Abstract
Schizophrenia is a severely debilitating psychiatric disease that is hypothesized to have its roots in neurodevelopment. Although the precise neuropathology underlying schizophrenia has remained elusive, there are consistent reports of abnormalities in several brain areas. Chief among these is the hippocampus, an area which has displayed both structural and functional abnormalities in many schizophrenic patients. In order to better understand how disruption of hippocampal development may contribute to the etiology of psychiatric disease, we investigated the function of a highly promising schizophrenia susceptibility gene, DISC1 (Disrupted-In-Schizophrenia 1), in the development of the hippocampus. DISC1 is strongly expressed in the hippocampus from its early development through adulthood and has been implicated in hippocampal structure and function in human studies. However, its precise role in the development of the hippocampus is not yet known. Here, we show that in utero electroporation of Disc1 shRNA into the developing mouse hippocampus hinders the migration of dentate gyrus granule cells. Intriguingly, Disc1 knockdown does not affect the migration of CA1 pyramidal neurons, suggesting that Disc1's role in regulating neuronal migration is spatially restricted within the hippocampus. These findings support the idea that DISC1 abnormalities that contribute to the onset of schizophrenia may do so through their influences on hippocampal development.
INTRODUCTION
Schizophrenia is a devastating mental illness affecting ∼1% of the population worldwide. Although symptoms do not usually present until the late teens or twenties, schizophrenia is widely considered to be a neurodevelopmental disorder (1). In addition, twin and family studies indicate that there is a clear genetic component to the disease (2). Thus, a major focus in psychiatric genetics is to investigate the function of key risk genes in brain development in order to better understand how their disruption can lead to the symptoms of schizophrenia.
Among the most highly promising of such candidate genes is Disrupted-In-Schizophrenia 1 (DISC1). Originally discovered as a balanced translocation segregating with major mental illness in a large Scottish family (3), DISC1 has subsequently been associated with mental illness in several populations (reviewed in 4). Investigations into the function of DISC1 have revealed that it plays a role in several developmental processes, including neurite outgrowth (5), neuronal migration (6,7), and axon targeting (8). DISC1 is also highly expressed in areas associated with schizophrenia, most notably the hippocampus (9–12). In addition, DISC1 disruption has been linked to impaired hippocampal structure and function in both schizophrenic patients and healthy carriers (13), and transgenic mice expressing a mutated form of Disc1 display functional and anatomical hippocampal abnormalities (14,15). These findings suggest that abnormalities in DISC1 lead to deficits in hippocampal function, which could eventually manifest as the symptoms of schizophrenia. Further support for this idea comes from the fact that functional and structural abnormalities of the hippocampus are among the most consistently observed deficits in the schizophrenic brain, as well as numerous reports that schizophrenic patients display abnormalities in hippocampal-mediated forms of memory (16,17).
In order to understand how DISC1 abnormalities influence the development and subsequent function of the hippocampus, we used in utero electroporation to deliver Disc1 shRNA into the embryonic mouse brain. This technique has been previously employed in the developing cerebral cortex (7), where it resulted in a delay in neuronal migration. Intriguingly, we observe dual effects of Disc1 loss of function on neuronal migration within different principal cell populations of the hippocampus. Specifically, Disc1 knockdown hinders the migration of early born granule cells, while it fails to affect migration in CA1 pyramidal neurons. These spatially defined differences demonstrate that Disc1 plays a complex role in the regulation of neuronal migration within the developing brain. Furthermore, they suggest that Disc1 disruptions conferring an increased risk for schizophrenia may do so through their early influences on the functional connectivity of the hippocampus.
RESULTS
Disc1 regulates granule cell migration
Development of the dentate gyrus begins when precursors from the primary dentate matrix (the area of the ventricular zone adjacent to the fimbria) begin migration into the subpial space (18) (Fig. 1). These cells follow a migratory stream that extends through the subpial space toward the area of the future dentate gyrus. As migrating cells reach the vicinity of the dentate gyrus, they form the initial granule cell layer. Subsequent precursor cells stop migration in the future hilus and continue to divide, producing large numbers of granule cells. It is here that large-scale production of granule cells occurs during the first week of post-natal development. Eventually, the production of granule cells slows, and this proliferative pool becomes situated in the subgranular zone, where neurogenesis of granule neurons continues throughout life.
Figure 1.

Granule cell migration in the developing hippocampus. Beginning around E14 in the mouse, precursor cells originating from an area of primary neuroepithelium near the dentate notch (DN) migrate through the dentate migratory stream (DMS) toward the future dentate gyrus (DG). This stream of cells consists of both post-mitotic cells and their dividing precursors, and their migration persists through the first week of post-natal life.
Immunohistochemical analysis has revealed that Disc1 is expressed in the stream of cells migrating toward the future dentate gyrus during embryonic and early post-natal time points (10). Because Disc1 expression is robust throughout the entire hippocampus at these early developmental stages, we sought further confirmation that Disc1 is expressed specifically by migrating granule cell precursors. To do this, we performed immunohistochemistry to detect Disc1 in CXCR4-EGFP mice; CXCR4 is a chemokine receptor which is expressed in migrating granule cells and is known to play a key role in their migration toward the dentate gyrus (19,20). As expected, we found that Disc1 colocalizes with CXCR4 in these cells (Fig. 2A and B). In addition, we determined that Disc1 is expressed by proliferating cells within the dentate migratory stream (Fig. 2C). Taken together, these data indicate that both differentiated granule cells and dividing precursors in the dentate migratory stream express Disc1.
Figure 2.
