Abstract
Spinal cord neuronal restricted progenitor (NRP) cells, when transplanted into the neonatal anterior forebrain subventricular zone, migrate to distinct regions throughout the forebrain including the olfactory bulb, frontal cortex, and occipital cortex but not to the hippocampus. Their migration pattern and differentiation potential is distinct from anterior forebrain subventricular zone NRPs. Irrespective of their final destination, NRP cells do not differentiate into glia. Rather they synthesize neurotransmitters, acquire region-specific phenotypes, and receive synapses from host neurons after transplantation. Spinal cord NRPs express choline acetyl transferase even in regions where host neurons do not express this marker. The restricted distribution of transplanted spinal cord NRP cells and their acquisition of varied region-specific phenotypes suggest that their ultimate fate and phenotype is dictated by a combination of intrinsic properties and extrinsic cues from the host.
Multipotent neural stem cells within the developing mammalian central nervous system develop into neurons, astroglia, and oligodendrocytes (1–8). The transition from neural stem cells to differentiated neurons or glial cells likely requires the generation of more restricted precursors (reviewed in ref. 9). Such lineage-restricted precursors (glial restricted and neuronal restricted progenitors, GRPs and NRPs, respectively) have been identified (9, 10). Progenitor cells have been isolated and characterized from multiple brain regions (2–4, 11–15) whereas NRP cells have so far been identified in only a few locations (2, 16–23).
Irrespective of the region of isolation NRP cells share several properties: an ability to divide, the expression of polysialated neural cell adhesion molecule, the expression of neuronal markers such as type III β-tubulin and microtubule-associated protein 2 (MAP-2), and an inability to generate glial derivatives in conditions in which other precursors readily generate astrocytes and oligodendrocytes. The neuronal lineage commitment of the NRPs seems immutable and is in contrast to progenitor populations described by Roy et al. (24), where oligodendrocyte precursors in vitro generated a small number of type III β-tubulin-positive cells.
Despite their overall similarities, differences between neural progenitor cells isolated from different brain regions exist (reviewed in ref. 9). For example, progenitors from the hippocampus, but not from the cerebellum or midbrain, produce hippocampal pyramidal neurons. Likewise, Luskin and colleagues (25) have noted that neurons derived from the anterior forebrain subventricular zone (SVZa) undergo GABAergic differentiation when transplanted into the striatum. These and other results raise the possibility that the restriction in developmental potential arises early and cannot be reversed. Multiple classes of NRPs distinguished on the basis of their ability to generate specific subclasses of neurons may exist.
In this study, the ability of spinal cord NRP cells to migrate and differentiate after their transplantation into the neonatal SVZa was examined and compared with endogenous and homotypically transplanted SVZa NRP cells. Our results show that spinal cord NRP cells are restricted to generating neurons in vivo. NRPs, however, migrate extensively and incorporate into different brain regions, and subsets of cells synthesize cholinergic, glutaminergic, and GABAergic neurotransmitters. Spinal cord NRPs differ from SVZa-derived NRPs in their migration and differentiation, indicating that cell intrinsic mechanisms play an important role in regulating differentiation.
Materials and Methods
Isolation and Labeling of NRP and GRP Cells.
Cells were isolated as described (12). Immunoselected cells were labeled by using an enhanced green fluorescent protein (GFP) retroviral construct (gift from Ray White, University of Utah). The construct was packaged by using the Phoenix cell line (gift from Gary Nolan, Stanford University, Stanford, CA). Viral supernatant was collected from infected cells grown in neuroepithelial basal medium. GFP expression in infected cells (10%) was evident within 48 h in vitro, and its expression persisted for at least 10 days.
Transplantation of GFP-Labeled NRP and GRP Cells.
The procedure described by Luskin and coworkers (16, 25) was used with slight modifications to transplant cells into the right SVZa of postnatal day 1 rat pups. About 3 μl of the GFP-labeled NRP or GRP cell suspension (approximately 3 × 104 cells) was injected into the SVZa. The needle was left in position for approximately 3 min and gradually withdrawn, the skull flap was repositioned, and the skin overlying the incision site was sealed with surgical glue. The animals were revived under a heat lamp and returned to their mothers.
Tissue Processing and Immunocytochemistry.
