mice, within the same time bin. Data are shown as mean ± SEM.
Differential expression of genes relevant to schizophrenia and cognitive function in SrrY269* mice
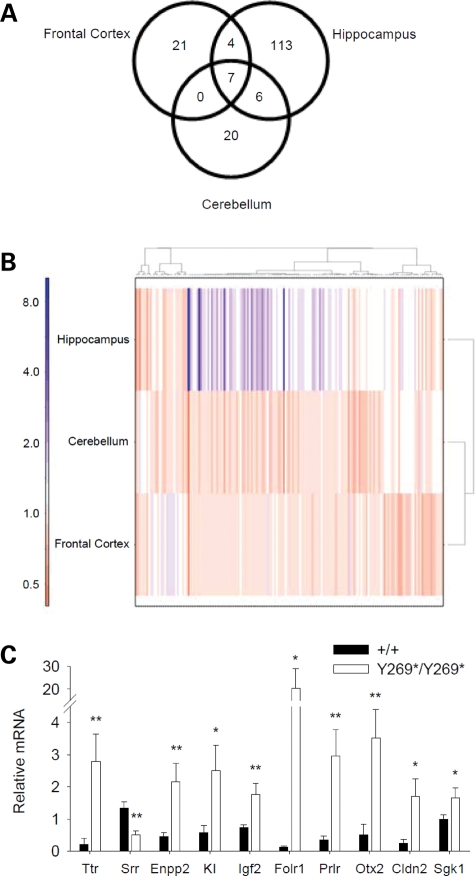
The recent discoveries of Srr and d-serine as a neurotransmitter in the brain, combined with their potential role in schizophrenia, indicate that the molecular perturbations resulting from reduced Srr activity are of significant interest. We adopted a functional genomics approach and performed transcriptome-wide screening of changes in mRNA levels. Adult wild-type and SrrY269* mutant mice were compared using total mRNA extracted from the hippocampus, frontal cortex and cerebellum to probe Illumina BeadChip expression arrays that survey 25 697 unique mouse transcripts. We identified 171 transcripts altered between wild-type and mutant mice at a 5% false-discovery rate (Fig. 5A). Since prominent reductions in d-serine concentrations were found in the hippocampus and frontal cortex, but not the cerebellum of adult SrrY269* mutant mice (Fig. 1D), the cerebellum may have comparatively fewer alterations in adult animals, although d-serine levels are elevated in this region during development (28). Indeed, the hippocampus was observed to have the largest changes, both in the number of transcripts (130 altered) and in the magnitudes of change (range: 0.5- to 8.2-fold change) (Fig. 5A and B). Fewer differences were observed in the frontal cortex (32 transcripts; 0.6- to 1.6-fold change) and cerebellum (33 transcripts; 0.5- to 1.4-fold change) (Fig. 5A and B). Only a modest degree of overlap was observed across the three brain regions (Fig. 5A).
Figure 5.
Changes in mRNA levels due to a loss of Srr function. (A) Venn diagram demonstrating the number of altered transcripts in response to the SrrY269* mutation and the overlapping transcripts in the hippocampus, frontal cortex and cerebellum. (B) Heatmap displaying the clustering patterns of all differentially expressed genes in the hippocampus, cerebellum and frontal cortex of SrrY269* mice. The color scale indicates the fold change. The hippocampus shows the greatest number and magnitude of changes. (C) Real-time RT–PCR analysis. In the hippocampus of mutant mice (Y269*/Y269*; n = 5) the mRNA levels of Ttr, Enpp2, KI, Igf2, Folr1, Prlr, Otx2, Cldn2 and Sgk1 were increased, while Srr mRNA levels were reduced relative to wild-type animals (+/+; n = 7) (genotype, F1,10 = 11.5, P < 0.01). *P < 0.05, **P < 0.01 compared with wild-type mice, within the same gene.
To determine if our gene lists indicated perturbation of specific pathways or biological functions, we performed a Gene Ontology (GO) enrichment analysis of the genes with differential mRNA levels in SrrY269* mice. Twenty-eight separate functional categories were enriched, with none overlapping across tissues (Supplementary Material, Table S2). The hippocampus was primarily enriched for genes mediating transport activity and constituents of the extracellular space, the frontal cortex for genes regulating gas exchange and the cerebellum for genes involved in neurogenesis.
Several techniques were employed to confirm and extend our microarray results. First, real-time RT–PCR assessment of hippocampal mRNA reproduced the differential expression of several of the identified genes (Fig. 5C). The magnitude of the changes observed tended to be greater using the real-time RT–PCR method, consistent with previous microarray literature (43). Second, western blots indicated that protein levels of transthyretin, ectonucleotide pyrophosphatase/phosphodiesterase 2, klotho, insulin-like growth factor 2, prolactin receptor and claudin 2 were elevated in the hippocampus (Supplementary Material, Fig. S8). Additionally, western blots demonstrating a lack of Srr protein (Fig. 1A and Supplementary Material, Fig. S2) further validated the reduced Srr expression seen in the microarray data.
Several genes implicated in schizophrenia were found among the differentially expressed genes identified in our analysis (Table 1), related to neurodevelopment, myelination and cognitive ability. The greatest degree of change was observed in transthyretin (Ttr, 8.2-fold in the hippocampus), a carrier protein for thyroxine and retinol that has been associated with amyloid diseases and is a proposed protein biomarker of schizophrenia (44). Individuals with schizophrenia have been reported to have reduced levels of prolactin (45), and the prolactin receptor (Prlr) was induced in SrrY269* mice. Mutant animals also showed increased mRNA levels of insulin-like growth factor 2 (Igf2), an important contributor to brain development, and elevations in IGF2 have been observed in the serum of schizophrenia patients (46). Abnormalities in myelination and oligodendroglial function are widely observed in schizophrenia (47), and SrrY269* mutant mice demonstrated perturbations in myelin-associated genes, including ectonucleotide pyrophosphatase/phosphodiesterase 2 (Enpp2), myelin-associated oligodendrocytic basic protein (Mobp) and myelin basic protein (Mbp). Sulfatase 1 (Sulf1), a modulator of cell growth, signaling and oligodendrocyte differentiation, also had elevated mRNA levels in the mutant animals (48). Differential expression was also observed for genes that modulate neuronal plasticity and learning, including klotho (Kl), a gene involved in ageing (49) and serum/glucocorticoid regulated kinase 1 (Sgk1), which influences activation of potassium, sodium and chloride channels, and of early growth response 1 (Egr1), an immediate-early transcription factor (50,51).
Table 1.
Gene expression changes in SrrY269* mice identified by microarray analysis
| Symbol | Gene | Entrez Gene ID | Fold change |
||
|---|---|---|---|---|---|
| Hippocampus | Cerebellum | Frontal cortex | |||
| Ttr | Transthyretin | 22139 | 8.24*** | 0.49** | 0.56*** |
| Srr | Serine racemase | 27364 | 0.53*** | 0.88 | 0.61*** |
| Enpp2 | Ectonucleotide pyrophosphatase/phosphodiesterase 2 | 18606 | 4.06*** | 0.70* | 0.96 |
| Clic6 | Chloride intracellular channel 6 | 209195 | 5.79*** | 0.89 | 1.03 |
| Kl | Klotho | 16591 | 2.76*** | 0.86 | 0.99 |
| Igf2 | Insulin-like growth factor 2 | 16002 | 1.87*** | 0.91 | 0.89 |
| Kcne2 | Potassium voltage-gated channel, Isk-related subfamily, gene 2 | 246133 | 2.30*** | 0.85 | 1.00 |
| Sulf1 | Sulfatase 1 | 240725 | 2.12*** | 0.93 | 0.90 |
| Folr1 | Folate receptor 1 (adult) | 14275 | 2.38*** | 0.86 | 1.01 |
| Prlr | Prolactin receptor | 19116 | 2.32*** | 0.88 | 0.95 |
| Otx2 | Orthodenticle homolog 2 (Drosophila) | 18424 | 2.35*** | 0.91 | 0.98 |
| Cldn2 | Claudin 2 | 12738 | 2.20*** | 0.95 | 0.99 |
| Fos | FBJ osteosarcoma oncogene | 14281 | 0.78* | 0.61*** | 0.79* |
| Arc | Activity regulated cytoskeletal-associated protein | 11838 | 0.78* | 0.94 | 0.67** |
| Sgk1 | Serum/glucocorticoid regulated kinase 1 | 20393 | 1.42** | 1.14 | 1.13 |
| Egr1 | Early growth response 1 | 13653 | 0.90 | 0.84 | 0.77* |
| Mobp | Myelin-associated oligodendrocytic basic Protein | 17433 | 1.27* | 1.21 | 1.15 |
| Mbp | Myelin basic protein | 17196 | 1.27* | 1.00 | 0.94 |
*P < 0.05, **P < 0.01, ***P < 0.001 compared with wild-type mice, within the same brain region, false discovery rate adjusted for multiple-testing.