Disc1 is expressed by cells of the dentate migratory stream. (A and B) Disc1 (red) colocalizes with CXCR4 (green), a chemokine receptor involved in the regulation of granule cell migration. Disc1 immunohistochemistry was performed on CXCR4-EGFP BAC transgenic mice at post-natal day 1 (P1), a time when migration of granule cells through the dentate migratory stream is well underway. (A) Dentate migratory stream closer to the dentate notch; (B) granule cell migration in the area of the dentate gyrus. Arrows indicate notably strong colocalization between Disc1 and CXCR4 in cells (yellow) migrating toward the dentate gyrus. (C) Disc1 immunohistochemistry on the E19 hippocampus of a wild-type mouse shows that Disc1 (red) is present in proliferating cells expressing Ki67 (green) of the dentate migratory stream. Arrows show cells with particularly strong colocalization. Scale bars represent 65 µm (A and B), 80 µm (C). DN, dentate notch.
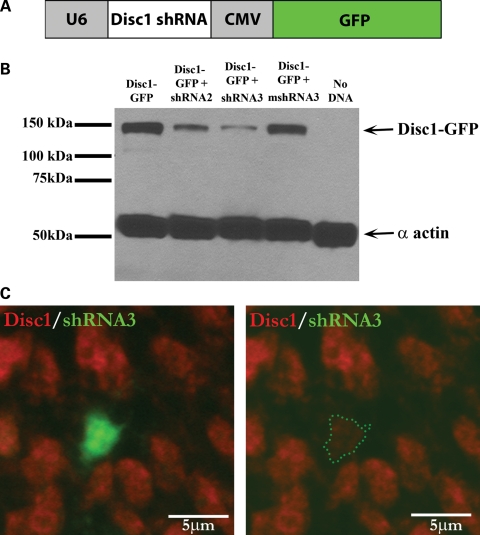
To determine the effects of Disc1 loss of function on granule cell migration, we used in utero electroporation to deliver Disc1 shRNA into the developing mouse hippocampus. We designed two Disc1 shRNAs (shRNA2 and shRNA3) that target different regions within exon 2 of Disc1. Exon 2 is the largest exon in Disc1 and is present in all currently identified Disc1 splice variants (4). These shRNAs were inserted into a dual-promoter expression vector containing an internal GFP reporter [modified from Rubinson et al. (21)] (Fig. 3A), and their ability to reduce Disc1 levels was confirmed in cultured HEK293 cells (Fig. 3B). While shRNA3 gave consistently strong levels of Disc1 knockdown, shRNA2 caused an intermediate level of Disc1 reduction (Fig. 3B). We also generated a control mismatch shRNA (mshRNA3), which has the same targeting sequence as shRNA3, but with three mismatched base pairs. The mshRNA3 construct is impaired in its ability to reduce Disc1 levels (Fig. 3B), which ensures that the effects of shRNA3 are due specifically to the knockdown of Disc1 and not to any effects caused by injection of the shRNA. Finally, we confirmed that the reduction of Disc1 levels can be achieved in vivo by using in utero electroporation to deliver shRNA3 into the developing hippocampus followed by immunohistochemistry to detect Disc1 (Fig. 3C).
Figure 3.
Disc1 shRNAs achieve varying degrees of Disc1 knockdown. (A) Disc1 shRNAs were inserted into a dual-promoter vector (pLL3.7) in which the U6 promoter drives shRNA expression and the CMV promoter drives GFP expression. (B) HEK293 cells were co-transfected with GFP-tagged Disc1 and various Disc1 shRNAs. Protein was then isolated and used for western blot analysis. Probing with an anti-GFP antibody indicates that shRNA3 produces the most dramatic decrease in Disc1 levels, shRNA2 gives an intermediate decrease, and a mismatch shRNA3 (mshRNA3) is impaired in its ability to decrease Disc1 levels. The level of α actin serves as a loading control. (C) In utero electroporation of shRNA3 into the hippocampus at E15–E17 followed by Disc1 immunohistochemistry reveals reduced Disc1 protein levels (red) in cells receiving the shRNA (green).
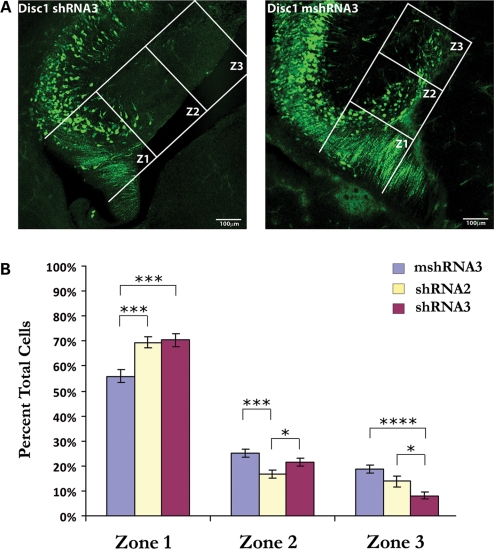
To visualize the effects of Disc1 loss of function on granule cell migration, we then introduced our shRNAs into the developing hippocampus. In utero electroporation was performed at embryonic day 15 (E15), which is soon after granule cell migration has begun, and animals were sacrificed 4 days later (E15–E19). To measure granule cell migration, the distance from the primary dentate neuroepithelium (where migration begins) to the tip of the dentate gyrus was measured for each individual section. On the basis of this distance, three equal zones of migration were measured, and the number of cells in each zone was manually counted. When Disc1 shRNA3 was electroporated, it caused a clear deficit in granule cell migration (Fig. 4A). Specifically, fewer cells migrated to the dentate gyrus (into zones 2 and 3) than was observed following electroporation of mshRNA3 [percent total cells, zone 2: 21.5% (shRNA3) versus 25.3% (mshRNA3); zone 3: 8.1% (shRNA3) versus 18.8% (mshRNA3); Fig. 4B]. Additionally, a higher percentage of cells remained near the ventricular zone (zone 1) in the shRNA3-electroporated brains than in the mshRNA3-electroporated brains (70.4% versus 55.9%, respectively). This effect was not due to higher induction of cell death or to altered levels of proliferation (Supplementary Material, Fig. S1). Thus, these data suggest that Disc1 positively regulates granule cell migration during early hippocampal development.