Animals were allowed to survive for either 3, 7, or 14 days after surgery and then were perfused with paraformaldehyde as described (25). At each time point single-label immunocytochemistry was done with anti-GFP to determine the location of transplanted cells. Double-label immunohistochemistry was performed to evaluate the phenotype of the transplanted cells. The following primary antibodies were used: anti-choline acetyl transferase (ChAT) (Chemicon, 1:2,000), anti-γ-aminobutyric acid (GABA) (Sigma, 1:5,000), anti-glial fibrillary acidic protein (GFAP) (Dako, 1:500), anti-glutamate (Signature, 1:100), anti-neurofilament (NF)-160 (Sigma, 1:50) and anti-NF-200 (Sigma, 1:500), anti-MAP-2 (Sigma, 1:500), anti-proteolipid protein-DM20 (Chemicon, 1:200), anti-synaptophysin (Sigma, 1:200), and TuJ1 (Babco, Richmond, CA, 1:2,000). Fluorescence (Zeiss Axiophot) and confocal microscopy (Zeiss Axiophot equipped with LSM 510) were used to capture representative images. A region in the host brain where large groups of GFP-NRP cells were unambiguously visualized was considered a destination for the NRP cells if similar groups were seen in 10 or more animals. A minimum of 100 GFP-NRP cells were counted at each site by using a 0.5-mm × 0.5-mm grid at ×200 magnification with the fluorescein filter. The percentage of cells expressing a particular phenotype within these regions was expressed as # double-labeled cells/# GFP-NRP cells.
Results
Before transplantation aliquots of immunopanned cells were cultured, retrovirally labeled, and tested for purity and their ability to differentiate into neurons. Only isolations that yielded 95% or more of NRP or GRP cells were used for transplantation. Fifteen independent isolations were performed, and on average, 10% of the isolated cells were infected by the retrovirus. Viability, determined by the trypan blue exclusion test before injection, was greater than 95%. Cells were injected into the SVZa of newborn Sprague–Dawley rat pups, by using the coordinates established by Luskin and her associates (25).
Transplanted Spinal Cord NRP Cells Migrate to the Olfactory Bulb (OB) and Anterior Olfactory Nucleus (AON).
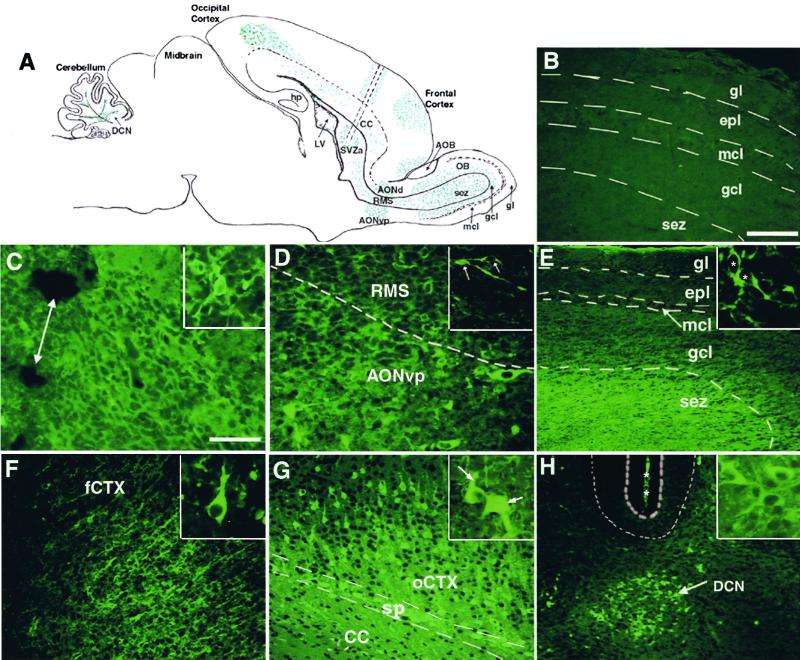
The distribution of the GFP-NRP cells was analyzed in 30 animals to determine whether the GFP-NRP cells migrated or remained at the implantation site. Similar to the SVZa progenitors (20, 25), transplanted GFP-NRP cells migrated along the rostral migratory stream (RMS)—the pathway traversed by the SVZa-derived cells —from the implantation site to the OB (Fig. 1 C–E). GFP-positive cells were present at the SVZa transplant site (Fig. 1C), along the RMS (Fig. 1D), and in the bulb (Fig. 1E) 3 days posttransplantation.
Figure 1.