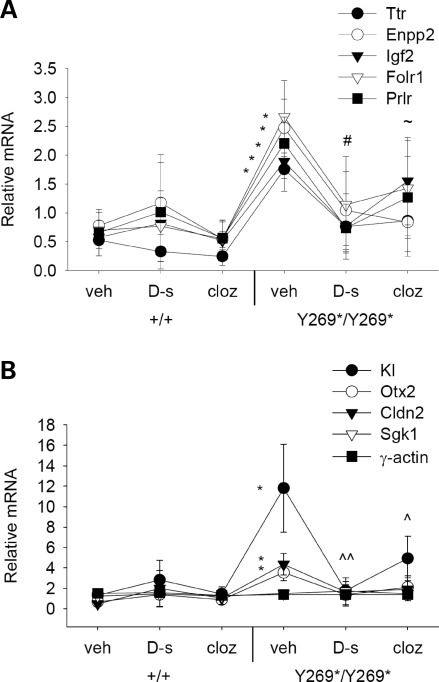
To assess the capacity for exogenous d-serine and clozapine treatment to modulate the transcripts altered by the SrrY269* mutation, real-time RT–PCR was again used to examine mRNA levels in hippocampal tissue. In mutant animals, d-serine and clozapine treatment were found to normalize several of the differentially expressed transcripts. Vehicle-treated SrrY269* mice displayed an elevated level of Ttr, Enpp2, Kl, Igf2, Folr1, Prlr, Otx2 and Cldn2 and these elevations were reversed by d-serine or clozapine administration (Fig. 6A and B). In particular, significant reductions in mRNA were observed for Igf2 in d-serine-treated mutant mice, for Enpp2 in clozapine-treated mutant mice and for Kl in d-serine- or clozapine-treated mutant animals. Drug treatments did not influence levels of γ-actin in mutant mice, signifying that the modulatory effects of d-serine and clozapine were specific and not transcriptome-wide.
Figure 6.
Differentially expressed mRNA transcripts in SrrY269* mice are normalized by d-serine or clozapine treatment. (A and B) Levels of mRNA in the hippocampus of wild-type (+/+) and mutant mice (Y269*/Y269*) were assessed 30 min following vehicle, d-serine (600 mg/kg) or clozapine (0.75 mg/kg) administration. Vehicle-treated mutant mice (n = 7) demonstrated an enhancement in Ttr, Enpp2, KI, Igf2, Folr1, Prlr, Otx2 and Cldn2 mRNA that was reversed by d-serine (n = 5) or clozapine (n = 5) treatment to a level similar to wild-type mice administered vehicle (n = 6), d-serine (n = 7) or clozapine (n = 7) (genotype, F1,30 = 4.5, P < 0.05; genotype×drug treatment×gene interaction, F16,240 = 2.6, P < 0.01). γ-actin was used as a negative control. *P < 0.05 compared with vehicle-treated wild-type mice, within the same gene; #P < 0.05 Igf2 mRNA levels in d-serine-treated mutant mice compared with vehicle-treated mutant mice; ∼P < 0.05 Enpp2 mRNA levels in clozapine-treated mutant mice compared with vehicle-treated mutant mice; ^P < 0.05; ^^P < 0.01 Kl mRNA levels in d-serine- or clozapine-treated mutant mice compared with vehicle-treated mutant mice.
A genetic variant of the SRR gene is associated with schizophrenia
To further assess the relevance of the SRR gene as a contributing factor in the pathophysiology of schizophrenia, four single nucleotide polymorphism (SNP) markers within the human SRR gene were analyzed in two independent samples: 117 nuclear families and 221 schizophrenia case–control pairs. The SNPs examined included two in intron 1 (rs4523957, rs391300), one in intron 4 (rs8081273) and one in intron 6 (rs7222251). In both samples, genotype distributions of all SRR polymorphisms investigated were in Hardy–Weinberg equilibrium and none of the markers showed strong linkage disequilibrium (D' < 0.9; Supplementary Material, Fig. S9). A significant genetic association between schizophrenia and a SNP marker in SRR (rs4523957) was present in the nuclear family sample (Table 2). This association was robust, as it persisted after Bonferroni correction (Table 2). The SRR gene in humans is known to have several isoforms that are derived from variations in the first exon (52). Among the tested markers, the significant polymorphism was the one closest to the 5′ end of SRR and was near exon 1b, the variant which composes the majority of Srr in the human brain (52). Analysis of the case–control sample did not reveal any significant associations with schizophrenia, though a trend for both genotype and allele frequencies (P ≤ 0.07) was observed in the same marker (rs4523957) found significant in the family sample (Supplementary Material, Table S3).
Table 2.
Family-based association test results for SNP markers in the SRR gene
| SNP | Allele | Frequency | Informative families | S | E(S) | Var(S) | Z score | P-value | Corrected P-value |
|---|---|---|---|---|---|---|---|---|---|
| rs4523957 | T | 0.59 | 37 | 36 | 45.75 | 12.97 | −2.71 | 0.006 | 0.024 |
| G | 0.41 | 42 | 32.25 | ||||||
| rs391300 | A | 0.39 | 36 | 36 | 31.50 | 13.20 | 1.24 | 0.21 | |
| G | 0.61 | 40 | 44.50 | ||||||
| rs8081273 | T | 0.44 | 40 | 34 | 36.20 | 15.11 | −0.56 | 0.57 | |
| C | 0.56 | 50 | 47.80 | ||||||
| rs7222251 | T | 0.70 | 34 | 46 | 46.33 | 11.84 | −0.097 | 0.92 | |
| C | 0.30 | 26 | 25.67 |
S, test statistic for observed number of alleles; E, expected value of S under null hypothesis; Var(S), variance between the observed and expected transmission. Significant results are shown in bold font.
Haplotype analyses in the nuclear family and case–control samples were completed using a two-marker window. In the family sample, haplotypes constructed by the rs4523957 and rs391300 markers demonstrated a significant overall association (Table 3). Individual haplotype analyses indicated that the G-A haplotype was associated with schizophrenia, while the T-G haplotype was protective (Table 3). When haplotype frequencies were compared in the cases and controls, no significant associations were observed (Supplementary Material, Table S4).
Table 3.
Haplotype analysis of schizophrenia family samplea
| Haplotype |
Frequency | Families | S | E(S) | Var(S) | Z score | P-value | Global P-value | |
|---|---|---|---|---|---|---|---|---|---|
| rs4523957–rs391300 | T-G | 0.68 | 27 | 48.35 | 55.67 | 11.92 | −2.12 | 0.033 | 0.006 |
| G-A | 0.28 | 28 | 39.35 | 31.67 | 10.83 | 2.34 | 0.020 | ||
| rs391300–rs8081273 | G-T | 0.46 | 36 | 47.96 | 49.55 | 12.03 | −0.46 | 0.63 | 0.64 |
| A-C | 0.35 | 34 | 49.96 | 47.05 | 11.44 | 0.86 | 0.43 | ||
| G-C | 0.18 | 26 | 31.04 | 47.05 | 7.64 | −0.66 | 0.51 | ||
| rs8081273–rs7222251 | C-T | 0.66 | 40 | 71.00 | 69.84 | 14.27 | 0.31 | 0.72 | 0.62 |
| T-C | 0.20 | 25 | 25.00 | 26.20 | 8.02 | −0.42 | 0.71 | ||
| T-T | 0.12 | 26 | 21.00 | 22.50 | 7.42 | −0.55 | 0.53 | ||
S, test statistic for observed number of alleles; E, expected value for S under null hypothesis of no biased transmission; Var(S), variance between the observed and expected transmission. Significant results are shown in bold font.
aRare haplotypes (frequencies less than 0.05) were omitted from haplotype analysis.
DISCUSSION
Reduced Srr activity resulted in diminished levels of d-serine and behavioral impairments resembling aspects of the negative and cognitive symptoms of schizophrenia, including social approach deficits, reduced PPI and deficient spatial recognition and memory. These behavioral deficits could be ameliorated by exogenous d-serine or the atypical antipsychotic clozapine, and aggravated by an NMDAR antagonist. Furthermore, abnormal expression of several genes implicated in schizophrenia and cognitive function were observed in mutant mice, including Pick1 and Ttr. In humans, the SRR gene was associated with schizophrenia in a nuclear family sample. These results indicate an important role for Srr in influencing biochemical and behavioral perturbations associated with schizophrenia.