Figure 4.
Disc1 knockdown hinders migration of cells in the dentate migratory stream. In utero electroporation of Disc1 shRNA into the developing hippocampus at E15 inhibits the migration of cells in the dentate migratory stream when assessed at E19. (A) Representative confocal images of brain sections from shRNA3- and mshRNA3-electroporated mice showing cells that have migrated into each of the three zones of migration. Zones were determined by measuring the distance from the dentate notch to the tip of the dentate gyrus for each individual brain section and dividing it into three equal zones (labeled Z1, Z2 and Z3). (B) Quantification of granule cell migration in mice electroporated with shRNA3 (n = 13), shRNA2 (n = 5) or mshRNA3 (n = 9). The percentage of total cells in the shRNA3 group that migrated to zone 3 was significantly lower than the percentage that reached this area following mshRNA3 electroporation (8.1% versus 18.8%, respectively). In addition, the percentage of total cells that remained in zone 1 was significantly greater in the shRNA3 group than in the mshRNA3 group (70.4% versus 55.9%, respectively). The percentage of cells in zones 1 and 3 for the shRNA2 (intermediate knockdown) group was between the shRNA3 and mshRNA3 groups, indicating that the degree of Disc1 knockdown is directly related to the severity of the granule cell migration deficit. Values are presented as mean ± SEM. *P < 0.05; ***P < 0.0005; ****P < 0.0001.
To determine whether the degree to which Disc1 levels are decreased affects the degree to which granule cell migration is hindered, we injected shRNA2, which gives intermediate levels of Disc1 knockdown (Fig. 3B). Indeed, we found that electroporation of shRNA2 at E15–E19 resulted in slightly more advanced granule cell migration than what was observed following electroporation of shRNA3. Although more cells migrated into zone 3 than in the shRNA3-electroporated animals [13.7% (shRNA2) versus 8.1% (shRNA3)], there were still fewer cells in this zone than in the control (mshRNA3) group (Fig. 4B). Accordingly, the percentage of total cells that remained in zone 1 following shRNA2 injection (69.5%) was slightly less than that observed for shRNA3 (70.4%) but greater than that observed for mshRNA3 (55.9%), and the percentage of cells in zone 2 following shRNA2 injection (16.8%) was less than that observed for both shRNA3 (21.5%) and mshRNA3 (25.3%) (Fig. 4B). Thus, while intermediate reduction of Disc1 still causes more cells to remain in zone 1 than in the control condition, it allows a greater percentage of cells to reach the dentate gyrus (zone 3) than is observed with the strong reduction of Disc1 by shRNA3. These results indicate that the level of Disc1 knockdown directly correlates with the degree to which granule cell migration is hindered.
Migration deficits are rescued with Disc1 overexpression
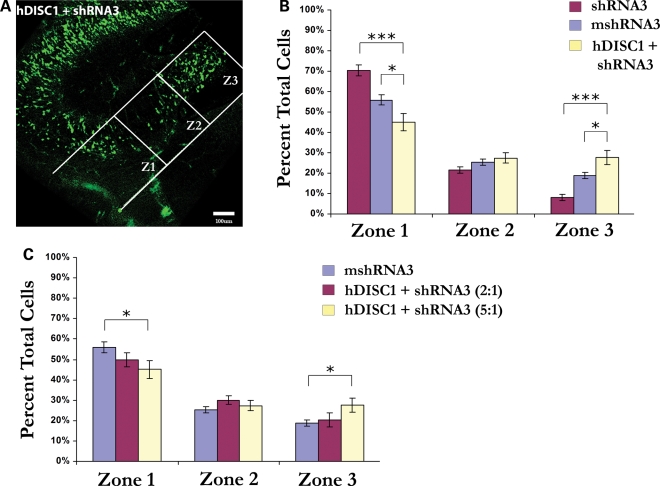
To ensure that the observed deficits in granule cell migration were specifically caused by the knockdown of Disc1, we attempted to rescue migration defects with overexpression of DISC1. To do this, we co-electroporated an expression construct containing full-length human DISC1 (pCAG-hDISC1) with shRNA3 into the mouse hippocampus at E15 and analyzed granule cell migration at E19 (Fig. 5A). We then compared the results of co-electroporation of hDISC1 and shRNA3 to the electroporation of shRNA3 and mshRNA3 alone (Fig. 5B). As expected, a greater percentage of hDISC1/shRNA3-containing (GFP-positive) cells migrated into zones 2 and 3 (27.4 and 27.6%, respectively) than when shRNA3 alone was electroporated (21.5 and 8.1%, respectively) (Fig. 5B). Also, a smaller percentage of hDISC1/shRNA3-containing cells remained in zone 1 (45.1%) than in brains receiving shRNA3 alone (70.4%). Intriguingly, the percentage of hDISC1/shRNA3-containing cells migrating into zone 3 was significantly greater than the percentage migrating this far in the control (mshRNA3) experiments (27.6% versus 18.8%, respectively) (Fig. 5B). This may be explained by the high ratio of hDISC1:shRNA3 that was injected (5:1), which could result in high hDISC1 expression levels in these cells. Indeed, when we lowered the ratio of hDISC1:shRNA3 that was injected from 5:1 to 2:1, the percentage of cells migrating into zone 3 was no longer significantly greater than in the mshRNA3 condition (20.3% versus 18.8%, respectively) (Fig. 5C). Combined with our previous finding that the level of Disc1 reduction influences the degree to which granule cell migration is hindered (Fig. 4B), these data lend further support to the idea that proper migration of embryonic granule cells depends on the amount of Disc1 that is present. In addition, by demonstrating that overexpression of hDISC1 rescues the migration deficit caused by Disc1 knockdown, we have confirmed that the disruption of granule cell migration caused by shRNA3 is specifically due to decreased levels of Disc1 and not to off-target effects of the Disc1 shRNA.