Transplanted embryonic spinal cord NRP cells migrate to discrete regions spread throughout the brain. GFP-labeled NRP cells were transplanted into the right SVZa, and GFP-NRP cells were visualized by using an antibody to GFP with a FITC-conjugated secondary. (A) A line drawing of a parasagittal view of the neonatal rat brain showing the injection tract (parallel dotted lines) and the pattern of distribution of the GFP-NRP cells, which was similar 3, 7, and 14 days posttransplantation. hp, hippocampus; LV, lateral ventricle; CC, corpus callosum; sez, subependymal zone; mcl, mitral cell layer; gcl, granule cell layer; gl, granule layer. (B) A representative photomicrograph of the left OB showing no GFP-NRP cells, proving that the cells did not cross the midline. gl, granule layer; epl, external plexiform; mcl, mitral cell layer; gcl, granule cell layer; sez, subependymal zone. (Bar = 100 μm.) (C–H) Representative photomicrographs of GFP-NRP cells at various locations throughout the host brain, 3 days after transplantation. The OB is to the right in each panel. (C) The site of implantation in the SVZa still containing numerous GFP-NRP cells and sites of tissue damage caused by the injection (double arrow). (Bar = 50 μm, also applies to D–H.) (Inset) Transplanted GFP-NRP cells with a round soma and short processes. (D) GFP-NRP cells in the mid-RMS and in the surrounding AONvp, demonstrating that some GFP-NRP cells leave the RMS (dotted line) and enter the AONvp and AONd. (Inset) Two GFP-NRP cells in the RMS with an elongated soma and leading processes. (E) Numerous GFP-NRP cells in the sez in the middle of the OB with the Inset showing bipolar GFP-NRP neurons in the sez. * indicates the cell bodies. (F) GFP-NRP cells in the frontal cortex (fCTX) of the brain with apical processes extending toward the pial surface. (Inset) A representative GFP-NRP cell resembling a differentiating pyramidal neuron. (G) GFP-NRP cells occipital cortex (oCTX), concentrated in the lower layers (IV-VI) of the brain and the subplate. CC, corpus callosum; sp, subplate. (Inset) Two GFP-NRP cells show a multipolar morphology (short arrow) and a pyramidal morphology (long arrow). (H) A photomicrograph showing the DCN where the GFP-NRP cells migrate, with the Inset showing multipolar GFP-NRP cells with short processes. The thick dotted lines overly the Purkinje cell layer, and the * marks the pial surface between adjacent folia in the cerebellum. The morphology of the transplanted GFP-NRP cells after migration resembles the neurons of the host brain present at each particular site.
Despite overt similarities between the spinal cord GFP-NRP cells and SVZa progenitors, major differences also were observed. The GFP-NRP cells exited the RMS, which the endogenous or homotypically transplanted SVZa progenitors never do (21) and were found in the adjacent ventroposterior AON (AONvp) and dorsal AON (AONd) (Fig. 2D). Moreover, unlike SVZa cells, once the NRP cells entered the subependymal zone of the OB, they never migrated past the granule cell-mitral cell border even at 15 days posttransplantation (the latest time point studied). Thus, spinal cord NRP cells do not obey the same cues as SVZa cells en route to and within the OB.
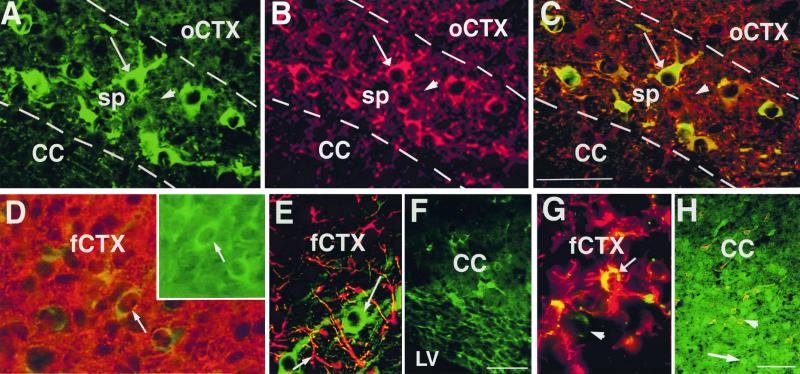
Figure 2.