We identified one functional mutation in the Srr gene in 7502 mice. In ENU mutagenesis, functional mutation rates at a specific locus are typically 1 in 1000 gametes. However, Srr has a small coding region with only 339 codons, and based on the Poisson distribution and our screening of 239 residues within Srr, the probability of discovering only one functional mutation among 7502 mice is 20% (27). Consequently, it was not unexpected that we found only one functional mutation in our Srr screen. Though we considered a functional mutation to be one that changes an amino acid in an exon, regulatory elements outside exons can influence gene expression (9), and the study of such point mutations may also reveal functional effects. ENU induces random single-base substitutions, and based on the average mutation rate, the F1 founder for the SrrY269* line would have ∼29 other functional heterozygous mutations randomly distributed among the ∼30 000 genes of the mouse genome (53). After the seven generations of backcrossing onto the C57BL/6J strain, these additional heterozygous mutations would be reduced to 0.23. Moreover, only 25% of the 0.23 additional mutations were expected to be present in both breeding mice of an N8 intercross (54), and the use of multiple breeding pairs further ensures independent segregation of these unlikely mutations. These steps effectively eliminated the likelihood that the abnormal phenotypes found in SrrY269* mice were related to a mutation other than the nonsense mutation in Srr. Also, the capacity for d-serine to reverse the behavioral deficits supports that the Srr mutation is responsible for the observed mutant phenotypes.
The degree to which d-serine is depleted in SrrY269* mice is noteworthy. Both enzyme activity assays and western blot analysis indicate a complete loss of Srr protein expression, a result that might not be predicted from the introduction of a premature stop signal at codon 269. Mutated mRNAs containing a premature stop codon are detected and degraded by processes collectively termed nonsense-mediated mRNA decay (NMD) (55). The best-understood mechanism of NMD in mammals relies on proteins that remain at the last splice junction until the first round of translation and thereby detect translational termination occurring greater than 50–55 nucleotides upstream of the last splice junction. Codon 269 is precisely at the beginning of exon 9, the canonical last exon of murine Srr, and therefore does not fit this rule. This may signify that there are alternative mechanisms of NMD that depend on somewhat different requirements. It is also possible that a truncated protein is produced from the mutant Srr mRNA but is inherently unstable. Regardless of the process(es) by which Srr protein is depleted in SrrY269* mice, a detectable level of d-serine remains in the brains of these animals, potentially rendering the phenotype somewhat less severe than NMDA-NR1 ablation. A considerable portion of the residual d-serine may come from the diet, as other d-amino acids can reach the CNS via this route (56). Alternatively, it has been suggested that the glycine cleavage system or other enzymes may contribute to d-serine levels in the brain (57).
In mice with the SrrY269* mutation, diminished Srr activity was accompanied by a substantial decrease in d-serine in the whole brain (95% loss), hippocampus, and frontal cortex, and an increase in l-serine in the frontal cortex. Drug-naïve schizophrenic patients have similar changes in l- and d-serine (13,14), and improvements in schizophrenic symptoms are correlated with an elevation in d-serine (58). These changes in d-serine have been proposed to be mediated by alterations in Srr function. In post-mortem studies, Srr protein levels have been shown to be decreased in the frontal cortex and hippocampus of schizophrenia patients (13). Activity of Srr is closely linked to NMDAR activation, as antagonism of the NMDAR has been found to upregulate Srr mRNA and protein expression (59). In our study, loss of Srr activity did not produce any detectable changes in frontocortical or hippocampal levels of the NMDAR subunit NR1, or in other regulators of NMDAR-mediated neurotransmission, including GluR1, GluR2 and GlyT-1, although it remains possible that there could be more subtle alterations in specific subregions or subcellular compartmentalization. In contrast, the SrrY269* mutation induced a decrease in GlyT-1 and DAO in the cerebellum, possibility related to the role of d-serine in NMDAR-dependent neuronal migration in the cerebellum of the developing brain (60). Furthermore, an increase in PICK1 was observed in SrrY269* animals. In addition to Srr, PICK1 interacts with and regulates numerous other proteins, many of which have been implicated in schizophrenia, including glutamate receptors, dopamine transporters, neuregulin receptors and ephrins (29). The PICK1 gene has been associated with schizophrenia risk (30) and abnormal PICK1 transcript levels have been reported in the prefrontal cortex of schizophrenia patients (61). Thus, the ability of the Srr mutation to reproduce some of the changes observed in schizophrenia supports the relevance of this model to the pathophysiology of this disease.
In addition to its racemization activity, Srr is also possesses α,β-elimination activity capable of transforming l- or d-serine into pyruvate and ammonia (62). Since the SrrY269* mice did not express any Srr protein or racemase activity, the α,β-eliminase action of Srr is likely absent in these mice. The physiological significance of the Srr α,β-eliminase activity is presently unclear, and it is unlikely to be a substantial contributor of cellular pyruvate, as glycolysis produces approximately 1000 times more pyruvate than could be produced by Srr (63). Instead, the Srr α,β-eliminase reaction may be involved in modulating intracellular levels of d-serine, avoiding excessive accumulation of d-serine by degrading it into pyruvate (62). In our study, lack of Srr α,β-eliminase activity is not likely responsible for the behavioral abnormalities observed, as these were reversible by d-serine and further induced by MK-801. However, there is a possibility that the loss of α,β-eliminase activity contributes to the alterations in certain transcripts and proteins reported, such as those described in the microarray analysis.
We found that SrrY269* mutant mice displayed behavioral endophenotypes related to the negative and cognitive symptoms of schizophrenia (Table 4). The social deficits exhibited by mutant animals were specific to a test of sociability. In the affiliations task, the sociability phase is an examination of social approach and avoidance-related motivation, while the social novelty phase is an assessment of social memory and the ability to discriminate a socially novel stimulus (64,65). Exploratory activity, olfactory acuity and anxiety were unaffected in mutant mice, and therefore these parameters are not likely to account for the observed social approach impairment. Mutant animals also displayed a disruption in PPI, a cross-species measure of pre-attentive information processing. Reductions in PPI are consistently demonstrated in schizophrenia (66) and the degree to which PPI is affected correlates with the severity of symptoms (33). Although PPI deficits were demonstrated in multiple cohorts of SrrY269* mice, a recent analysis of an Srr exon 1 knock-out (KO) model did not identify changes in PPI (Table 4) (25), and discrepancies between these genetic models may be related to differing procedural demands and degree of d-serine loss (89% d-serine reduction in Srr exon 1 KO).
Table 4.
Summary of phenotypes of SrrY269* mutant mice and Srr exon 1 KO mice
| SrrY269* | Srr exon1 KO | ||
|---|---|---|---|
| Biochemical changes | d-serine | ↓ | ↓ |
| l-serine | ↑ (frontal cortex) | = | |
| Srr | No Srr protein,↓Srr mRNA | No Srr protein | |
| PICK1 | ↑ | n/a | |
| Proteins relevant to NMDAR signaling | = NR1, GluR1, GluR2 | n/a | |
| ↓ GlyT-1, DAO in cerebellum | |||
| Other mRNA and protein levels | altered (i.e.↑Ttr, Enpp2, Kl, Igf2, Prlr, Cldn2) | n/a | |
| Electrophysiology | n/a | ↓ NMDAR glycine site occupancy,↓NMDAR-mediated neurotransmission, No LTP, slower decay kinetics of NMDAR-mediated EPSCs | |
| Behavioral phenotypea | Physical appearance and neurological reflexes | = | = |
| Motor coordination and motor learning (rotarod) | = (trend to decreased motor coordination) | = | |
| Locomotor activity | = | ↑ (males), =(females) | |
| Forced swim immobility | = | n/a | |
| Anxiety (elevated plus-maze, open field) | = | = (males), ↑(females) | |
| Sociability | ↓ | n/a | |
| Social novelty | = | n/a | |
| Olfactory function | = | n/a | |
| Prepulse inhibition (PPI) | ↓ | = | |
| Startle reactivity | = | = (males), ↑(females) | |
| Spatial object recognition | ↓ | n/a | |
| Non-spatial object recognition | = | n/a | |
| Motor abilities, sensory functions and motivation in visible platform MWM task | = | n/a | |
| Spatial learning and memory (MWM) | ↓ memory | ↓ memory (males), =(females) | |
| Pharmacological responses | d-serine | ++ sociability, PPI, spatial recognition and memory deficits | ↑ NMDAR-mediated EPSCs and LTP |
| ++ altered mRNA levels | |||
| Clozapine | ++ PPI and spatial recognition deficits | n/a | |
| ++ altered mRNA levels | |||
| MK-801 | ↑ locomotor hyperactivity and PPI deficits | n/a |
↑ increased; ↓decreased; ++, normalized; =, no change; n/a, not applied.
aOnly male mice examined in SrrY269* mice.