Figure 5.
Granule cell migration defects are rescued with DISC1 overexpression. In utero electroporation following co-injection of human DISC1 and Disc1 shRNA3 (5:1 ratio) into the mouse hippocampus at E15–E19 rescues the deficits in granule cell migration observed with shRNA3 injection alone. (A) Representative image showing that cells receiving hDISC1 + shRNA3 are able to migrate to the dentate gyrus. (B) Quantification of cells reaching each migration zone shows that a significantly greater percentage of cells reaches zone 3 following the injection of hDISC1/shRNA3 (27.6%) than shRNA3 alone (8.1%). Similarly, a significantly lower percentage of cells remains in zone 1 following hDISC1/shRNA3 injection (45.1%) than with shRNA3 alone (70.4%). Interestingly, a significantly greater percentage of cells migrates to zone 3 after hDISC1/shRNA3 injection (27.6%) compared with mshRNA3 (18.8%). (C) When the ratio of hDISC1:shRNA3 injected was lowered from 5:1 to 2:1, the percentage of cells migrating to zone 3 was no longer significantly different from mshRNA3 injection (20.3% versus 18.8%). Values are presented as mean ± SEM. n = 4 (hDISC1 + shRNA3 5:1), n = 5 (hDISC1 + shRNA3 2:1), n = 9 (mshRNA3), n = 13 (shRNA3). *P < 0.05; ***P < 0.0001.
Inhibition of granule cell migration does not affect cell fate
Because Disc1 loss of function hinders the migration of granule cell precursors toward the dentate gyrus, we examined whether it also compromises their ability to properly differentiate into granule cells. To investigate whether Disc1 knockdown alters fate determination in granule cell precursors, we performed immunohistochemistry on hippocampal slices from shRNA3-electroporated brains to detect the granule cell marker Prox1. In utero electroporation was performed at E15 and the animals were sacrificed at post-natal day 2 (P2), to ensure that cells migrating to the dentate gyrus would have sufficient time to properly differentiate. When we compared Prox1 expression on the injected side of the brain with the non-injected side, we observed no general difference in Prox1 expression (Fig. 6A). Using high magnification confocal microscopy, we then determined whether shRNA3-containing cells in the dentate gyrus expressed Prox1 (Fig. 6B). We observed no significant difference in the percentage of GFP-positive cells that were Prox1-positive between shRNA3- and mshRNA3-electroporated brains (83.9% versus 81.3%, respectively; Fig. 6C), indicating that Disc1 knockdown does not influence granule cell fate. Thus, although Disc1 is necessary for the proper migration of granule cell precursors, it does not play a role in granule cell differentiation.
Figure 6.
Disc1 knockdown does not affect granule cell fate. Immunohistochemistry to detect the granule cell marker Prox1 was performed following in utero electroporation of Disc1 shRNA3 at E15–P2. (A) Comparison of the uninjected and injected sides of the same brain reveals no gross abnormalities in Prox1 expression (red) following Disc1 knockdown (green) (top panel: ×5 magnification; bottom panel: ×10 magnification). In the bottom panel, several GFP/shRNA3-containing cells that also express Prox1 can be visualized (arrows). (B) A representative high magnification confocal image shows a cell in the dentate gyrus receiving GFP/shRNA3 (green) which also expresses Prox1 (red). (C) Quantification of Prox1-positive cells in the dentate gyrus following in utero electroporation of shRNA3 (n = 4) or mshRNA3 (n = 6) shows no significant difference in the ability of cells to properly differentiate (shRNA3: 83.9%, mshRNA3: 81.3%; P = 0.6762). Values are presented as mean ± SEM.
Disc1 knockdown does not influence pyramidal cell migration
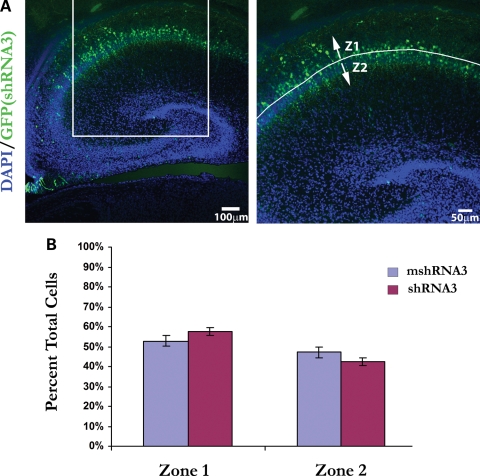
Some studies have reported findings of pyramidal cell disarray in the hippocampus of schizophrenics (22–25), and it has been suggested that such deficits are due to abnormal migration during hippocampal development (26). Because Disc1 is expressed in pyramidal neurons of the hippocampus (10,11), we decided to investigate whether Disc1 knockdown affects their migration as well. To accomplish this, we targeted pyramidal cells in the CA1 region with in utero electroporation of Disc1 shRNA3 or mshRNA3. In utero electroporation was performed at E15 and animals were sacrificed at post-natal day 2 (P2), as this timeframe overlaps the period during which CA1 pyramidal cells are born (27) and when they have migrated to their destination in the ammonic plate (28). During hippocampal development, pyramidal cells migrate in an inside-out fashion, with early-born cells forming the deep layers of stratum pyramidale (closer to the alveus) and subsequent cells migrating past them to populate the more superficial layers (closer to the hippocampal fissure) (29). To analyze the effects of Disc1 loss of function on migration in this area, we quantified the number of cells in each half of the pyramidal cell layer (Fig. 7A; zone 1, deeper half; zone 2, more superficial half). Intriguingly, we found no significant difference in the percentage of cells migrating to either zone in shRNA3- versus mshRNA3-electroporated brains [zone 1: 57.6% (shRNA3), 52.9% (mshRNA3); zone 2: 42.4% (shRNA3), 47.1% (mshRNA3)] (Fig. 7B). Thus, although Disc1 knockdown hinders the migration of dentate gyrus granule cells, it fails to influence the migration of pyramidal neurons within CA1 stratum pyramidale.
