Abstract
Frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17) is caused by mutations in the MAPT gene, encoding the tau protein that accumulates in intraneuronal lesions in a number of neurodegenerative diseases. Several FTDP-17 mutations affect alternative splicing and result in excess exon 10 (E10) inclusion in tau mRNA. RNA reprogramming using spliceosome-mediated RNA trans-splicing (SMaRT) could be a method of choice to correct aberrant E10 splicing resulting from FTDP-17 mutations. SMaRT creates a hybrid mRNA through a trans-splicing reaction between an endogenous target pre-mRNA and a pre-trans-splicing RNA molecule (PTM). However, FTDP-17 mutations affect the strength of cis-splicing elements and could favor cis-splicing over trans-splicing. Excess E10 inclusion in FTDP-17 can be caused by intronic mutations destabilizing a stem-loop protecting the 5′ splice site at the E10/intron 10 junction. COS cells transfected with a minigene containing the intronic +14 mutation produce exclusively E10+ RNA. Generation of E10− RNA was restored after co-transfection with a PTM designed to exclude E10. Similar results were obtained with a target containing the exonic N279K mutation which strengthens a splicing enhancer within E10. Conversely, increase or decrease in E10 content was achieved by trans-splicing from a target carrying the Δ280K mutation, which weakens the same splicing enhancer. Thus E10 inclusion can be modulated by trans-splicing irrespective of the strength of the cis-splicing elements affected by FTDP-17 mutations. In conclusion, RNA trans-splicing could provide the basis of therapeutic strategies for impaired alternative splicing caused by pathogenic mutations in cis-acting splicing elements.
INTRODUCTION
Tauopathies are major diseases of the central nervous system characterized neuropathologically by intracellular filamentous inclusions formed by the microtubule-associated protein tau in affected neurons (1,2). Tauopathies include dementias such as Alzheimer's disease and some forms of frontotemporal dementia (3). Definitive evidence for the pathogenic importance of tau was provided by the discovery of dominant mutations in the MAPT gene, the gene encoding tau, in the rare dementia, frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17) (4–6).
The MAPT gene comprises 16 exons and alternative splicing of exons 2, 3 and 10 generates six isoforms in the brain. Exon 10 (E10) encodes the second of four imperfect 31–32 amino-acid microtubule-binding repeats in the C-terminal half of the protein. Exclusion or inclusion of E10 generate tau isoforms with three (3R tau, E10−) or four (4R tau, E10+) microtubule-binding repeats, the latter having an increased affinity for microtubules. E10 is expressed only in adults and E10+ and E10− isoforms are expressed in approximately equal amounts in adult human brain.
To date, more than thirty-five MAPT mutations have been associated with FTDP-17. Most missense mutations reduce the affinity of tau for microtubules (7), whereas silent or intronic mutations affect E10 splicing and can result in an up to 6-fold excess of tau mRNA containing E10 and in an elevated 4R/3R ratio (4,6,8,9). Elevated 4R/3R ratio is likely to have functional consequences, for example in the regulation of axonal transport (10).
From a therapeutic perspective, correcting defective alternative splicing is best achieved by direct intervention at the RNA level. RNA can be reprogrammed by using spliceosome-mediated RNA trans-splicing (SMaRT®) (11). SMaRT creates a hybrid mRNA through a trans-splicing reaction mediated by the spliceosome between the 5′ splice site of an endogenous target pre-mRNA and the 3′ splice site of an exogenously delivered pre-trans-splicing RNA molecule (PTM). Conversely, a PTM can be designed to carry the 5′ splice site and trans-splice to the 3′ splice site of a specific target (12). A typical application of SMaRT is the correction of loss-of-function mutations. We have shown previously that E10− to E10+ tau RNA conversion could be achieved using SMaRT (13). Thus, SMaRT could be a method of choice to correct aberrant E10 splicing resulting from FTDP-17 mutations. However, FTDP-17 mutations affect the strength of cis-splicing elements and could favor cis-splicing over trans-splicing. For instance, most intronic mutations are clustered at the exon 10-intron 10 junction and disrupt a stem-loop structure protecting the 5′ splice site (14,15). Furthermore, with the exception of the P301L/S mutations, mutations within E10 are located in cis-acting splicing regulatory elements and promote E10 retention (16,17). We therefore addressed the question of the potential effectiveness of SMaRT to correct aberrant E10 splicing caused by FTDP-17 mutations. Here we show that elevated or reduced E10 inclusion caused by intronic or exonic FTDP-17 mutations can be reversed by trans-splicing. Hence, RNA trans-splicing could provide the basis for the development of promising therapeutic strategies for impaired alternative splicing, especially in the context of neurological diseases.