The phenotype of transplanted GFP-NRP cells and GFP-GRP cells. Sections were double-labeled with antibodies against GFP to identify NRP (A–F) and GRP cells (G and H) and with neuronal markers MAP-2 (A–C), neuron-specific type III β-tubulin (D), the astrocyte marker GFAP (E and G), and the oligodendrocyte marker PLP-DM20 (F and H) are shown. The GFP is identified with an FITC-conjugated secondary antibody in all sections. (A–C) Representative section from the subplate showing NRP cells (A) and MAP-2 (+) cells (B). MAP-2 (+) NRP cells appear yellow (long arrows A–C). The arrowhead points to a MAP-2 (+)/GFP (−) host cell. (D) A representative confocal section from the frontal cortex shows some of the β-tubulin (+) cells in the cortex are GFP-NRP cells (yellow, arrow). (Inset) The same cell visualized with an FITC-filter (arrow). (E and F) Representative sections from either the frontal cortex (E) or corpus callosum (F) demonstrate that the NRP cells do not express either the astrocyte marker GFAP (E) or the oligodendrocyte marker PLP-DM 20 (F). (G) A representative field from the frontal cortex showing both GFP (+)/GFAP (+) (yellow) and GFP (+)/GFAP (−) (green, arrowhead) GRP cells. (H) A representative photomicrograph from the corpus callosum demonstrating some GFP-GRP cells expressing PLP-DM20 (yellow, short arrow) interspersed with GFP-GRP cells that do not (green, long arrow). These experiments therefore show that the NRP cells are committed to a neuronal lineage, whereas the GRP cells differentiate into glia only. CC, corpus callosum; sp, subplate; oCTX, occipital cortex; LV, lateral ventricle; fCTX, frontal cortex. (Bars = 100 μm.)
Transplanted NRP Cells Migrate to Discrete Regions Throughout the Brain.
In addition to the RMS and AON (see above), and in contrast to the behavior of SVZa-derived NRP cells, labeled spinal cord NRP cells were found in multiple sites (summarized in Table 1). Spinal cord NRP cells migrated both anteriorly and posteriorly through the overlying corpus callosum to the frontal and occipital cortices as well as to the cerebellum. In the cortex, cells were found in layers II, III, and IV in the frontal cortex and the subplate (Fig. 1F) and layers IV-VI in the occipital cortex (Fig. 1G). Large numbers of spinal cord NRP cells also were found in the cerebellum, and they were restricted to the deep cerebellar nuclei (DCN) (Fig. 1H). The GFP-NRP cells, therefore, were less restricted in their migratory behaviors when compared with SVZa cells.
Table 1.
Distribution of transplanted GFP-labeled spinal cord NRP cells
| Animal no. | SVZa | RMS | OB | AONvp | AONd | Frontal cortex | Occipital cortex | Hippocampus | Midbrain | Cerebellum |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | + | + | + | + | + | − | − | + |
| 2* | + | + | + | + | + | + | + | + | − | + |
| 3 | + | + | + | + | + | + | + | − | − | + |
| 4 | + | + | + | + | + | + | + | − | − | + |
| 5 | + | + | + | + | + | + | + | − | − | + |
| 6 | + | + | + | + | + | + | + | − | − | + |
| 7 | + | + | + | + | + | + | + | − | − | + |
| 8 | + | + | + | + | + | + | + | − | − | + |
| 9 | + | + | + | + | + | + | + | − | − | + |
Distribution of transplanted GFP-NRP cells in various brain regions. Table summarizes the regions in the brain where GFP-NRP cells were identified 3, 7, and 14 days after transplantation into the SVZa of postnatal day 1 Sprague–Dawley rats. The transplanted cells were identified immunohistochemically by using an antibody against GFP and a fluorescein-conjugated secondary antibody. NRP cells were identified in the hippocampus only in animal 2 (*) presumably caused by a leakage of some transplanted GFP-NRP cells into the lateral ventricle.
GFP-NRP cells were not detected in the hippocampus unless transplanted cells leaked into the lateral ventricle. NRP cells were never detected in the midbrain or in the striatum. The absence of spinal cord NRP cells in the contralateral OB (Fig. 1B) indicated that spinal cord NRP cell migration was restricted to the ipsilateral hemisphere. Thus, NRPs can migrate in multiple directions, although the pathway(s) selected by the GFP-NRP cells are not random.
Transplanted NRP Cells at Their Final Destinations Are Morphologically Similar to Host Neurons.