Figure 7.
Disc1 knockdown does not influence pyramidal cell migration. In utero electroporation of shRNA3 (n = 4) or mshRNA3 (n = 5) at E15–P2 was followed by analysis of pyramidal cell migration in CA1 stratum pyramidale. (A) Image of a brain section following electroporation of shRNA3 (green); the higher magnification image (right) with labeled migration zones (Z1 = zone 1; Z2 = zone 2) indicates the boxed area in the image on the left. (B) There was no significant difference in the number of cells migrating to either half of stratum pyramidale when shRNA3 or mshRNA3 was injected (zone 1: 57.6% (shRNA3) versus 52.9% (mshRNA3), P = 0.1751; zone 2: 42.4% (shRNA3) versus 47.1% (mshRNA3), P = 0.1751). Values are presented as mean ± SEM.
DISCUSSION
We present here data which uncover a spatially restricted role for Disc1 in neuronal migration within the developing mouse brain, demonstrating that the in utero electroporation technique can be successfully used to ascertain the function of a disease risk gene in the developing hippocampus. Using this technique, we have determined that Disc1 positively regulates the migration of early-born granule cells as they make their way toward the future dentate gyrus. This finding is particularly intriguing given reports that Disc1 knockdown (6) or mutant Disc1 expression (15) in adult-born granule cells leads to an enhancement of migration in the dentate gyrus. These differences might represent two distinct roles of Disc1 in mediating the migration of early- and adult-born granule cells, or they may be indicative of differing extracellular cues in the developing and adult hippocampus which differentially affect how Disc1 mediates granule cell migration. Adding further complexity to the picture is our finding that Disc1 knockdown does not affect the migration of pyramidal cells in developing CA1. Thus, not only does Disc1 loss of function have opposing influences on the migration of early- and adult-born granule cells, but it differentially affects migration within distinct principal cell populations in the developing hippocampus as well.
One possible explanation for the differential effects of Disc1 knockdown on neuronal migration is that spatially and temporally regulated external cues differentially influence the outcome of Disc1 function. For instance, it may be that certain extracellular signals which work to either inhibit or promote neuronal migration do so by triggering intracellular signaling cascades that involve Disc1. The presence of such cues may also change within different brain areas or at different times during brain development. Such a scenario would explain why Disc1 knockdown inhibits migration in early-born granule cells and enhances it in adult-born granule cells. Similarly, spatial differences in extracellular cues present in the embryonic hippocampus may underlie the finding that Disc1 loss of function in the developing hippocampus hinders granule cell migration while failing to influence neuronal migration in CA1. Further support for this idea comes from the finding that both the Disc1 knockdown-mediated inhibition of embryonic cortical neuron migration and enhancement of adult-born granule cell migration are enhanced by concomitant knockdown of the interacting protein Ndel1 (Nudel) (6,7). Disc1 and Nudel form a complex with Lis1 (30) and are together thought to contribute to migration through their interaction with cytoplasmic dynein and the stabilization of nucleus-centrosome coupling. Thus, perhaps Disc1's role in this complex is governed by the presence of extracellular signals whose influence is spatially and temporally controlled.
However, we still cannot exclude the possibility that Disc1 has different intracellular functions within specific neuronal subtypes. Complicating this scenario is that Disc1 interacts with over 50 proteins (31), many of which are involved in intracellular processes contributing to neuronal migration. Thus, there exists the possibility that an as yet uncharacterized interaction between Disc1 and another protein essential for the process of migration is stronger in one neuronal population over another. Similarly, proteins with which Disc1 interacts may have opposite effects on migration in different neuronal populations. Furthermore, the existence of several Disc1 isoforms (32) whose function and localized expression patterns remain largely unknown are another possible key to understanding the spatial and temporal restrictions on Disc1's regulation of neuronal migration. Certainly, elucidating which Disc1 isoforms are important for migration and understanding their function in different brain regions will be a crucial step in unraveling the complexity of Disc1's role in neuronal migration.
The data presented here contribute to the growing amount of evidence that Disc1 plays a profound role in the development and function of the hippocampus. The discovery of spatial restrictions on how Disc1 influences neuronal migration within the hippocampus is an important step toward understanding the key neurodevelopmental changes which accompany Disc1 disruption. In addition, the finding that Disc1 positively regulates the migration of early-born granule cells, while enhancing it in adult-born granule cells (6), suggests the existence of temporal restrictions on Disc1 function as well.
Although Disc1 knockdown in either the embryonic or adult (6) hippocampus does not compromise granule cell differentiation, the long-term effects of abnormal migration of both early- and adult-born granule cells could have profound consequences for their ability to properly integrate into local hippocampal networks. Indeed, Disc1 knockdown in adult-born granule cells causes abnormalities in both pre- and post-synaptic synapse formation (6,8) as well as neuronal excitability (6). Taken together, these findings have consequences not only for our knowledge of the mechanisms underlying proper hippocampal function, but for psychiatric disease as well. In the context of schizophrenia, a picture is emerging whereby Disc1 disruption inhibits migration during development and leads to misplaced neurons that may disrupt hippocampal connectivity. As the brain matures, this disruption continues as newborn granule cells are abnormally integrated into the adult hippocampus. Eventually, the altered hippocampal function induced by Disc1 disruption, perhaps when combined with Disc1's effects in other brain areas, the effects of other disrupted risk genes, or with the particular experiences to which an individual is exposed, culminates in the onset of schizophrenia. Continuing the investigation into Disc1's function in the developing and adult hippocampus and how this function is spatially and temporally regulated will be an important contribution to our understanding of hippocampal function under both normal and pathological conditions.