RESULTS
Tau PTM constructs
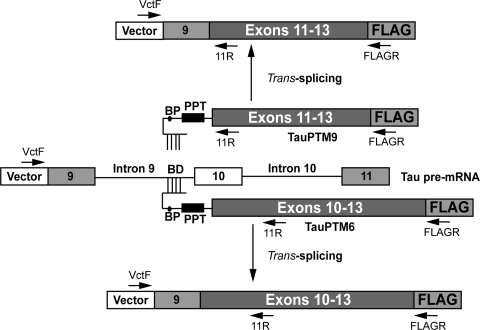
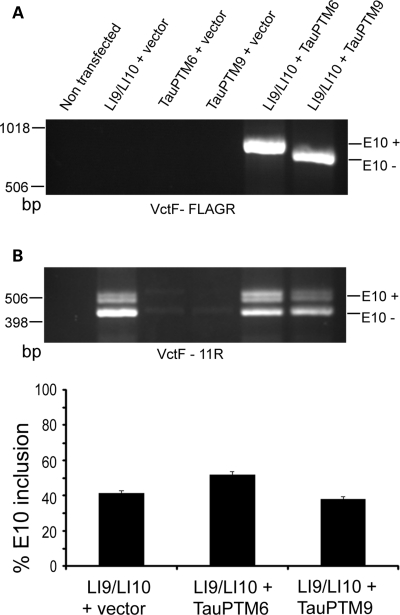
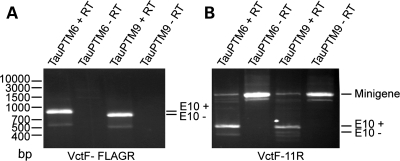
We have shown previously that E10 could be introduced in tau RNA by SMaRT (13). We have now designed a PTM, TauPTM9, to exclude E10. TauPTM9 comprises a binding domain hybridizing with the 3′ end of intron 9 and human tau exons 11–13 followed by a FLAG epitope sequence in the coding sequence (Fig. 1). COS cells were co-transfected with TauPTM9 and the LI9/LI10 tau minigene consisting of the full genomic MAPT sequence from exon 9 to exon 11, including exons 9, 10, 11 and the entire introns 9 and 10 (18). As a positive control, cells were also co-transfected with the minigene and TauPTM6, designed to introduce E10 in tau RNA (13). To detect trans-spliced products, RNA was analyzed by RT–PCR with a minigene-specific forward primer, VctF, corresponding to a pCIneo vector sequence, and the PTM-specific reverse primer, FLAGR, complementary to the FLAG epitope sequence. Using this primer combination, a product of 773 bp was detected in cells transfected with TauPTM9 (Fig. 2A). Trans-splicing was also detected in cells transfected with the minigene and TauPTM6, that has the same binding and trans-splicing domains as TauPTM9. The 93 bp difference in size between the TauPTM6 (866 bp) and TauPTM9 products correspond to E10 (Fig. 2A). Sequencing of VctF-FLAGR products confirmed that exact exon 9–exon 10 and exon 9–exon 11 junctions had been generated using TauPTM6 and TauPTM9, respectively, and demonstrates that trans-splicing has occurred between minigene transcripts and either PTM.
Figure 1.
Structure of TauPTM6 and TauPTM9 and resulting trans-spliced products. The coding sequence of TauPTM6 and TauPTM9 consists of exons 10–13 or exons 11–13, respectively, followed by a 24 nt FLAG epitope sequence. The trans-splicing domain of the PTMs comprises a 125 nt binding domain (BD) complementary to the 3′ end of tau intron 9 as well as a strong conserved yeast branch point sequence (BP), a 26 nt polypyrimidine tract (PPT), an AG acceptor site and a spacer sequence separating the binding domain and the branch point. Arrows indicate the locations of the PCR primers used to detect cis- and trans-splicing events.
Figure 2.
Trans-splicing modulation of E10 usage in tau transcripts. COS cells were co-transfected with the LI9/LI10 minigene and TauPTM6 or TauPTM9. (A) RT–PCR analysis with the forward primer VctF, specific for the target minigene and the PTM-specific reverse primer FLAGR, complementary to the FLAG epitope sequence, demonstrates trans-splicing between minigene transcripts and PTMs. The difference in size between the products generated using Tau PTM6 and TauPTM9 corresponds to E10. (B) Change in E10 inclusion after co-transfection of LI9/LI10 with TauPTM6 and TauPTM9. Both cis- and trans-spliced products were detected by RT–PCR with the forward primer VctF and the reverse primer 11R, corresponding to an exon 11 sequence. Co-transfection with TauPTM6 or TauPTM9 resulted in an increase or decrease of E10 inclusion, respectively.
We next analyzed whether trans-splicing with TauPTM9 was accompanied by a reduction in E10 inclusion in the total mature RNA (i.e. cis-spliced and trans-spliced) derived from LI9/LI10. Reverse transcribed RNA was amplified with the VctF primer, as above, and the reverse primer 11R complementary to an exon 11 sequence, that detects both cis- and trans-spliced products. Co-transfection of LI9/LI10 with TauPTM9 resulted in a decrease in E10 inclusion (Fig. 2B). Conversely, and as reported previously (13), co-transfection with TauPTM6 resulted in increased E10 inclusion (Fig. 2B). These results show that E10 usage can be modulated in either direction, irrespective of the cis-splicing fate on an individual targeted pre-mRNA.
Trans-splicing reduction of E10 excess caused by intronic MAPT mutations
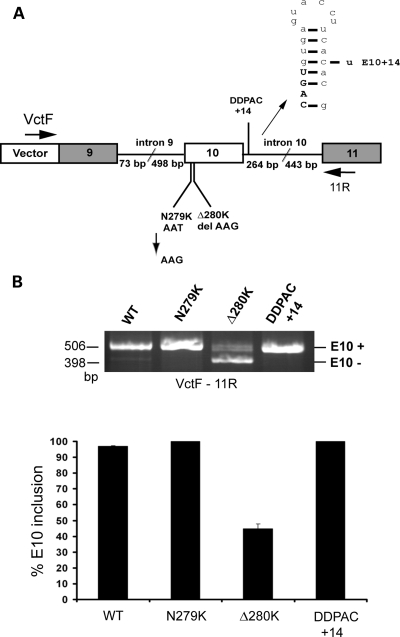
FTDP-17 MAPT mutations affecting splicing are located in splicing regulatory elements and most produce excess E10 inclusion. We assessed whether the level of E10 inclusion in tau RNA could be reduced by trans-splicing in the context of FTDP-17 mutations. As mutations are within or close to E10, trans-splicing assays with mutant targets were performed using the TauEx9-11WT minigene backbone, with minimal intronic sequences flanking E10 (19) (Fig. 3A). The sequence of the exon 10/intron 10 junction predicts a stem-loop structure regulating E10 splicing by protecting the 5′ splice site. FTDP-17 mutations +3 to +16 destabilize the stem loop and enhance E10 inclusion (14,15,19). Among these the +14 DDPAC mutation is one of the most potent at increasing E10 inclusion (4). To confirm that the +14 mutation resulted in E10 excess in mature tau RNA, tau splicing was compared between COS cells transfected with the wild-type minigene, TauEx9-11WT or the TauEx9-11DDPAC minigene, containing the +14 mutation. TauEx9-11WT generated mostly E10+ RNA with a small amount of E10− RNA (Fig. 3B). In contrast, the TauEx9-11DDPAC minigene produced exclusively E10+ RNA (Fig. 3B).