Excitatory post-synaptic currents (EPSCs), long-term potentiation (LTP).
Since cognitive dysfunctions are recognized to be a primary and enduring core deficit in schizophrenia, SrrY269* animals were evaluated in a visuospatial object discrimination task and in the MWM. In the spatial recognition task, mutant mice displayed a selective inability to detect a spatial change. Conversely, the responses of mutant animals to novelty remained unaltered, concordant with their normal ability to detect novelty in a social environment. Prior studies have shown that NMDAR antagonists are capable of impairing spatial recognition while leaving intact the capacity to respond to non-spatial novelty (37,38). In addition, SrrY269* mice exhibited diminished long-term retention of a spatial memory in the MWM, consistent with reports of spatial memory deficits in the MWM in Srr exon 1 KO mice (Table 4) (25). The cognitive deficits displayed in SrrY269* mice were not related to perturbations in exploration, motivation or motor abilities, as these animals performed adequately in locomotor activity and MWM visible platform tests. Furthermore, the observed deficits in spatial tasks may be comparable to the deficits in perception and representation of spatial relationships, motion and orientation described in schizophrenia (35,36). In the rotarod task, SrrY269* mice displayed a slight and non-significant reduction in motor coordination, which may be relevant to the soft neurological and cerebellar signs reported in schizophrenia that are correlated with a greater prevalence of negative symptoms, poor psychosocial performance and neurocognitive disturbances (67,68).
Behavioral impairments in SrrY269* mutant mice were ameliorated by d-serine and clozapine. In the social affiliation task, d-serine was more effective than clozapine in reversing the reduced sociability in mutant animals. The ability of clozapine to normalize social deficits induced by NMDAR antagonists is controversial in rodent studies (31), and investigations in patients do not support a direct improvement of primary negative symptoms by clozapine (69). In clinical trials with d-serine and other activators of the NMDAR glycine site administered as adjuvant treatments, symptomatic improvements have been observed (4,70). These glutamatergic therapies have demonstrated to be particularly beneficial in alleviating negative and cognitive symptoms, with some studies achieving negative symptom response independent of anti-psychotic effects, suggestive of direct improvement in primary negative symptoms (69,71,72). Likewise, our findings support the efficacy of d-serine to ameliorate these chronically debilitating disturbances. Importantly, long-term administration of d-serine and similar compounds at therapeutic doses have not been found to produce adverse effects in humans (73), although large doses are required to overcome difficulties with penetrating the blood–brain-barrier. There is also concern with the possibility that under conditions promoting excitotoxicity and neuroinflammation, high levels of d-serine could compromise neuronal survival through excessive NMDAR activation (74,75). Consequently, the development of novel therapies targeting enzymes that lead to more modest rises in d-serine is ongoing (76).
Abnormal behavioral responses in SrrY269* mice may be related to aberrant NMDAR neurotransmission and/or to the alterations we identified in our microarrays. The greatest changes in transcripts were found in the hippocampus, supporting previous reports of an important role for d-serine and Srr activity in hippocampal-dependent processes (77,78). Surprisingly few transcripts were changed in the frontal cortex of mutant mice, though d-serine levels in this area were severely reduced relative to wild-type animals. This might indicate that d-serine has less of a crucial role in this region, that the little remaining d-serine was sufficient, or that the activity of d-serine in the frontal cortex is only evident under specific stimuli. Importantly, several of the genes we identified have been demonstrated to be involved in schizophrenia and processes relevant to this disorder, including myelination, neurodevelopment, and cognitive ability. Previous microarray studies examining the effects of chronic treatments of NMDAR antagonists have observed an upregulation of Ttr, Enpp2, Igf2 and potassium channels (79,80), indicating that some of the changes related to the SrrY69* mutation resemble the effects of reduced NMDAR activation. Acute administration of MK-801 was also found to increase Igf2 mRNA levels in the rat brain, possibly through NMDAR-induced release of nitric oxide (81). Furthermore, d-serine and NMDAR activation can influence the expression of immediate-early genes (82), several of which were altered in our mutant mice. NMDAR activity has been shown to closely regulate prolactin secretion in rodents and humans (6,83), and the prolactin receptor was found to be elevated in SrrY269* mice. In mutant animals exogenous d-serine treatment influenced the mRNA levels of several of the genes identified in the microarray analysis, suggesting that d-serine availability dynamically regulates the abundance of these transcripts. The mechanistic link between these genes and the d-serine pathway is unknown; however, the reproducibility of these alterations by NMDAR antagonists suggests that expression of these genes is regulated by signaling cascades that follow NMDAR-mediated neurotransmission. Overall, the microarray analysis identified specific molecules relevant to perturbations in the d-serine pathway and supports the notion that reduced Srr activity may be involved in schizophrenia pathogenesis.
A genetic variant in the human SRR gene was associated with schizophrenia in a nuclear family sample. Association of SRR with schizophrenia in previous studies has been somewhat inconsistent (9,84,85). Investigations examining the 5′-promoter region of SRR have demonstrated a significant association with schizophrenia (9,84), contrary to a study examining polymorphisms in the central and 3′ region of SRR (85). Since the significant SNP found in our study is also closest to the 5′ end of SRR, it is probable that the genetic region of SRR analyzed is of importance. Moreover, our significant marker (rs4523957) is relatively close to the associated SNP (rs408067) in the Morita et al. (9) study, which was found to induce a 60% reduction in SRR promoter function. The proximity of these SNPs to exon 1b, the major Srr isoform in the brain (52), suggests a potential modulation of SRR transcription in the brain. Consequently, whether Srr activity is reduced through a polymorphism in a 5′-regulatory region or through a premature stop codon further downstream in the genomic sequence, the resulting effects support the contention that abnormal Srr function is involved in the pathophysiological mechanism of schizophrenia.
In contrast to mice with global losses of the NMDA-NR1 subunit (86), diminished Srr activity and d-serine do not impact viability nor produce any gross physical abnormalities. Instead, our findings suggest that low d-serine and Srr function is relevant to modulating the more subtle phenotypes characteristic of psychiatric illness. Aberrant regulation of d-serine has also been implicated in neuropathologies involving excitotoxicity, including Alzheimer's disease, stroke and amyotrophic lateral sclerosis (74,78,87), and studying the SrrY269* mice could further elucidate the role of altered d-serine in these neurodegenerative diseases. Moreover, the SrrY269* mice may be a useful preclinical model to assess the efficacy of novel therapeutic interventions for schizophrenia and other neurological disorders influenced by d-serine availability.
MATERIALS AND METHODS
Mutation screening
The temperature gradient capillary electrophoresis (TGCE) heteroduplex detection method was used to screen the Srr gene for ENU-induced mutations in 7502 mice, as described (27).
Mice
All animal procedures were approved by the Animal Management Committee of Mount Sinai Hospital and complied with the requirements of the Province of Ontario Animals for Research Act 1971 and the Canadian Council on Animal Care. Groups of 3–5 littermates were housed by sex in polycarbonate cages, and given ad libitum sterile food (Purina mouse chow) and water. The vivarium was maintained under controlled temperature (21°C ± 1°C) and humidity (50–60%), with a 12-h diurnal cycle (lights on: 0700–1900). All studies were conducted on experimentally naïve mice that were 9–12 weeks of age, except where stated. Biochemical studies were conducted on approximately equal numbers of male and female mice. As no sex differences were found, male and female data were pooled for greater subject numbers. Behavioral studies and microarray experiments were conducted with male subjects. Animals with the SrrY269* mutation were genotyped using a PCR-amplicon restriction endonuclease protocol that detected the absence of an HpyCH4V (Fermentas) restriction site in homozygous mutants.