MATERIALS AND METHODS
Transfection
HEK293 cells were grown to ∼85% confluency and transfected with 4 µg DNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Cells were incubated 48 h at 37°C, 5% CO2 and lysed by the addition of 200 µl freshly prepared lysis buffer (Laemmli buffer plus 5% 0.5 m NaF, 2% 0.5 m Na3VO4, 2% Protease Inhibitor, 0.2% 1 m DTT) and gently scraping the cells off the wells. Cell lysates were transferred to 1.5 ml eppendorf tubes and incubated on ice for 1 h. Lysates were then spun down for 30 min at 4°C, 16 000 rcf, at which point the supernatants were collected and transferred on ice to clean eppendorf tubes. Protein concentrations were determined using BCA protein assays.
Western blot
Equal protein amounts of HEK293 cell lysates were subjected to SDS–PAGE and transferred to PVDF membranes. Membranes were then blocked for 4–6 h in blocking buffer (PBS with 0.1% Tween 20 and 5% non-fat dry milk). Primary antibodies were added [Rabbit anti-GFP (Molecular Probes) 1:10 000; Mouse anti-α tubilin (Abcam) 1:6000; all diluted in PBS with 0.1% Tween 20] and allowed to incubate overnight at 4°C. Antibodies were then removed and membranes washed five times for 5 min in PBS with 0.1% Tween 20. Secondary antibody [Jackson Laboratories: peroxidase-conjugated donkey anti-rabbit (1:15 000) or goat anti-mouse (1:7500)] was diluted in blocking buffer and incubated over the membranes for 1.5 h at room temperature. After undergoing five 5 min washes in PBS with 0.1% Tween 20, membranes were incubated for 1 min in ECL developing solutions mixed 1:1 (Pierce) and immediately developed.
Animals
All methods were approved by the Children's Memorial Research Center Animal Care and Use Committee. Animals used were timed pregnant (E15) Swiss Webster mice and CXCR4-EGFP BAC transgenic mice (generous gift of Dr Richard Miller). For mice sacrificed on day E17 or E19, the mother was killed by CO2 and cervical dislocation, and embryos were removed by cesarean section. Embryos were placed in ice cold HBSS and decapitated, and their brains were isolated and placed in 4% paraformaldehyde at 4°C overnight. Fixed brains were then embedded in 4% low melt agarose and stored in PBS until sectioning. Post-natal (P1 or P2) mouse pups were sacrificed by decapitation and their brains isolated and stored as described above.
Constructs
Disc1 shRNAs were ordered as single stranded oligonucleotides and annealed according to the Invitrogen protocol. Initially, shRNAs were inserted into the pENTR-U6 vector (Invitrogen) and tested for knockdown ability using western blot. Select shRNAs were then inserted into a modified pLL3.7 vector (Addgene plasmid 11795), subjected to western blot analysis to confirm knockdown ability and used for in utero electroporation experiments. In the case of shRNA2, one brain was injected with shRNA2/pENTR-U6 and EGFP-N3 (5:1); the other four brains were injected with shRNA2/pLL3.7. For rescue experiments, human DISC1 with a C-terminal V5 epitope tag was inserted into the pCAG-DsRed vector (33) (Addgene plasmid 11151). Targeting sequences for shRNAs are as follows: 5′ GCATACATTGCTCAGGGAATG 3′ (shRNA3); 5′ GGCTTCCAAGACACCTTTACT 3′ (shRNA2); 5′ GCATACATTCCTGACGGAATG 3′ (mshRNA3).
In utero electroporation
For in utero electroporation experiments, timed-pregnant (E15) Swiss Webster mice were anesthetized with isofluorane inhalation, and a ventral midline incision was made through the skin and underlying muscle. The uterine horns were exposed and the embryos pulled out, and various DNA constructs (2 µl total volume, ∼2 µg total DNA plus 0.5% fast green to monitor the injection) were injected into the lateral ventricle of selected embryos using a micropump syringe coupled to an electronic controller (UMP2 and Micro4, World Precision Instruments). Electroporation (five pulses, 42 mV, 50 ms duration, 950 ms interval) was then carried out using small electrode tweezertrodes connected to a square-wave generator. The tweezertrodes were positioned around the skull in the lateral to medial direction, which allowed for DNA incorporation into the primary dentate matrix of the developing neuroepithelium. Following electroporation, the embryos were carefully placed back into the mother, whose muscle and skin incisions were closed with sutures. Antibacterial and anesthetic creams were applied to the abdomen and the mouse was given oxygen and placed in a recovery chamber before being returned to her home cage.
Immunohistochemistry
Embedded brains were sectioned into 50 µm slices on a vibrating microtome (Leica VT1000S). For select brains used for Disc1 immunohistochemistry, tissue was fixed in 1% paraformaldehyde, cryoprotected in 30% sucrose overnight and cryosectioned into 12 µm slices. Sections were washed briefly in PBS and blocked in PBS+(PBS, 10% normal donkey serum, 0.1% Triton X-100) for 1 h at room temperature. Sections were then incubated in primary antibodies diluted in PBS+[rabbit anti-Disc1 Mid (Zymed) 3 µg/ml; mouse anti-Prox1 (Chemicon) 1:500; mouse anti-human Ki67 (BD Pharmingen) 1:300; rabbit anti-GFP (Molecular Probes) 1:1000] overnight at 4°C. Following three 5 min washes in PBS, secondary antibody was added for 1 h at room temperature (Jackson Laboratories: FITC- or Texas Red-conjugated goat anti-rabbit 1:200, Texas Red-conjugated donkey anti-mouse 1:200; Invitrogen: Alexa Fluor 680 goat anti-mouse 1:200). Sections were then washed 2 × 5 min in PBS and incubated for 5 min in 4′,6-diamidino-2-phenylindole dihydrochloride [DAPI (Sigma); diluted 1:1000 in PBS]. After another 5 min wash in PBS, sections were mounted onto glass slides, coverslipped and imaged.