Figure 3.
Splicing pattern of minigenes containing FTDP-17 mutations. (A) Structure of the MAPT minigenes incorporating the exonic N279K and Δ280K mutations or the intronic DDPAC +14 mutation. The backbone minigene, TauEx9-11, consists of tau exons 9–10–11 and 498 bp 5′ and 264 bp 3′ of intronic sequences flanking E10. (B) Splicing was assayed by RT–PCR using primers VctF and 11R in transfected COS cells. TauEx9-11WT produces mainly E10+ RNA; the mutants minigenes, TauEx9-11DDPAC and TauEx9-11N279K, produce exclusively E10+ RNA, whereas TauEx9-11Δ280K produces an excess of E10− RNA. Arrows indicate the locations of the PCR primers used to detect splice isoforms.
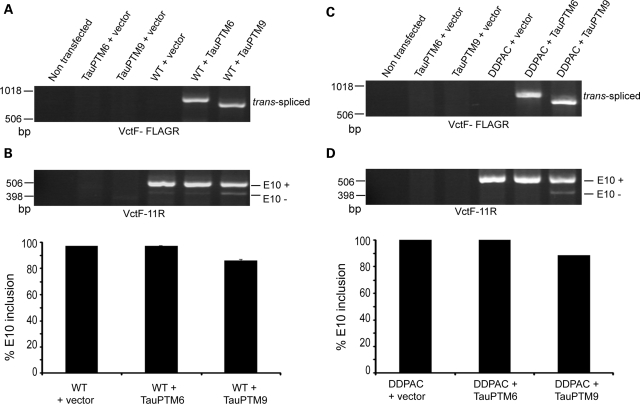
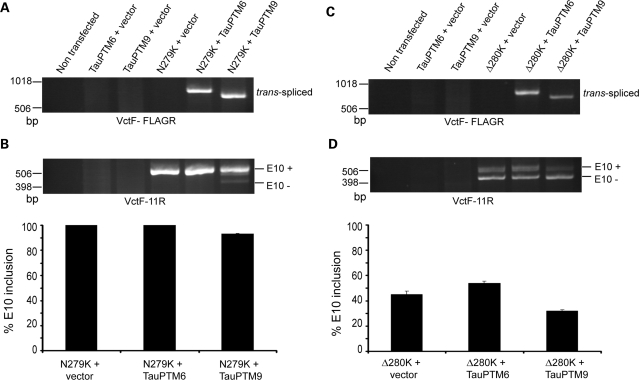
To determine whether RNA lacking E10 could be generated by trans-splicing from a target containing the +14 mutation COS cells were co-transfected with the TauEx9-11DDPAC minigene and TauPTM9 and analyzed for occurrence of trans-splicing and levels of E10 inclusion using the VctF-FLAGR or VctF-11R primer combinations. Trans-splicing was first confirmed for the wild-type minigene, TauEx9-11WT, and Tau PTM6 or TauPTM9 (Fig. 4A, B). Analysis of cells co-transfected with the TauEx9-11DDPAC minigene and TauPTM6 or TauPTM9 demonstrated trans-splicing between transcripts from the minigene and either PTM (Fig. 4C). In the absence of PTM, the TauEx9-11DDPAC minigene produced E10+ RNA only. In contrast, products lacking E10 were generated in cells co-transfected with TauPTM9 (Fig. 4D). Based on the reduction in the percentage of E10 inclusion estimated by semi-quantitative PCR, restoration of E10− RNA was achieved with a ∼11% efficiency. The generation of E10− RNA is not the consequence of a direct change in cis-splicing activity secondary to the blocking of a potential splicing enhancer in intron 9 by the binding of the PTM as such products were not observed with TauPTM6 which has the same binding domain as TauPTM9 (Fig. 4D).
Figure 4.
Trans-splicing reduction of E10 inclusion in tau transcripts with the intronic +14 mutation. COS cells were co-transfected with a wild-type tau minigene, TauEx9-11WT (A and B) or the TauEx9-11DDPAC minigene containing the +14 mutation (C and D) and TauPTM6 or TauPTM9 and analyzed by RT–PCR. (A and C) PCR analysis with primers VctF and FLAGR demonstrates trans-splicing between minigene transcripts and PTMs. (B and D) E10 usage after co-transfection with the minigenes was analyzed by RT–PCR using the VctF–11R primer combination. (B) Co-transfection of TauEx9-11WT with TauPTM9 reduced E10 inclusion. (D) The TauEx9-11DDPAC minigene produces E10+ RNA only and E10− products were generated after co-transfection with TauPTM9. Co-transfection with TauPTM6 had no effect on E10 inclusion.