The morphology of the transplanted cells varied in different brain regions. In the SVZa the GFP-NRP cells were primarily round with short processes (Fig. 1C Inset), but in the RMS, many NRP cells had elongated cell bodies with leading processes, consistent with the morphology of migrating neurons (Fig. 1D Inset). Once in the OB, many cells acquired a bipolar morphology (Fig. 1E Inset). In contrast, NRP cells in the frontal and occipital cortices had a distinct pyramidal appearance (Fig. 1 F and G Insets) with dendritic processes; however, the cells within the subplate were multipolar (Fig. 1 G Inset, short arrow). The NRP cells in the DCN were also multipolar with short processes (Fig. 1H Inset). Taken together, these results show that GFP-NRP cells assumed a range of morphologies that resemble the morphologies found in the local regions within the host brain.
Transplanted NRP Cells Express Neuronal Cell-Type Specific Markers Exclusively.
The differentiation ability of NRP cells was assessed by using a panel of cell-type specific markers. Table 2 summarizes the pattern of expression of all markers tested. The expression of MAP-2 (Fig. 2 A–C) and β-tubulin III (Fig. 2D) was evident in all of the migrated NRP cells at all of the locations at all ages and time points studied.
Table 2.
Marker expression profile of transplanted GFP-NRP cells
| Marker | SVZa | RMS | OB | AONvp | AONd | Frontal cortex | Occipital cortex | Cerebellum |
|---|---|---|---|---|---|---|---|---|
| β-tubulin III | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| MAP-2 | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| NF-160 (3 DPT only) | − | − | + | + | + | − | − | − |
| NF-200 (7 DPT only) | − | − | ++ | ++ | ++ | ++ | ++ | ++ |
| GFAP | − | − | − | − | − | − | − | − |
| ChAT | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| Glutamate | − | − | ++ | ++ | ++ | − | − | − |
| GABA | − | − | ++ | + | + | − | − | − |
| Synaptophysin | + | ++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
In all the areas examined, the average percentage of double-labeled cells is represented by using the following symbols: −, no cells expressing the phenotype; +, up to 25% of cells expressing the phenotype; ++, 25–50% of cells expressing the phenotype; +++, 50–75% of cells expressing the phenotype; ++++, >75% of cells expressing the phenotype. (DPT, days after transplantation).
At no stage did we detect expression of GFAP or PLP-DM20 by GFP-labeled NRP cells (Fig. 3 E and F), confirming that NRP cells do not adopt astroglial or oligodendrocyte identities. This finding is in distinct contrast to other neural progenitors, which predominantly mature into glia after transplantation (8, 26, 27), but similar to SVZa neuronal progenitors, which retain their neuronal identity following heterotypic transplantation (20, 25).
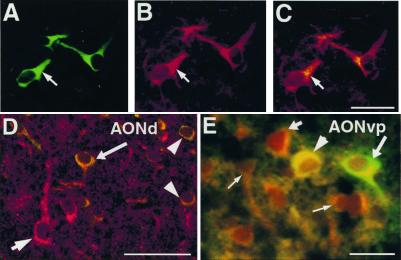
Figure 3.
Neurotransmitter expression by transplanted NRP cells. Sections from brains transplanted with GFP-NRP cells were double-labeled with anti-GFP along with anti-ChAT (A–C), antiglutamate (D), and anti-GABA (E). Anti-GFP is visualized with a FITC-conjugated secondary. (A–C) A representative section from the occipital cortex. Images from A and B are superimposed in C showing NRP cells uniformly expressing ChAT (arrow). (Bar = 100 μm.) (D) A representative confocal image from the AONd shows a host neuron expressing glutamate (short arrow) and transplanted NRP cells expressing GFP and glutamate (long arrow, arrowheads). (Bar = 100 μm.) (E) A representative section from the AONvp showing a GABA (−) NRP cell, (long arrow), GABA (+) host neurons (short arrow, thin arrows), and a GABA (+) GFP-NRP cell (arrowhead). (Bar = 50 μm.)
To rule out the possibility that the host environment failed to initiate oligodendrocyte or astrocyte differentiation or support the survival of newly formed glial cells, we analyzed the differentiation of GRP cells isolated from the embryonic rat spinal cord at the same developmental stage as NRP cells. In contrast to NRP cells, GRP cells readily differentiated into GFAP immunoreactive astrocytes (Fig. 3G) and PLP-DM20-positive oligodendrocytes (Fig. 3H). Moreover, GRP cells did not differentiate into cells that exhibited a neuronal morphology and did not express neuronal markers (data not shown). Thus, NRPs do not respond to the glial differentiation cues that GRP cells obey.