Brain imaging and analysis
Mouse brains were sectioned into 50 µm slices on a vibrating microtome (Leica VT1000S). Sections were collected and then processed for immunohistochemistry using a rabbit anti-GFP antibody to strengthen the GFP signal and DAPI to mark cell bodies. Select brain sections were then mounted onto glass slides and coverslipped following application of Gel Mount (Sigma). For the detection of Disc1 immunoreactivity, brain sections were imaged using a Leica DM-IRB inverted microscope. For all other brain sections, imaging was carried out by using a confocal laser scanning microscope (Zeiss 510 META) to generate Z stack images of each individual brain section. Z stacks were then analyzed in the Zeiss AIM LSM software. Specifically, three zones of migration were determined by first measuring the distance from the area around the dentate notch (where the precursor cells are born) to the tip of the two blades of the dentate gyrus (where the cells are migrating to). This distance was then divided into thirds to determine where the boundaries for each of the three zones of migration would be made. Finally, in order to determine how far above and below the dentate notch the cell counting should extend to, a distance was measured that was ×0.22 the distance from the dentate notch to the tip of the dentate gyrus (the value of 0.22 was determined by making measurements of multiple tissue sections in which the dentate migratory stream could be well visualized and then averaging the results). On the basis of these measurements, three zones of migration were created, with zone 1 being closest to the dentate neuroepithelium and zone 3 being the furthest and overlaying the dentate gyrus. For area CA1, migration zones were determined by bisecting the width of the pyramidal layer in CA1. Measurements were performed and migration zones created for individual slices of each Z stack or for projection images of combined Z slices. To ensure consistency in the location within the hippocampus at which cell migration was analyzed, only the sections from the same general hippocampal region (the more dorsal region, in which the dentate migratory stream could be easily visualized and measured) were included in the analysis.
Cell counts
The number of cells in each migration zone was determined in a blinded manner by manually marking cells in each zone for a minimum of 2–3 brain slices per animal. Cell markings were generated as individual layers in Adobe Photoshop and counted manually or exported to ImageJ (http://rsbweb.nih.gov/ij/) for counting. The mean percentages of GFP+ cells in each migration zone were compared for the various electroporation conditions using one-way analysis of variance (ANOVA) and Student's t-test.
TUNEL assays
Analysis of cell death was done using the In Situ Cell Death Detection Kit, TMR Red (Roche) according to the manufacturer's instructions. Individual sections were then mounted onto glass slides, coverslipped and imaged. The number of TUNEL+ and GFP+ cells in the three zones of migration was manually counted, and the percentage of GFP+ cells that were TUNEL+ was calculated. Analysis of cell proliferation and cell fate was performed in a similar manner following immunohistochemistry to detect Ki67 or Prox1, respectively (above). The mean percentages for each analysis (TUNEL, Ki67 or Prox1) were compared between shRNA3 and mshRNA3 conditions using the Student's t-test.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
FUNDING
This work was supported by the McKnight Brain Disorders Award, National Alliance for Research on Schizophrenia and Depression and National Institute of Mental Health [5T32 MH067564-04].
ACKNOWLEDGEMENTS
We would like to thank Dr Anjen Chenn for instruction on the in utero electroporation technique as well as helpful commentary on the manuscript. In addition, we are grateful to Dr Richard Miller for the use of CXCR4-EGFP mice. Finally, we are thankful to James Rebesco for guidance on statistical analyses.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Häfner H. Prodrome, onset and early course of schizophrenia. In: Murray R., Jones P.B., Susser E., can Os J., Cannon M., editors. The Epidemiology of Schizophrenia. Cambridge: Cambridge University Press; 2003. pp. 124–147. [Google Scholar]
- 2.Sullivan P.F., Kendler K.S., Neale M.C. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch. Gen. Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 3.Millar J.K., Wilson-Annan J.C., Anderson S., Christie S., Taylor M.S., Semple C.A., Devon R.S., Clair D.M., Muir W.J., Blackwood D.H., et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum. Mol. Genet. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- 4.Chubb J.E., Bradshaw N.J., Soares D.C., Porteous D.J., Millar J.K. The DISC locus in psychiatric illness. Mol. Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- 5.Miyoshi K., Honda A., Baba K., Taniguchi M., Oono K., Fujita T., Kuroda S., Katayama T., Tohyama M. Disrupted-In-Schizophrenia 1, a candidate gene for schizophrenia, participates in neurite outgrowth. Mol. Psychiatry. 2003;8:685–694. doi: 10.1038/sj.mp.4001352. [DOI] [PubMed] [Google Scholar]
- 6.Duan X., Chang J.H., Ge S., Faulkner R.L., Kim J.Y., Kitabatake Y., Liu X.B., Yang C.H., Jordan J.D., Ma D.K., et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamiya A., Kubo K., Tomoda T., Takaki M., Youn R., Ozeki Y., Sawamura N., Park U., Kudo C., Okawa M., et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat. Cell Biol. 2005;7:1167–1178. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- 8.Faulkner R.L., Jang M.H., Liu X.B., Duan X., Sailor K.A., Kim J.Y., Ge S., Jones E.G., Ming G.L., Song H., et al. Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proc. Natl Acad. Sci. USA. 2008;105:14157–14162. doi: 10.1073/pnas.0806658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipska B.K., Peters T., Hyde T.M., Halim N., Horowitz C., Mitkus S., Weickert C.S., Matsumoto M., Sawa A., Straub R.E., et al. Expression of DISC1 binding partners is reduced in schizophrenia and associated with DISC1 SNPs. Hum. Mol. Genet. 2006;15:1245–1258. doi: 10.1093/hmg/ddl040. [DOI] [PubMed] [Google Scholar]