Western blot analysis
Protein extracts from whole brain were resolved by SDS–PAGE and then subjected to Western blot analysis that involved incubation with the following antibodies: mouse anti-Srr monoclonal antibody (BD Biosciences; 1:500), Srr polyclonal antibody, T-16 (Santa Cruz Biotechnology; 1:100), anti-PICK1 monoclonal antibody, clone L20/8 (UC Davis/NINDS/NIMH NeuroMab Facility; 1:1000), NR1 (Millipore; 1:500), GluR1 (Calbiochem; 1:100), GluR2 (Calbiochem; 1:100), glycine transporter-1 (Millipore; 1:500), d-amino acid oxidase (Nordic Immunological; 1:1000), Ttr, FL-147 (Santa Cruz Biotechnology; 1:50), Enpp2, H-180 (Santa Cruz Biotechnology; 1:100), Kl (Abcam; 1:250), Igf2 (Abcam; 1:250), Folr1, M-19 (Santa Cruz Biotechnology; 1:100), Prlr, B10 (Santa Cruz Biotechnology; 1:75), Otx2, Y-23 (Santa Cruz Biotechnology; 1:100) and Cldn2 (Abcam; 1:75). Srr antibodies were specific to epitopes in the N-terminus region.
Real-time RT–PCR
The mRNA levels of Srr, PICK1, Ttr, Enpp2, Kl, Igf2, Folr1, Prlr, Otx2, Cldn2, Sgk1 and γ-actin were quantified in SrrY269* mice using real-time RT–PCR, as described (88).
Srr activity
The procedure to measure the activity of Srr in whole brain, hippocampus, frontal cortex and cerebellum was adapted from a previously described protocol (12; Supplementary Material, Methods).
High-performace liquid chromatography
To measure d-serine concentrations in whole brain, hippocampus, frontal cortex and cerebellum of SrrY269* mice a high-performace liquid chromatography procedure was conducted, as described (89).
Behavioral studies
Behavioral testing was performed during the light phase between 0900 and 1600 h using male mice. Experiments were randomized with regard to day and drug treatment, and experimenters were blind to genotype. A physical examination was performed on adult mice that involved assessment of weight, fur condition, whisker presence, eye-blink, ear-twitch, whisker-twitch, righting reflex and vision, as described (90,91). Performance in an accelerating rotarod task and motor learning was examined using a modification of a previously described protocol (92; Supplementary Material, Methods). Locomotor activity was measured for 30 min (5-min bins) in a transparent Plexiglas open field (41 × 41 × 31 cm) equipped with infrared beams to detect horizontal movements (model 7420/7430; Ugo Basile). Floating duration in the forced swim test was evaluated, as described (92). Anxiety-like responses were measured in the elevated plus-maze, as described (93). In the social affiliations task, sociability was examined by comparing the time spent in a chamber with a caged stranger mouse to the time spent in an opposite empty cage chamber, while preference for social novelty was assessed by comparing the time spent with a familiar versus a non-familiar partner, as described (94). Olfactory acuity was evaluated by the latency to find a buried food pellet, as described (91). Prepulse inhibition of the acoustic startle response and startle reactivity was assessed, as described (42). The spatial and non-spatial recognition task compared responses to displaced and non-displaced objects, as well as reactivity to novel and familiar objects, as described (94). The MWM task was used to measure spatial learning and memory, and was adapted from a described protocol (77; Supplementary Material, Methods).
Drugs
d-serine (600 mg/kg; Sigma) and MK-801 (0.1 mg/kg; Sigma) were dissolved in a saline (0.9% NaCl) solution. Clozapine (0.75 mg/kg; Tocris) was dissolved in 0.9% NaCl with 0.3% Tween. d-serine and MK-801 were injected subcutaneously, while clozapine was injected intraperitoneally. All drugs were administered at a volume of 10 ml/kg and injection-testing intervals were 30 min. Drug doses were chosen based on previous work (42,77,94) and pilot experiments.
Statistical analysis of biochemical and behavioral data
Statistical analyses were performed using Statistica (Statsoft). Biochemical and behavioral data were analyzed using one-way, two-way or repeated-measures (RM) ANOVA with the appropriate between-subjects and within-subjects factors. Significant main effects or interactions were followed by Fisher's least significant difference post hoc comparisons.
Microarrays
Microarray analysis was performed using eight male animals (four wild-type and four mutant). For each animal, RNA was isolated from three brain regions (hippocampus, frontal cortex and cerebellum) and hybridized to a separate MouseRef-8 v2.0 Expression BeadChip array (Illumina) by the McGill University and Genome Quebec Innovation Centre following manufacturer's protocols. Microarray data pre-processing and downstream statistical, machine-learning and functional analyses were largely as described previously (95,96; Supplementary Material, Methods). All raw and pre-processed data are available in the GEO repository (accession GSE13974).
Human SNP analysis
Analysis of SNP markers in the human SRR gene was completed with two types of genetic sample configurations (family and case–control). The family sample consisted of 117 DSM-IV schizophrenia probands with at least one first-degree relative. The matched case–control sample consisted of 221 genetically unrelated pairs (DSM-IV schizophrenia patients and controls) who were matched for sex, ethnicity and age. All recruitment and clinical assessments were conducted with written informed consent in accordance with the University of Toronto and Canadian Institutes of Health Research (CIHR) guidelines for the ethical treatment of human subjects. Genomic DNA was extracted from peripheral venous blood specimens using a non-enzymatic high-salt procedure, as described (97). Four SRR SNPs were studied: rs4523957 (intron 1, G1651T), rs391300 (intron 1, A9010G), rs8081273 (intron 4, C15077T), rs7222251 (intron 6, T18868C). The SRR markers were genotyped by TaqMan allele-specific assays (Applied Biosystems) using the ABI Prism 7000 Sequence Detection System (Applied Biosystems). Polymorphisms and haplotype distribution were analyzed for genetic association in the family and matched case–control samples, as described (98,99).
SUPPLEMENTARY MATERIAL
Supplementary material is available at HMG online.
FUNDING
V.L. was supported by a Natural Sciences and Engineering Research Council (NSERC, Canada) studentship. This work was supported by the Canadian Institutes of Health Research (GMH-79044 to J.C.R.); and the United States National Institutes of Health (P01AG12411 to S.W.B.).