Transplanted NRP Cells Express ChAT, GABA, and Glutamate.
NRP cells mature, synthesize, and respond to neurotransmitters in vitro. We therefore evaluated the ability of transplanted NRP cells to express excitatory or inhibitory neurotransmitters. A substantial number of the transplanted NRP cells throughout the brain expressed ChAT as early as 3 days posttransplantation even in regions like the occipital cortex (Fig. 3 A–C) where endogenous ChAT-positive neurons have not been. GABA and glutamate expression (Fig. 3 D and E), however, was restricted to the NRP cells that migrated to the OB, AONd, and AONvp. Furthermore, fewer than 50% of the GFP-NRP cells in these locations expressed GABA or glutamate. These results, summarized in Table 2, show that the neurotransmitter phenotype expressed by the GFP-NRP cells is a function of both intrinsic and extrinsic factors.
Transplanted NRP Cells Differentiate in the Host Brain and Receive Synapses.
To determine whether the GFP-NRP cells can synaptically integrate and mature within the host environment, we examined synaptophysin expression and the expression of NF (NF-160 and NF-200), markers of neuronal differentiation. Synaptophysin expression on transplanted NRP cells was faint in the SVZa and RMS, suggesting some vesicle formation. However, greater expression of synaptophysin was seen in regions with more mature neurons such as the cerebral cortex (Fig. 4D). This finding suggests that the NRP cells assimilate with mature host neurons.
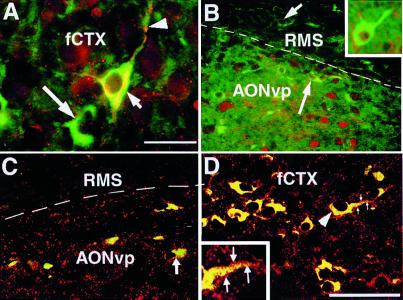
Figure 4.
Transplanted NRP cells mature in the host brain and integrate with mature host neurons. Brains transplanted with GFP-NRP cells were stained with antibodies against NF-160 (A), NF-200 (B), and synaptophysin (C and D) and GFP (visualized with a FITC-conjugated secondary). (A) A representative section from the frontal cortex (fCTX) shows a pyramidal GFP-NRP cell (short arrow) expressing NF-160 with a long process (arrowhead). Not all NRP cells express NF-160 (long arrow). (Bar = 50 μm.) (B) A representative section encompassing the RMS and AONvp. Within the RMS, NRP cells are NF-200 (−) (short arrow), but some NRP cells in the AONvp are NF-200 (+) (long arrow). Host GFP (−), NF (+) neurons also are seen (arrowhead). (Inset) A representative GFP-NRP cell from the frontal cortex, showing perinuclear NF-200 staining is seen. (C) Representative confocal image encompassing the RMS and AONvp showing NRP cells (yellow) surrounded by synaptophysin-positive vesicles (red). (D) A similar confocal image from the frontal cortex shows NRP cells, again surrounded by synaptophysin (+) vesicles. The arrowhead points to a representative GFP-NRP cell; the process emanating from the cell is magnified in the Inset, and the synaptophysin (+) vesicles surrounding the process are clearly seen (arrows). (Bar = 100 μm, also applies to B and C.)
NF isoforms identify maturing neurons that extend axons or processes, with NF-200 expression increasing as development progresses. Staining with anti-NF antibodies showed that NRP cells underwent a process of maturation. Three days after transplantation, NRP cells in the cerebral cortex (Fig. 4A) expressed NF-160. Seven days posttransplantation, however, NF-160 was extinguished and NF-200 was present in the transplanted cells (Fig. 4B). NF-200 expression was evident only in NRP cells present in host areas with mature postmitotic neurons and was not seen in the SVZa or RMS. Thus, NRP cells not only retain their neuronal phenotype, but also continue to mature in vivo by expressing developmentally regulated proteins like NF.
Discussion
Spinal cord NRP cells migrate extensively, integrate into the host brain, and differentiate after transplantation into the host SVZa. Transplanted cells generate extensive processes, make synapses, and acquire region-specific phenotypic characteristics. They generate exclusively into neurons, even in regions such as the corpus callosum, at a time of active gliogenesis. This finding contrasts with the behavior of GRP cells, which readily differentiated into astrocytes and oligodendrocytes (but not neurons) in the same environment. Thus, the lineage restriction in the two populations seen in vitro also is reflected in vivo.