- 10.Meyer K.D., Morris J.A. Immunohistochemical analysis of Disc1 expression in the developing and adult hippocampus. Gene Expr. Patterns. 2008;8:494–501. doi: 10.1016/j.gep.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Austin C.P., Ky B., Ma L., Morris J.A., Shughrue P.J. Expression of Disrupted-In-Schizophrenia-1, a schizophrenia-associated gene, is prominent in the mouse hippocampus throughout brain development. Neuroscience. 2004;124:3–10. doi: 10.1016/j.neuroscience.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Austin C.P., Ma L., Ky B., Morris J.A., Shughrue P.J. DISC1 (Disrupted in Schizophrenia-1) is expressed in limbic regions of the primate brain. Neuroreport. 2003;14:951–954. doi: 10.1097/01.wnr.0000074342.81633.63. [DOI] [PubMed] [Google Scholar]
- 13.Callicott J.H., Straub R.E., Pezawas L., Egan M.F., Mattay V.S., Hariri A.R., Verchinski B.A., Meyer-Lindenberg A., Balkissoon R., Kolachana B., et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc. Natl Acad. Sci. USA. 2005;102:8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hikida T., Jaaro-Peled H., Seshadri S., Oishi K., Hookway C., Kong S., Wu D., Xue R., Andrade M., Tankou S., et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc. Natl Acad. Sci. USA. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kvajo M., McKellar H., Arguello P.A., Drew L.J., Moore H., MacDermott A.B., Karayiorgou M., Gogos J.A. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc. Natl Acad. Sci. USA. 2008;105:7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichenberg A., Harvey P.D. Neuropsychological impairments in schizophrenia: integration of performance-based and brain imaging findings. Psychol. Bull. 2007;133:833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- 17.Boyer P., Phillips J.L., Rousseau F.L., Ilivitsky S. Hippocampal abnormalities and memory deficits: new evidence of a strong pathophysiological link in schizophrenia. Brain Res. Rev. 2007;54:92–112. doi: 10.1016/j.brainresrev.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Altman J., Bayer S.A. Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. J. Comp. Neurol. 1990;301:325–342. doi: 10.1002/cne.903010302. [DOI] [PubMed] [Google Scholar]
- 19.Bagri A., Gurney T., He X., Zou Y.R., Littman D.R., Tessier-Lavigne M., Pleasure S.J. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- 20.Lu M., Grove E.A., Miller R.J. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc. Natl Acad. Sci. USA. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubinson D.A., Dillon C.P., Kwiatkowski A.V., Sievers C., Yang L., Kopinja J., Rooney D.L., Zhang M., Ihrig M.M., McManus M.T., et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat. Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 22.Casanova M.F., Rothberg B. Shape distortion of the hippocampus: a possible explanation of the pyramidal cell disarray reported in schizophrenia. Schizophr. Res. 2002;55:19–24. doi: 10.1016/s0920-9964(01)00201-8. [DOI] [PubMed] [Google Scholar]
- 23.Kovelman J.A., Scheibel A.B. A neurohistological correlate of schizophrenia. Biol. Psychiatry. 1984;19:1601–1621. [PubMed] [Google Scholar]
- 24.Conrad A.J., Abebe T., Austin R., Forsythe S., Scheibel A.B. Hippocampal pyramidal cell disarray in schizophrenia as a bilateral phenomenon. Arch. Gen. Psychiatry. 1991;48:413–417. doi: 10.1001/archpsyc.1991.01810290025003. [DOI] [PubMed] [Google Scholar]
- 25.Jonsson S.A., Luts A., Guldberg-Kjaer N., Brun A. Hippocampal pyramidal cell disarray correlates negatively to cell number: implications for the pathogenesis of schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 1997;247:120–127. doi: 10.1007/BF03033065. [DOI] [PubMed] [Google Scholar]
- 26.Conrad A.J., Scheibel A.B. Schizophrenia and the hippocampus: the embryological hypothesis extended. Schizophr. Bull. 1987;13:577–587. doi: 10.1093/schbul/13.4.577. [DOI] [PubMed] [Google Scholar]
- 27.Angevine J.B., Jr Time of neuron origin in the hippocampal region. An autoradiographic study in the mouse. Exp. Neurol. Suppl. 1965;(Suppl 2):1–70. [PubMed] [Google Scholar]
- 28.Nakahira E., Yuasa S. Neuronal generation, migration, and differentiation in the mouse hippocampal primoridium as revealed by enhanced green fluorescent protein gene transfer by means of in utero electroporation. J. Comp. Neurol. 2005;483:329–340. doi: 10.1002/cne.20441. [DOI] [PubMed] [Google Scholar]
- 29.Altman J., Bayer S.A. Prolonged sojourn of developing pyramidal cells in the intermediate zone of the hippocampus and their settling in the stratum pyramidale. J. Comp. Neurol. 1990;301:343–364. doi: 10.1002/cne.903010303. [DOI] [PubMed] [Google Scholar]
- 30.Brandon N.J., Handford E.J., Schurov I., Rain J.C., Pelling M., Duran-Jimeniz B., Camargo L.M., Oliver K.R., Beher D., Shearman M.S., et al. Disrupted in Schizophrenia 1 and Nudel form a neurodevelopmentally regulated protein complex: implications for schizophrenia and other major neurological disorders. Mol. Cell. Neurosci. 2004;25:42–55. doi: 10.1016/j.mcn.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Camargo L.M., Collura V., Rain J.C., Mizuguchi K., Hermjakob H., Kerrien S., Bonnert T.P., Whiting P.J., Brandon N.J. Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol. Psychiatry. 2007;12:74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- 32.Ishizuka K., Paek M., Kamiya A., Sawa A. A review of Disrupted-In-Schizophrenia-1 (DISC1): neurodevelopment, cognition, and mental conditions. Biol. Psychiatry. 2006;59:1189–1197. doi: 10.1016/j.biopsych.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda T., Cepko C.L. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl Acad. Sci. USA. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.