ACKNOWLEDGEMENTS
J.C.R. and G.B.B. are Canada Research Council (CRC) chairs. A.H.C.W. is a Canadian Institutes of Health Research Clinician-Scientist Fellow. The authors gratefully thank G. Rauw and E. Weiss for expert technical assistance.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Lewis D.A., Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nature Med. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- 2.Ross C.A., Margolis R.L., Reading S.A., Pletnikov M., Coyle J.T. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Insel T.R., Scolnick E.M. Cure therapeutics and strategic prevention: raising the bar for mental health research. Mol. Psychiatry. 2006;11:11–17. doi: 10.1038/sj.mp.4001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coyle J.T. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell. Mol. Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Javitt D.C., Zukin S.R. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 6.Krystal J.H., Karper L.P., Seibyl J.P., Freeman G.K., Delaney R., Bremner J.D., Heninger G.R., Bowers M.B.J., Charney D.S. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 7.Chumakov I., Blumenfeld M., Guerassimenko O., Cavarec L., Palicio M., Abderrahim H., Bougueleret L., Barry C., Tanaka H., La Rosa P., et al. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc. Natl Acad. Sci. USA. 2002;99:13675–13680. doi: 10.1073/pnas.182412499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumacher J., Jamra R.A., Freudenberg J., Becker T., Ohlraun S., Otte A.C.J., Tullius M., Kovalenko S., Bogaert A.V., Maier W., et al. Examination of G72 and D-amino acid oxidase as genetic risk factor for schizophrenia and bipolar affective disorder. Mol. Psychiatry. 2004;9:203–207. doi: 10.1038/sj.mp.4001421. [DOI] [PubMed] [Google Scholar]
- 9.Morita Y., Ujike H., Tanaka Y., Otani K., Kishimoto M., Morio A., Kotaka T., Okahisa Y., Matsushita M., Morikawa A., et al. A genetic variant of the serine racemase gene is associated with schizophrenia. Biol. Psychiatry. 2006;61:1200–1203. doi: 10.1016/j.biopsych.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 10.Corvin A., McGhee K.A., Murphy K., Donohoe G., Nangle J.M., Schwaiger S., Kenny N., Clarke S., Meagher D., Quinn J., et al. Evidence for association and epistasis at the DAOA/G30 and D-amino acid oxidase loci in an Irish schizophrenia sample. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007;144B:949–953. doi: 10.1002/ajmg.b.30452. [DOI] [PubMed] [Google Scholar]
- 11.Madeira C., Freitas M.E., Vargas-Lopes C., Wolosker H., Panizzutti R. Increased brain D-amino acid oxidase (DAAO) activity in schizophrenia. Schizophr. Res. 2008;101:76–83. doi: 10.1016/j.schres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Wolosker H., Sheth K.N., Takahashi M., Mothet J., Brady R.O.J., Ferris C.D., Snyder S.H. Purification of serine racemase: Biosynthesis of the neuromodulator D-serine. Proc. Natl Acad. Sci. USA. 1999;96:721–725. doi: 10.1073/pnas.96.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bendikov I., Nadri C., Amar S., Panizzutti R., De Miranda J., Wolosker H., Agam G. A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. Schizophr. Res. 2007;90:41–51. doi: 10.1016/j.schres.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto K., Fukushima T., Shimizu E., Komatsu N., Watanabe H., Shinoda N., Nakazato M., Kumakiri C., Okada S., Hasegawa H., et al. Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch. Gen. Psychiatry. 2003;60:572–576. doi: 10.1001/archpsyc.60.6.572. [DOI] [PubMed] [Google Scholar]
- 15.Clements J.D., Westbrook G.L. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron. 1991;7:605–613. doi: 10.1016/0896-6273(91)90373-8. [DOI] [PubMed] [Google Scholar]
- 16.Johnson J.W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 17.Matsui T., Sekiguchi M., Hashimoto A., Tomita U., Nishikawa T., Wada K.J. Functional comparison of D-serine and glycine in rodents: the effects on cloned NMDA receptors and the extracellular concentration. J. Neurochem. 1995;65:454–458. doi: 10.1046/j.1471-4159.1995.65010454.x. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs S.A., Berger R., Klomp L.W., de Koning T.J. D-amino acids in the central nervous system in health and disease. Mol. Genet. Metab. 2005;85:168–180. doi: 10.1016/j.ymgme.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Panatier A., Theodosis D.T., Mothet J.P., Touquet B., Pollegioni L., Poulain D.A., Oliet S.H. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 20.Shleper M., Kartvelishvily E., Wolosker H. D-serine is the dominant endogenous coagonist for NMDA receptor neurotoxicity in organotypic hippocampal slices. J. Neurosci. 2005;25:9413–9417. doi: 10.1523/JNEUROSCI.3190-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams S.M., Diaz C.M., Macnab L.T., Sullivan R.K., Pow D.V. Immunocytochemical analysis of D-serine distribution in the mammalian brain reveals novel anatomical compartmentalizations in glia and neurons. Glia. 2006;53:401–411. doi: 10.1002/glia.20300. [DOI] [PubMed] [Google Scholar]
- 22.Yoshikawa M., Nakajima K., Takayasu N., Noda S., Sato Y., Kawaguchi M., Oka T., Kobayashi H., Hashimoto A. Expression of the mRNA and protein of serine racemase in primary cultures of rat neurons. Eur. J. Pharmacol. 2006;548:74–76. doi: 10.1016/j.ejphar.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 23.Schell M.J., Brady R.O.J.r., Molliver M.E., Snyder S.H. D-serine as a neuromodulator: regional and developmental localizations in rat brain glia resemble NMDA receptors. J. Neurosci. 1997;17:1604–1615. doi: 10.1523/JNEUROSCI.17-05-01604.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panizzutti R., Rausch M., Zurbrügg S., Baumann D., Beckmann N., Rudin M. The pharmacological stimulation of NMDA receptors via co-agonist site: an fMRI study in the rat brain. Neurosci. Lett. 2005;380:111–115. doi: 10.1016/j.neulet.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 25.Basu A.C., Tsai G.E., Ma C.L., Ehmsen J.T., Mustafa A.K., Han L., Jiang Z.I., Benneyworth M.A., Froimowitz M.P., Lange N., et al. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol. Psychiatry. 2008 doi: 10.1038/mp.2008.130. in press, doi:10.1038/mp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman H.E., Jirásková J., Ingr M., Zvelebil M., Konvalinka J. Recombinant human serine racemase: enzymologic characterization and comparison with its mouse ortholog. Protein Expr. Purif. 2009;63:62–67. doi: 10.1016/j.pep.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Sakuraba Y., Sezutsu H., Takahasi K.R., Tsuchihashi K., Ichikawa R., Fujimoto N., Kaneko S., Nakai Y., Uchiyama M., Goda N., et al. Molecular characterization of ENU mouse mutagenesis and archives. Biochem. Biophys. Res. Commun. 2005;336:609–616. doi: 10.1016/j.bbrc.2005.08.134. [DOI] [PubMed] [Google Scholar]
- 28.Wang L.Z., Zhu X.Z. Spatiotemporal relationships among D-serine, serine racemase, and D-amino acid oxidase during mouse postnatal development. Acta Pharmacol. Sin. 2003;24:965–974. [PubMed] [Google Scholar]
- 29.Dev K.K., Henley J.M. The schizophrenic faces of Pick1. Trends Pharmacol. Sci. 2006;27:574–579. doi: 10.1016/j.tips.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujii K., Maeda K., Hikida T., Mustafa A.K., Balkissoon R., Xia J., Yamada T., Ozeki Y., Kawahara R., Okawa M., et al. Serine racemase binds to Pick1: potential relevance to schizophrenia. Mol. Psychiatry. 2006;11:150–157. doi: 10.1038/sj.mp.4001776. [DOI] [PubMed] [Google Scholar]
- 31.Ellenbroek B.A., Cools A.R. Animal models for the negative symptoms of schizophrenia. Behav. Pharmacol. 2000;11:223–233. doi: 10.1097/00008877-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Karlsson R.M., Tanaka K., Saksida L.M., Bussey T.J., Heilig M., Holmes A. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacol. 2008;34:1578–1589. doi: 10.1038/npp.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swerdlow N.R., Braff D.L., Taaid N., Geyer M.A. Assessing the validity of an animal model of deficitent sensorimotor gaiting in schizophrenia patients. Arch. Gen. Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- 34.Geyer M.A., Krebs-Thomson K., Braff D.L., Swerdlow N.R. Pharmacological studies of prepulse inhibition models sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 35.Barnett J.H., Croudace T.J., Jaycock S., Blackwell C., Hynes F., Sahakian B.J., Joyce E.M., Jones P.B. Improvement and decline of cognitive function in schizophrenia over 1 year: a longitudinal investigation using latent growth modelling. BMC Psychiatry. 2007;7:1–10. doi: 10.1186/1471-244X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Donnell B.F., Swearer J.M., Smith L.T., Nestor P.G., Shenton M.E., McCarley R.W. Selective deficits in visual perception and recognition in schizophrenia. Am. J. Psychiatry. 1996;153:687–692. doi: 10.1176/ajp.153.5.687. [DOI] [PubMed] [Google Scholar]