To exclude the possibility that the hybrid RNA observed was the result of recombination events at the DNA level between minigene and PTM PCR analysis was conducted with or without reverse transcription and with an extension time sufficient to amplify potential recombination products. No trans-splicing (Fig. 5A) or cis- and trans-splicing (Fig. 5B) products were detected when the reverse transcription step was omitted. In addition, no large size species that would have been indicative of recombination were amplified from the traces of DNA present in the RNA preparation using the VctF-FLAGR primer combination (Fig. 5A). In contrast minigene DNA was readily detected using the VctF-11R primer combination (Fig. 5B). Therefore E10 can be skipped by trans-splicing in the presence of a constitutively available 5′ splice site in the target.
Figure 5.
Absence of recombination between minigene and PTM at the DNA level. RNA isolated from cells co-transfected with TauEx9-11DDPAC and TauPTM6 or TauPTM9 was analyzed by PCR with (+RT) or without (−RT) prior reverse transcription. No cis- or trans-splicing (A and B) products were detected without RT. (A) Trans-splicing products only are amplified using the VctF-FLAGR primer combination; no larger size species were detected with or without RT. (B) In contrast, in addition to cis- and trans-splicing products, minigene DNA was readily amplified from the traces of DNA present in the RNA preparation using the VctF–11R primer combination.
Trans-splicing reduction of E10 excess caused by exonic MAPT mutations
The N279K mutation is located within an exonic splicing enhancer (ESE) in E10, and increases E10 inclusion by facilitating the use of the 3′ splice site of intron 9 (16,20). In transfected cells, a minigene carrying the N279K mutation, TauEx9-11N279K, produces exclusively E10+ RNA (Fig. 3B). COS cells were co-transfected with the TauEx9-11N279K minigene and analyzed for occurrence of trans-splicing and levels of E10 inclusion, as earlier. Analysis with the VctF and FLAGR primers demonstrated trans-splicing between transcripts from the TauEx9-11N279K minigene and both TauPTM6 and Tau PTM9 (Fig. 6A). Co-transfection of TauEx9-11N279K with TauPTM9 resulted in the appearance of E10− products with a ∼7% efficiency, whereas co-transfection with TauPTM6 had no effect (Fig. 6B).
Figure 6.
Trans-splicing modulation of E10 usage in tau transcripts with the exonic N279K and Δ280K mutations. COS cells were co-transfected with the TauEx9-11N279K (A and B) or the TauEx9-11Δ280K (C and D) tau minigenes and TauPTM6 or TauPTM9 and analyzed by RT–PCR. (A and C) PCR analysis with primers VctF–FLAGR demonstrates trans-splicing between minigene transcripts and PTMs. (B and D) E10 usage after co-transfection with the minigenes was analyzed by RT–PCR using the VctF-11R primer combination. (B) Co-transfection of TauEx9-11N279K with TauPTM9 resulted in the appearance of E10− products. Co-transfection with TauPTM6 had no effect on E10 inclusion. (D) The TauEx9-11Δ280K minigene produces both E10+ and E10− RNA, with a slight excess of E10− RNA. Co-transfection with TauPTM6 or TauPTM9 increased or reduced E10 inclusion, respectively.
The ESE strengthened by the N279 mutation is weakened by the Δ280K deletion, causing reduced E10 inclusion (16). In transfected cells, an excess of E10− RNA is produced from a minigene containing the Δ280K mutation, TauEx9-11Δ280K (Fig. 3B). RT–PCR analysis demonstrated trans-splicing between TauEx9-11Δ280K transcripts and either TauPTM6 or Tau PTM9 and a resulting increase or decrease in the level of E10 inclusion, respectively (Fig. 6C, D). Taken together, these results show that E10 usage can be modulated by trans-splicing independently of the strength of the cis-regulatory elements in the targets.
DISCUSSION
In this study, we have demonstrated that aberrant tau E10 splicing resulting from FTDP-17 mutations can be partially corrected by trans-splicing. We showed previously that cis-splicing exclusion of E10 could be by-passed by trans-splicing and that E10− to E10+ tau RNA conversion could be achieved from wild-type tau sequences (13). The demonstration that E10 inclusion can be reduced as well as increased by trans-splicing shows that trans-splicing can occur irrespective of the outcome of the cis-splicing event determined by the combination of splicing factors recruited by an individual pre-mRNA. Importantly, correction of aberrant splicing caused by FTDP-17 mutations shows that trans-splicing can be achieved in the presence of cis-splicing elements of altered strength.
Most intronic FTDP-17 mutations are clustered at the exon 10–intron 10 junction. This region is predicted to form a stem-loop structure regulating E10 splicing by protecting the 5′ splice site. FTDP-17 mutations +3 to +16, as well as the exonic S305N mutation, destabilize the stem loop making the 5′ splice site of intron 10 constitutively available, hence promoting E10 inclusion (14,15,20). E10 retention results in a 1.5- to 6-fold increase in the E10+/E10− ratio (4,9). Trans-splicing was achieved using a target carrying the very potent DDPAC +14 mutation and produced mature RNA lacking E10 demonstrating that the constitutive availability of the 5′ splice site of intron 10 does not interfere with trans-splicing.