- 37.Mandillo S., Rinaldi A., Oliverio A., Mele A. Repeated administration of phencyclidine, amphetamine and MK-801 selectively impairs spatial learning in mice: a possible model of psychotomimetic drug-induced cognitive deficits. Behav. Pharmacol. 2003;14:533–544. doi: 10.1097/00008877-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Roullet P., Mele A., Ammassari-Teule M. Involvement of glutamatergic and dopaminergic systems in the reactivity of mice to spatial and non-spatial change. Psychopharmacology. 1996;126:55–61. doi: 10.1007/BF02246411. [DOI] [PubMed] [Google Scholar]
- 39.Mumby D.G., Gaskin S., Glenn M.J., Schramek T.E., Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn. Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sargolini F., Roullet P., Oliverio A., Mele A. Effects of lesions to the glutamatergic afferents to the nucleus accumbens in the modulation of reactivity to spatial and non-spatial novelty in mice. Neuroscience. 1999;93:855–867. doi: 10.1016/s0306-4522(99)00259-6. [DOI] [PubMed] [Google Scholar]
- 41.Arguello P.A., Gogos J.A. Modeling madness in mice: one piece at a time. Neuron. 2006;52:179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 42.Lipina T., Labrie V., Weiner I., Roder J. Modulators of the glycine site on NMDA receptors, D-serine and ALX-5407, display similar beneficial effects to clozapine in mouse models of schizophrenia. Psychopharmacology. 2005;179:54–67. doi: 10.1007/s00213-005-2210-x. [DOI] [PubMed] [Google Scholar]
- 43.Rajeevan M.S., Vernon S.D., Taysavang N., Unger E.R. Validation of array-based gene expression profiles by real-time (kinetic) RT-PCR. J. Mol. Diagn. 2001;3:26–31. doi: 10.1016/S1525-1578(10)60646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J.T., Leweke F.M., Oxley D., Wang L., Harris N., Koethe D., Gerth C.W., Nolden B.M., Gross S., Schreiber D., et al. Disease biomarkers in cerebrospinal fluid of patients with first-onset psychosis. PLoS Med. 2006;3:e428. doi: 10.1371/journal.pmed.0030428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warner M.D., Walker A.M., D'souza D.C., Lee D., Nasseri D., Peabody C.A. Lower prolactin bioactivity in unmedicated schizophrenic patients. Psychiatry Res. 2001;102:249–254. doi: 10.1016/s0165-1781(01)00256-6. [DOI] [PubMed] [Google Scholar]
- 46.Akanji A.O., Ohaeri J.U., Al-Shammri S.A., Fatania H.R. Associations of blood levels of insulin-like growth factor (IGF)-I, IGF-II and IGF binding protein (IGFBP)-3 in schizophrenic Arab subjects. Clin. Chem. Lab. Med. 2007;45:1229–1231. doi: 10.1515/CCLM.2007.265. [DOI] [PubMed] [Google Scholar]
- 47.Davis K.L., Stewart D.G., Friedman J.I., Buchsbaum M., Harvey P.D., Hof P.R., Buxbaum J., Haroutunian V. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch. Gen. Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 48.Danesin C., Agius E., Escalas N., Ai X., Emerson C., Cochard P., Soula C. Ventral neural progenitors switch toward an oligodendroglial fate in response to increased Sonic hedgehog (Shh) activity: involvement of sulfatase 1 in modulating Shh signaling in the ventral spinal cord. J. Neurosci. 2006;26:5037–5048. doi: 10.1523/JNEUROSCI.0715-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagai T., Yamada K., Kim H.C., Kim Y.S., Noda Y., Imura A., Nabeshima Y., Nabeshima T. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J. 2003;17:50–52. doi: 10.1096/fj.02-0448fje. [DOI] [PubMed] [Google Scholar]
- 50.Knapska E., Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog. Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Tyan S.W., Tsai M.C., Lin C.L., Ma Y.L., Lee E.H. Serum- and glucocorticoid-inducible kinase 1 enhances zif268 expression through the mediation of SRF and CREB1 associated with spatial memory formation. J. Neurochem. 2008;105:820–832. doi: 10.1111/j.1471-4159.2007.05186.x. [DOI] [PubMed] [Google Scholar]
- 52.Yamada K., Ohnishi T., Hashimoto K., Ohba H., Iwayama-Shigeno Y., Toyoshima M., Okuno A., Takao H., Toyota T., Minabe Y., et al. Identification of multiple serine racemase (SRR) mRNA isoforms and genetic analyses of SRR and DAO in schizophrenia and D-serine levels. Biol. Psychiatry. 2005;57:1493–1503. doi: 10.1016/j.biopsych.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 53.Gondo Y. Trends in large-scale mouse mutagenesis: from genetics to functional genomics. Nat. Rev. Genet. 2008;9:803–810. doi: 10.1038/nrg2431. [DOI] [PubMed] [Google Scholar]
- 54.Michaud E.J., Culiat C.T., Klebig M.L., Barker P.E., Cain K.T., Carpenter D.J., Easter L.L., Foster C.M., Gardner A.W., Guo Z.Y., et al. Efficient gene-driven germ-line point mutagenesis of C57BL/6J mice. BMC Genomics. 2005;6:164. doi: 10.1186/1471-2164-6-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang Y.F., Imam J.S., Wilkinson M.F. The nonsense-mediated decay RNA surveillance pathway. Annu. Rev. Biochem. 2007;76:51–74. doi: 10.1146/annurev.biochem.76.050106.093909. [DOI] [PubMed] [Google Scholar]
- 56.D'Aniello A., D'Onofrio G., Pischetola M., D'Aniello G., Vetere A., Petrucelli L., Fisher G.H. Biological role of D-amino acid oxidase and D-aspartate oxidase. Effects of D-amino acids. J. Biol. Chem. 1993;268:26941–26949. [PubMed] [Google Scholar]
- 57.Wood P.L., Hawkinson J.E., Goodnough D.B. Formation of D-serine from L-phosphoserine in brain synaptosomes. J. Neurochem. 1996;67:1485–1490. doi: 10.1046/j.1471-4159.1996.67041485.x. [DOI] [PubMed] [Google Scholar]
- 58.Ohnuma T., Sakai Y., Maeshima H., Hatano T., Hanzawa R., Abe S., Kida S., Shibata N., Suzuki T., Arai H. Changes in plasma glycine, L-serine, and D-serine levels in patients with schizophrenia as their clinical symptoms improve: Results from the Juntendo University Schizophrenia Projects (JUSP) Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:1905–1912. doi: 10.1016/j.pnpbp.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 59.Hashimoto A., Yoshikawa M., Andoh H., Yano H., Matsumoto H., Kawaguchi M., Oka T., Kobayashi H. Effects of MK-801 on the expression of serine racemase and D-amino acid oxidase mRNAs and on the D-serine levels in rat brain. Eur. J. Pharmacol. 2007;555:17–22. doi: 10.1016/j.ejphar.2006.09.062. [DOI] [PubMed] [Google Scholar]
- 60.Kim P.M., Aizawa H., Kim P.S., Huang A.S., Wickramasinghe S.R., Kashani A.H., Barrow R.K., Huganir R.L., Ghosh A., Snyder S.H. Serine racemase: activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc. Natl Acad. Sci. USA. 2005;102:2105–2110. doi: 10.1073/pnas.0409723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beneyto M., Meador-Woodruff J.H. Lamina-specific abnormalities of AMPA receptor trafficking and signaling molecule transcripts in the prefrontal cortex in schizophrenia. Synapse. 2006;60:585–598. doi: 10.1002/syn.20329. [DOI] [PubMed] [Google Scholar]
- 62.Foltyn V.N., Bendikov I., De Miranda J., Panizzutti R., Dumin E., Shleper M., Li P., Toney M.D., Kartvelishvily E., Wolosker H. Serine racemase modulates intracellular D-serine levels through an alpha,beta-elimination activity. J. Biol. Chem. 2005;280:1754–1763. doi: 10.1074/jbc.M405726200. [DOI] [PubMed] [Google Scholar]
- 63.Dunlop D.S., Neidle A. Regulation of serine racemase activity by amino acids. Brain Res. Mol. Brain Res. 2005;133:208–214. doi: 10.1016/j.molbrainres.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 64.Brodkin E.S., Hagemann A., Nemetski S.M., Silver L.M. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002:151–157. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 65.O'Tuathaigh C.M., Babovic D., O'sullivan G.J., Clifford J.J., Tighe O., Croke D.T., Harvey R., Waddington J.L. Phenotypic characterization of spatial cognition and social behavior in mice with ‘knockout’ of the schizophrenia risk gene neuregulin 1. Neuroscience. 2007;147:18–27. doi: 10.1016/j.neuroscience.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 66.Braff D.L., Grillon C., Geyer M.A. Gating and habituation of the startle reflex in schizophrenic patients. Arch. Gen. Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- 67.Wong A.H.C., Voruganti L.N.P., Heslegrave R.J., Awad A.G. Neurocognitive deficits and neurological signs in schizophrenia. Schizophr. Res. 1997;23:139–146. doi: 10.1016/S0920-9964(96)00095-3. [DOI] [PubMed] [Google Scholar]