The N279K mutation, within E10, strengthens an ESE and facilitates the use of the 3′ splice site of intron 9. The affinity of the splicing factors, Tra2β and SF2/ASF, for the ESE is enhanced by the N279K mutation and, conversely, Tra2β and SF2/ASF affinity for the ESE is reduced by the Δ280K deletion (16,17,21). The Δ280K mutation is the only known MAPT mutation that reduces E10 inclusion and is associated with lesions containing exclusively, or, at least predominantly, 3R tau (22,23). Trans-splicing obtained with the N279K mutation and subsequent production of RNA lacking E10 shows that enhanced availability of the 3′ splice site of intron 9 in the target does not interfere with the use of the 3′ splice site from the PTM, which is required for the trans-splicing reaction. Therefore abnormal binding of Tra2β and/or SF2/ASF to the ESE within the target does not inhibit trans-splicing modulation of E10 usage in either direction. However, its efficiency might be influenced by the interplay between trans-acting factors recruited on the target and on the PTM.
The possibility of correcting E10 inclusion is relevant not only for FTDP-17 but also for sporadic tauopathies. A single molecule analysis of tau isoforms in Alzheimer's disease brain revealed an increase to 1.3 in the E10+/E10− ratio (24). In addition, the H1 haplotype of the MAPT gene, which is associated with an increased risk of Alzheimer's disease, expresses more E10+ tau mRNA than the H2 haplotype (25,26).
Several chemical inhibitors of E10 inclusion have been identified through high throughput screening and could provide drug leads (27,28). However, RNA-based therapies are gaining increasing popularity due to their specificity and versatility (29). Among those, SMaRT has proved to be a very powerful method to correct loss-of-function mutations at the RNA level (30–33). Recent applications have included correction of β-globin in sickle-cell anemia and β-thalassemia (34), plectin (PLEC1) in blistering skin disease epidermolysis bullosa simplex with muscular dystrophy (EBS-MD) (35), severe combined immune deficiency (SCID) (36), spinal muscular atrophy (37,38) and myotonic dystrophy (39). Gene therapy for brain diseases is still at an early stage, but several classes of viral vectors have been developed for the transduction of neurons (40,41). Furthermore, long-term expression of trans-splicing molecules obtained using viral vectors or transposon systems has been shown to result in recovery of functional defects compatible with a therapeutic benefit (36,38,42–44). The level of tau E10 splicing correction necessary for a detectable phenotypic effect is not known, neither is the precise consequence of tau isoform imbalance on microtubule properties and functions or on neurotoxicity. Trans-splicing reduction of E10 inclusion on endogenous tau could be optimized in induced pluripotent stem cells derived from FTDP-17 patients, when available, after differentiation into neurons (45).
An increasing number of neurological and other disorders are being associated with aberrant alternative splicing (46–49) and SMaRT would be an approach to be developed to correct aberrant splicing caused by pathogenic mutations in cis-acting splicing elements.
MATERIALS AND METHODS
Pre-trans-splicing molecules
The pre-trans-splicing molecule, TauPTM6, in the mammalian expression vector, pcDNA3.1, has been described previously (13). TauPTM6 is designed to include E10 in tau RNA; its coding sequence consists of tau exons 10–13, followed by a FLAG epitope sequence (5′-GACTACAAGGACGACGATGACAAG-3′). TauPTM9, designed to exclude E10 from tau RNA, was generated by deleting E10 from TauPTM6, using restriction digestions and ligation of synthetic oligonucleotides.
Tau minigene targets
The LI9/LI10 minigene in the pCIneo vector comprises tau exons 9–10–11 and full-length introns 9 and 10 (18) and was obtained from Dr Jianhua Zhou (University of Massachusetts Medical School, Worcester). Minigenes TauEx9-11WT and TauEx9-11DDPAC comprising tau exons 9–10–11 and 498 bp of 5′ and 264 bp 3′ of intronic sequences flanking E10 (19) (Fig. 3) were obtained from Dr Jane Wu (Northwestern University Feinberg School of Medicine, Chicago). The TauEx9-11DDPAC minigene carries the intronic +14 DDPAC FTDP-17 mutation. Two additional minigenes, TauEx9-11N279K and TauEx9-11Δ280K were constructed by introducing the exonic N279K and Δ280K mutations into TauEx9-11 using the Quick-Change site-directed mutagenesis kit (Stratagene). Wild-type and mutant minigenes based on TauEx9-11 were subcloned into pCIneo. All constructs were verified by sequencing.
Cell culture and transfection
COS-7 cells were maintained in DMEM supplemented with 10% (v/v) heat inactivated FBS (Invitrogen), 100 U/ml penicillin/100 µg/ml streptomycin and 2 mm l-glutamine, in a humidified atmosphere of 95% air/5% CO2 at 37°C. Cells were transfected with Lipofectamine 2000 reagent (Invitrogen). Briefly, cells plated on 35 mm dishes were grown to 60–70% confluency and exposed to the DNA/liposome complex for 5 h in Optimem (Invitrogen) before being returned to normal culture medium. Cells were routinely analyzed 24 h after transfection. Cells were transfected with either tau minigene plus empty vector or PTM plus empty vector, or co-transfected with minigene and PTM. Typically, 2 µg minigene and 2 µg PTM were used in each transfection.
RT–PCR analysis of cis- and trans-splicing
Total RNA was isolated from transfected cells using Trizol reagent (Invitrogen). Reverse transcription was performed using the Multiscribe™ RT kit (Applied Biosystem) and oligo-(dT)16. Reverse transcription was carried out under the following conditions: 10 min at 25°C, 30 min at 48°C and a final step of 5 min at 95°C. Reverse transcribed RNA was amplified by using Go Taq polymerase (Promega) under the following conditions: 94°C for 5 min, 30 cycles of 1 min at 94°C, 1 min at 58°C, 1 min at 72°C and a final step of 10 min at 72°C. An extension time of 3 min was used in reactions designed to visualize DNA. The sequence of the primers used for PCR and the sizes of cis- and trans-splicing products are listed in Table 1. PCR products were separated by electrophoresis in 2% (w/v) agarose gels and stained with ethidium bromide. Densitometric analysis of triplicate experiments was performed using the Innotech software (Alpha Innotech, San Leandro, CA, USA). Splicing activity was expressed as percentage of E10 inclusion. The efficiency of E10+ to E10− conversion was estimated from the percentage of E10 inclusion in cells transfected with minigene only (p1) and co-transfected with minigene and PTM (p2) as 100 × (p1−p2)/p1. For sequencing, PCR products were excised from agarose gels and cloned into the pCR2.1 vector using the TA cloning system (Invitrogen). Sequencing of both strands was performed using T3 and T7 primers.
Table 1.
Sequence of the PCR primers used to detect cis- and trans-splicing products and sizes of the corresponding products
| Name | Location | Sequence | Product size | |
|---|---|---|---|---|
| Cis- and trans-splicing | VctF | Vector | 5′-GGTGTCCACTCCCAGTTCAA | E10+518 bp |
| 11R | Exon 11 | 5′-CCCTGGTTTATGATGGATGTTGCCTAATGAG | E10- 425 bp | |
| Trans-splicing | VctF | Vector | 5′-GGTGTCCACTCCCAGTTCAA | E10+866 bp |
| FLAGR | FLAG sequence | 5′-TTGTCATCGTCGTCCTTGTAG | E10- 773 bp |
The forward primer, VctF, corresponds to a sequence in the pCIneo vector and is specific for the target minigenes. The FLAGR reverse primer, complementary to a FLAG epitope sequence, is specific for PTMs and was used to demonstrate trans-splicing. The reverse primer 11R is complementary to an exon 11 sequence and detects both cis- and trans-spliced products.
FUNDING
This work was supported by the Wellcome Trust, the Biotechnology and Biological Sciences Research Council, the Medical Research Council, Ataxia UK, the Alzheimer's Research Trust and the Psychiatry Research Trust. Funding to pay the Open Access publication charges for this article was provided by the Wellcome Trust.
ACKNOWLEDGEMENTS
We thank Drs Jianhua Zhou and Jane Wu for tau minigenes.
Conflict of Interest statement. M.A.G.-B. is a founder of Intronn, a company that was set up for the development and commercialization of the SMaRT technology. Intronn has been bought by VIRxSYS and M.G.-B. is a consultant for VIRxSYS. S.G.M. is an employee of VIRxSYS.
REFERENCES
- 1.Ballatore C., Lee V.M., Trojanowski J.Q. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat. Rev. Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 2.Gallo J.-M., Noble W., Rodriguez Martin T. RNA and protein-dependent mechanisms in tauopathies: consequences for therapeutic strategies. Cell. Mol. Life Sci. 2007;64:1701–1714. doi: 10.1007/s00018-007-6513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams D.R. Tauopathies: classification and clinical update on neurodegenerative diseases associated with microtubule-associated protein tau. Intern. Med. J. 2006;36:652–660. doi: 10.1111/j.1445-5994.2006.01153.x. [DOI] [PubMed] [Google Scholar]
- 4.Hutton M., Lendon C.L., Rizzu P., Baker M., Froelich S., Houlden H.H., Pickering Brown S., Chakraverty S., Isaacs A., Grover A., et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–704. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 5.Poorkaj P., Bird T.D., Wijsman E., Nemens E., Garruto R.M., Anderson L., Andreadis A., Wiederholt W.C., Raskind M., Schellenberg G.D. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann. Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- 6.Spillantini M.G., Murrell J.R., Goedert M., Farlow M.R., Klug A., Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc. Natl Acad. Sci. USA. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt R., Hundelt M., Shahani N. Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Biochim. Biophys. Acta. 2005;1739:331–354. doi: 10.1016/j.bbadis.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Hong M., Zhukareva V., Vogelsberg-Ragaglia V., Wszolek Z., Reed L., Miller B.I., Geschwind D.H., Bird T.D., McKeel D., Goate A., et al. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science. 1998;282:1914–1917. doi: 10.1126/science.282.5395.1914. [DOI] [PubMed] [Google Scholar]
- 9.Connell J.W., Rodriguez-Martin T., Gibb G.M., Khan N.M., Grierson A.J., Hanger D.P., Revesz T., Anderton B.H., Gallo J.-M. Quantitative analysis of tau isoform transcripts in sporadic tauopathies. Brain Res. Mol. Brain Res. 2005;137:104–109. doi: 10.1016/j.molbrainres.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Dixit R., Ross J.L., Goldman Y.E., Holzbaur E.L. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319:1086–1089. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puttaraju M., Jamison S.F., Mansfield S.G., Garcia-Blanco M.A., Mitchell L.G. Spliceosome-mediated RNA trans-splicing as a tool for gene therapy. Nat. Biotechnol. 1999;17:246–252. doi: 10.1038/6986. [DOI] [PubMed] [Google Scholar]
- 12.Mansfield S.G., Clark R.H., Puttaraju M., Kole J., Cohn J.A., Mitchell L.G., Garcia-Blanco M.A. 5′ exon replacement and repair by spliceosome-mediated RNA trans-splicing. RNA. 2003;9:1290–1297. doi: 10.1261/rna.5101903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Martin T., Garcia-Blanco M.A., Mansfield S.G., Grover A.C., Hutton M., Yu Q., Zhou J., Anderton B.H., Gallo J.-M. Reprogramming of tau alternative splicing by spliceosome-mediated RNA trans-splicing: Implications for tauopathies. Proc. Natl Acad. Sci. USA. 2005;102:15659–15664. doi: 10.1073/pnas.0503150102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grover A., Houlden H., Baker M., Adamson J., Lewis J., Prihar G., Pickering-Brown S., Duff K., Hutton M. 5′ splice site mutations in tau associated with the inherited dementia FTDP-17 affect a stem-loop structure that regulates alternative splicing of exon 10. J. Biol. Chem. 1999;274:15134–15143. doi: 10.1074/jbc.274.21.15134. [DOI] [PubMed] [Google Scholar]