- 68.Chan R.C., Gottesman I.I. Neurological soft signs as candidate endophenotypes for schizophrenia: a shooting star or a Northern star? Neurosci. Biobehav. Rev. 2008;32:957–971. doi: 10.1016/j.neubiorev.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 69.Murphy B.P., Chung Y.C., Park T.W., McGorry P.D. Pharmacological treatment of primary negative symptoms in schizophrenia: a systematic review. Schizophr. Res. 2006;88:5–25. doi: 10.1016/j.schres.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 70.Heresco-Levy U., Javitt D.C., Ebstein R., Vass A., Lichtenberg P., Bar G., Catinari S., Ermilov M. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol. Psychiatry. 2005;57:577–585. doi: 10.1016/j.biopsych.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 71.Evins A.E., Amico E., Posever T.A., Toker R., Goff D.C. D-cycloserine added to risperidone in patients with primary negative symptoms of schizophrenia. Schizophr. Res. 2002;56:19–23. doi: 10.1016/s0920-9964(01)00220-1. [DOI] [PubMed] [Google Scholar]
- 72.Heresco-Levy U., Javitt D.C., Ermilov M., Mordel C., Siliop G., Lichenstein M. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch. Gen. Psychiatry. 1999;56:29–36. doi: 10.1001/archpsyc.56.1.29. [DOI] [PubMed] [Google Scholar]
- 73.Tsai G., Yang P., Chung L.-C., Lange N., Coyle J.T. D-serine added to antipsychotics for the treatment of schizophrenia. Biol. Psychiatry. 1998;44:1081–1089. doi: 10.1016/s0006-3223(98)00279-0. [DOI] [PubMed] [Google Scholar]
- 74.Wu S.Z., Bodles A.M., Porter M.M., Griffin W.S., Basile A.S., Barger S.W. Induction of serine racemase expression and D-serine release from microglia by amyloid beta-peptide. J. Neuroinflammation. 2004;1:2. doi: 10.1186/1742-2094-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martineau M., Baux G., Mothet J.P. D-serine signalling in the brain: friend and foe. Trends Neurosci. 2006;29:481–491. doi: 10.1016/j.tins.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 76.Adage T., Trillat A.C., Quattropani A., Perrin D., Cavarec L., Shaw J., Guerassimenko O., Giachetti C., Gréco B., Chumakov I., et al. In vitro and in vivo pharmacological profile of AS057278, a selective d-amino acid oxidase inhibitor with potential anti-psychotic properties. Eur. Neuropsychopharmacol. 2008;18:200–214. doi: 10.1016/j.euroneuro.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 77.Duffy S., Labrie V., Roder J.C. D-serine augments NMDA-NR2B receptor-dependent hippocampal long-term depression and spatial reversal learning. Neuropsychopharmacology. 2008;33:1004–1018. doi: 10.1038/sj.npp.1301486. [DOI] [PubMed] [Google Scholar]
- 78.Wolosker H., Dumin E., Balan L., Foltyn V.N. D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration. FEBS J. 2008;275:3514–3526. doi: 10.1111/j.1742-4658.2008.06515.x. [DOI] [PubMed] [Google Scholar]
- 79.Matsuoka T., Tsunoda M., Sumiyoshi T., Takasaki I., Tabuchi Y., Seo T., Tanaka K., Uehara T., Itoh H., Suzuki M., et al. Effect of MK-801 on gene expressions in the amygdala of rats. Synapse. 2008;62:1–7. doi: 10.1002/syn.20455. [DOI] [PubMed] [Google Scholar]
- 80.Krügel H., Becker A., Polten A., Grecksch G., Singh R., Berg A., Seidenbecher C., Saluz H.P. Transcriptional response to the neuroleptic-like compound Ampullosporin A in the rat ketamine model. J. Neurochem. 2006;97:74–81. doi: 10.1111/j.1471-4159.2005.03621.x. [DOI] [PubMed] [Google Scholar]
- 81.Giannakopoulou M., Mansour M., Kazanis E., Bozas E., Philpipidis H., Stylianopoulou F. NMDA receptor mediated changes in IGF-II gene expression in the rat brain after injury and the possible role of nitric oxide. Neuropathol. Appl. Neurobiol. 2000;26:513–521. doi: 10.1046/j.0305-1846.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- 82.Lamas M., Lee-Rivera I., Ramírez M., López-Colomé A.M. D-serine regulates CREB phosphorylation induced by NMDA receptor activation in Müller glia from the retina. Neurosci. Lett. 2007;427:55–60. doi: 10.1016/j.neulet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 83.Aguilar E., Tena-Sempere M., Aguilar R., González D., Pinilla L. Interactions between N-methyl-D-aspartate, nitric oxide and serotonin in the control of prolactin secretion in prepubertal male rats. Eur. J. Endocrinol. 1997;137:99–106. doi: 10.1530/eje.0.1370099. [DOI] [PubMed] [Google Scholar]
- 84.Goltsov A.Y., Loseva J.G., Andreeva T.V., Grigorenko A.P., Abramova L.I., Kaleda V.G., Orlova V.A., Moliaka Y.K., Rogaev E.I. Polymorphism in the 5′-promoter region of serine racemase gene in schizophrenia. Mol. Psychiatry. 2006;11:325–326. doi: 10.1038/sj.mp.4001801. [DOI] [PubMed] [Google Scholar]
- 85.Strohmaier J., Georgi A., Schirmbeck F., Schmael C., Jamra R.A., Schumacher J., Becker T., Höfels S., Klopp N., Illig T., et al. No association between the serine racemase gene (SRR) and schizophrenia in a German case-control sample. Psychiatr. Genet. 2007;17:125. doi: 10.1097/YPG.0b013e3280143e43. [DOI] [PubMed] [Google Scholar]
- 86.Forrest D., Yuzaki M., Soares H.D., Ng L., Luk D.C., Sheng M., Stewart C.L., Morgan J.I., Connor J.A., Curran T. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron. 1994;13:325–338. doi: 10.1016/0896-6273(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 87.Sasabe J., Chiba T., Yamada M., Okamoto K., Nishimoto I., Matsuoka M., Aiso S. D-serine is a key determinant of glutamate toxicity in amyotrophic lateral sclerosis. EMBO J. 2007;26:4149–4159. doi: 10.1038/sj.emboj.7601840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fick L.J., Cai F., Belsham D.D. Hypothalamic preproghrelin gene expression is repressed by insulin via both PI3-K/Akt and ERK1/2 MAPK pathways in immortalized, hypothalamic neurons. Neuroendocrinol. 2009;89:267–275. doi: 10.1159/000167698. [DOI] [PubMed] [Google Scholar]
- 89.Labrie V., Duffy S., Wei W., Barger S., Baker G.B., Roder J.C. Genetic inactivation of D-amino acid oxidase enhances extinction and reversal learning in mice. Learn. Mem. 2008;16:28–37. doi: 10.1101/lm.1112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miyakawa T., Yared E., Pak J.H., Huang F.L., Huang K.P., Crawley J.N. Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus. 2001;11:763–775. doi: 10.1002/hipo.1092. [DOI] [PubMed] [Google Scholar]
- 91.Wersinger S.R., Ginns E.I., O'Carroll A.M., Lolait S.J., Young W.S., III Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol. Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- 92.Soleimani L., Roder J.C., Dennis J.W., Lipina T. Beta N-acetylglucosaminyltransferase V (Mgat5) deficiency reduces the depression-like phenotype in mice. Genes Brain Behav. 2008;7:334–343. doi: 10.1111/j.1601-183X.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- 93.Labrie V., Clapcote S.J., Roder J.C. Mutant mice with reduced NMDA-NR1 glycine affinity or lack of D-amino acid oxidase function exhibit altered anxiety-like behaviors. Pharmacol. Biochem. Behav. 2008;91:610–620. doi: 10.1016/j.pbb.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 94.Labrie V., Lipina T., Roder J.C. Mice with reduced NMDA receptor glycine affinity model some of the negative and cognitive symptoms of schizophrenia. Psychopharmacol. 2008;200:217–230. doi: 10.1007/s00213-008-1196-6. [DOI] [PubMed] [Google Scholar]
- 95.Boutros P.C., Yan R., Moffat I.D., Pohjanvirta R., Okey A.B. Transcriptomic responses to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in liver: comparison of rat and mouse. BMC Genomics. 2008;9:419. doi: 10.1186/1471-2164-9-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dunning M.J., Smith M.L., Ritchie M.E., Tavare S. Beadarray: R classes and methods for Illumina bead-based data. Bioinformatics. 2007;23:2183–2184. doi: 10.1093/bioinformatics/btm311. [DOI] [PubMed] [Google Scholar]
- 97.Lahiri D.K., Nurnberger J.I.J.r. A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martucci L., Wong A.H., De Luca V., Likhodi O., Wong G.W., King N., Kennedy J.L. N-methyl-D-aspartate receptor NR2B subunit gene GRIN2B in schizophrenia and bipolar disorder: Polymorphisms and mRNA levels. Schizophr. Res. 2006;84:214–221. doi: 10.1016/j.schres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 99.Wong A.H., Likhodi O., Trakalo J., Yusuf M., Sinha A., Pato C.N., Pato M.T., Van Tol H.H., Kennedy J.L. Genetic and post-mortem mRNA analysis of the 14-3-3 genes that encode phosphoserine/threonine-binding regulatory proteins in schizophrenia and bipolar disorder. Schizophr. Res. 2005;78:137–146. doi: 10.1016/j.schres.2005.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.