- 15.Donahue C.P., Muratore C., Wu J.Y., Kosik K.S., Wolfe M.S. Stabilization of the tau exon 10 stem loop alters pre-mRNA splicing. J. Biol. Chem. 2006;281:23302–23306. doi: 10.1074/jbc.C600143200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Souza I., Poorkaj P., Hong M., Nochlin D., Lee V.M., Bird T.D., Schellenberg G.D. Missense and silent tau gene mutations cause frontotemporal dementia with parkinsonism-chromosome 17 type, by affecting multiple alternative RNA splicing regulatory elements. Proc. Natl Acad. Sci. USA. 1999;96:5598–5603. doi: 10.1073/pnas.96.10.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Z., Tang H., Havlioglu N., Zhang X., Stamm S., Yan R., Wu J.Y. Mutations in tau gene exon 10 associated with FTDP-17 alter the activity of an exonic splicing enhancer to interact with Tra2β. J. Biol. Chem. 2003;278:18997–19007. doi: 10.1074/jbc.M301800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Q., Guo J., Zhou J. A minimal length between tau exon 10 and 11 is required for correct splicing of exon 10. J. Neurochem. 2004;90:164–172. doi: 10.1111/j.1471-4159.2004.02477.x. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Z., Cote J., Kwon J.M., Goate A.M., Wu J.Y. Aberrant splicing of tau pre-mRNA caused by intronic mutations associated with the inherited dementia frontotemporal dementia with parkinsonism linked to chromosome 17. Mol. Cell Biol. 2000;20:4036–4048. doi: 10.1128/mcb.20.11.4036-4048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasegawa M., Smith M.J., Iijima M., Tabira T., Goedert M. FTDP-17 mutations N279K and S305N in tau produce increased splicing of exon 10. FEBS Lett. 1999;443:93–96. doi: 10.1016/s0014-5793(98)01696-2. [DOI] [PubMed] [Google Scholar]
- 21.D'Souza I., Schellenberg G.D. Arginine/serine-rich protein interaction domain-dependent modulation of a tau exon 10 splicing enhancer: altered interactions and mechanisms for functionally antagonistic FTDP-17 mutations Δ280K and N279K. J. Biol. Chem. 2006;281:2460–2469. doi: 10.1074/jbc.M505809200. [DOI] [PubMed] [Google Scholar]
- 22.van Swieten J.C., Bronner I.F., Azmani A., Severijnen L.A., Kamphorst W., Ravid R., Rizzu P., Willemsen R., Heutink P. The ΔK280 mutation in MAP tau favors exon 10 skipping in vivo. J. Neuropathol. Exp. Neurol. 2007;66:17–25. doi: 10.1097/nen.0b013e31802c39a4. [DOI] [PubMed] [Google Scholar]
- 23.Momeni P., Pittman A., Lashley T., Vandrovcova J., Malzer E., Luk C., Hulette C., Lees A., Revesz T., Hardy J., et al. Clinical and pathological features of an Alzheimer's disease patient with the MAPT ΔK280 mutation. Neurobiol. Aging. 2009;30:388–393. doi: 10.1016/j.neurobiolaging.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conrad C., Zhu J., Conrad C., Schoenfeld D., Fang Z., Ingelsson M., Stamm S., Church G., Hyman B.T. Single molecule profiling of tau gene expression in Alzheimer's disease. J. Neurochem. 2007;103:1228–1236. doi: 10.1111/j.1471-4159.2007.04857.x. [DOI] [PubMed] [Google Scholar]
- 25.Caffrey T.M., Joachim C., Paracchini S., Esiri M.M., Wade-Martins R. Haplotype-specific expression of exon 10 at the human MAPT locus. Hum. Mol. Genet. 2006;15:3529–3537. doi: 10.1093/hmg/ddl429. [DOI] [PubMed] [Google Scholar]
- 26.Myers A.J., Pittman A.M., Zhao A.S., Rohrer K., Kaleem M., Marlowe L., Lees A., Leung D., McKeith I.G., Perry R.H., et al. The MAPT H1c risk haplotype is associated with increased expression of tau and especially of 4 repeat containing transcripts. Neurobiol. Dis. 2006;25:561–570. doi: 10.1016/j.nbd.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Donahue C.P., Ni J., Rozners E., Glicksman M.A., Wolfe M.S. Identification of tau stem loop RNA stabilizers. J. Biomol. Screen. 2007;12:789–799. doi: 10.1177/1087057107302676. [DOI] [PubMed] [Google Scholar]
- 28.Stoilov P., Lin C.H., Damoiseaux R., Nikolic J., Black D.L. A high-throughput screening strategy identifies cardiotonic steroids as alternative splicing modulators. Proc. Natl Acad. Sci. USA. 2008;105:11218–11223. doi: 10.1073/pnas.0801661105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood M., Yin H., McClorey G. Modulating the expression of disease genes with RNA-based therapy. PLoS. Genet. 2007;3:e109. doi: 10.1371/journal.pgen.0030109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Blanco M.A. Messenger RNA reprogramming by spliceosome-mediated RNA trans-splicing. J. Clin. Invest. 2003;112:474–480. doi: 10.1172/JCI19462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mansfield S.G., Chao H., Walsh C.E. RNA repair using spliceosome-mediated RNA trans-splicing. Trends Mol. Med. 2004;10:263–268. doi: 10.1016/j.molmed.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y., Walsh C.E. Spliceosome-mediated RNA trans-splicing. Mol. Ther. 2005;12:1006–1012. doi: 10.1016/j.ymthe.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell L.G., McGarrity G.J. Gene therapy progress and prospects: reprograming gene expression by trans-splicing. Gene Ther. 2005;12:1477–1485. doi: 10.1038/sj.gt.3302596. [DOI] [PubMed] [Google Scholar]
- 34.Kierlin-Duncan M.N., Sullenger B.A. Using 5′-PTMs to repair mutant β-globin transcripts. RNA. 2007;13:1317–1327. doi: 10.1261/rna.525607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wally V., Klausegger A., Koller U., Lochmuller H., Krause S., Wiche G., Mitchell L.G., Hintner H., Bauer J.W. 5′ trans-splicing repair of the PLEC1 gene. J. Invest Dermatol. 2008;128:568–574. doi: 10.1038/sj.jid.5701152. [DOI] [PubMed] [Google Scholar]
- 36.Zayed H., Xia L., Yerich A., Yant S.R., Kay M.A., Puttaraju M., McGarrity G.J., Wiest D.L., McIvor R.S., Tolar J., et al. Correction of DNA protein kinase deficiency by spliceosome-mediated RNA trans-splicing and Sleeping Beauty transposon delivery. Mol. Ther. 2007;15:1273–1279. doi: 10.1038/sj.mt.6300178. [DOI] [PubMed] [Google Scholar]
- 37.Coady T.H., Shababi M., Tullis G.E., Lorson C.L. Restoration of SMN function: Delivery of a trans-splicing RNA re-directs SMN2 pre-mRNA splicing. Mol. Ther. 2007;15:1471–1478. doi: 10.1038/sj.mt.6300222. [DOI] [PubMed] [Google Scholar]
- 38.Coady T.H., Baughan T.D., Shababi M., Passini M.A., Lorson C.L. Development of a single vector system that enhances trans-splicing of SMN2 transcripts. PLoS. ONE. 2008;3:e3468. doi: 10.1371/journal.pone.0003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen H.Y., Kathirvel P., Yee W.C., Lai P.S. Correction of dystrophia myotonica type 1 pre-mRNA transcripts by artificial trans-splicing. Gene Ther. 2009;16:211–217. doi: 10.1038/gt.2008.150. [DOI] [PubMed] [Google Scholar]
- 40.Azzouz M., Kingsman S.M., Mazarakis N.D. Lentiviral vectors for treating and modeling human CNS disorders. J. Gene Med. 2004;6:951–962. doi: 10.1002/jgm.600. [DOI] [PubMed] [Google Scholar]
- 41.Wong L.F., Azzouz M., Walmsley L.E., Askham Z., Wilkes F.J., Mitrophanous K.A., Kingsman S.M., Mazarakis N.D. Transduction patterns of pseudotyped lentiviral vectors in the nervous system. Mol. Ther. 2004;9:101–111. doi: 10.1016/j.ymthe.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Chao H., Mansfield S.G., Bartel R.C., Hiriyanna S., Mitchell L.G., Garcia-Blanco M.A., Walsh C.E. Phenotype correction of hemophilia A mice by spliceosome-mediated RNA trans-splicing. Nat. Med. 2003;9:1015–1019. doi: 10.1038/nm900. [DOI] [PubMed] [Google Scholar]
- 43.Tahara M., Pergolizzi R.G., Kobayashi H., Krause A., Luettich K., Lesser M.L., Crystal R.G. Trans-splicing repair of CD40 ligand deficiency results in naturally regulated correction of a mouse model of hyper-IgM X-linked immunodeficiency. Nat. Med. 2004;10:835–841. doi: 10.1038/nm1086. [DOI] [PubMed] [Google Scholar]
- 44.Liu X., Luo M., Zhang L.N., Yan Z., Zak R., Ding W., Mansfield S.G., Mitchell L.G., Engelhardt J.F. Spliceosome-mediated RNA trans-splicing with recombinant adeno-associated virus partially restores cystic fibrosis transmembrane conductance regulator function to polarized human cystic fibrosis airway epithelial cells. Hum. Gene Ther. 2005;16:1116–1123. doi: 10.1089/hum.2005.16.1116. [DOI] [PubMed] [Google Scholar]
- 45.Park I.H., Arora N., Huo H., Maherali N., Ahfeldt T., Shimamura A., Lensch M.W., Cowan C., Hochedlinger K., Daley G.Q. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faustino N.A., Cooper T.A. Pre-mRNA splicing and human disease. Genes Dev. 2003;17:419–437. doi: 10.1101/gad.1048803. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Blanco M.A., Baraniak A.P., Lasda E.L. Alternative splicing in disease and therapy. Nat. Biotechnol. 2004;22:535–546. doi: 10.1038/nbt964. [DOI] [PubMed] [Google Scholar]
- 48.Gallo J.-M., Jin P., Thornton C.A., Lin H., Robertson J., D'Souza I., Schlaepfer W.W. The role of RNA and RNA processing in neurodegeneration. J. Neurosci. 2005;25:10372–10375. doi: 10.1523/JNEUROSCI.3453-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Licatalosi D.D., Darnell R.B. Splicing regulation in neurologic disease. Neuron. 2006;52:93–101. doi: 10.1016/j.neuron.2006.09.017. [DOI] [PubMed] [Google Scholar]