NRP cells migrated extensively, and labeled cells were found in the cerebellum, OB, and the occipital and frontal cortices similar to the behavior of other neural stem cells transplanted into the neonatal brain. In the adult, however, multipotent cells do not appear to recognize normal migratory cues, and large numbers of cells are retained at the injection site (refs. 26–29; reviewed in ref. 9). In our experiments we observed few NRP cells at or near the injection site, and the cells present appeared to be dispersed rather than aggregated (Figs. 1 and 2). These observations are consistent with the normal behavior of stem cells during development. In vivo, multipotent progenitor cells are restricted to proliferating regions (30–32), and only their progeny appear to migrate (32).
Spinal cord NRPs migrated considerably more than SVZa NRPs (present results and ref. 25). Like SVZa progenitors the spinal cord NRP cells migrated independently of radial glia in the RMS. However, unlike SVZa cells, the spinal cord NRP cells also migrated to additional sites including the cerebral cortex. The final destinations of the spinal cord NRP cells were not simply a function of time. The cells could be seen at their targets 3 days after transplantation, and sites in the brain close to the site of implantation like the hippocampus or striatum were preferentially bypassed for cerebral cortical structures and the cerebellum.
In general when multipotent stem cells are transplanted only a fraction of the cells differentiate into neurons (usually 1–5%). Predominantly GABAergic neuronal differentiation has been reported. In contrast, virtually all NRP cells expressed neuronal markers and matured in the host environment to acquire a variety of different morphologies, and neurotransmitter phenotypes (Figs. 1, 3, and 4). An important finding was that a significant number of transplanted NRP cells expressed ChAT even in regions where no endogenous cholinergic cells are present. NRP cells may have differentiated into ChAT immunoreactive cells before transplantation. Alternatively cholinergic differentiation may represent a default pathway and an intrinsic bias in the developmental potential of spinal cord NRP cells, which is adopted when overriding cues are not present. The present results do not allow us to distinguish between these possibilities.
The present results combined with other transplantation experiments suggest that intrinsic and extrinsic signals regulate development (1). The overall evidence suggests that cues that direct migration and differentiation are present in the environment, and some progenitor cells retain the capacity to respond to them. Some progenitors lose or lack the ability to interpret and respond to these cues and thus fail to migrate. Loss of this ability may happen early in development well before target innervation. For example, a study comparing the migration potential of progenitors from the lateral and medial ganglionic eminences showed that the lateral ganglionic eminence cells were more restricted in their migration ability (33) even though the cells were harvested from embryonic animals at a stage when neurogenesis and migration are prevalent. Evidence of such differences at early developmental stages underscore the importance of carefully characterizing each cell type and selecting an appropriate source of cells especially when contemplating therapeutic transplantation.
Neuronal transplantation to correct congenital or acquired disorders using multipotent progenitor cells has two major limitations: migration of the transplanted cells is limited, and the cells seldom develop into neurons (26, 27, 34–36). In transplants using more restricted NRP cells from the spinal cord or the SVZa, the cells migrate more freely, and there is essentially no glial formation (20, 33), suggesting that these cells may be more useful therapeutically. Recently, it has been shown that NRP cells can be isolated from human spinal cords (37) and embryonic stem (ES) cells (38). NRP cells derived from ES cells may be sufficiently undifferentiated to allow the use of a single population of NRPs to correct acquired or congenital neurological disorders.
Abbreviations
- NRP
neuronal restricted progenitor
- SVZa
anterior forebrain subventricular zone
- GRP
glial restricted progenitor
- MAP-2
microtubule-associated protein 2
- GFP
green fluorescent protein
- ChAT
choline acetyl transferase
- GABA
γ-aminobutyric acid
- OB
olfactory bulb
- AON
anterior olfactory nucleus
- AONvp
ventro posterior AON
- AONd
dorsal AON
- RMS
rostal migratory stream
- NF
neurofilament
- DCN
deep cerebellar nuclei
- GFAP
glial fibrillary acidic protein
References
- 1.McKay R. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- 2.Kalyani A, Hobson K, Rao M S. Dev Biol. 1997;186:202–223. doi: 10.1006/dbio.1997.8592. [DOI] [PubMed] [Google Scholar]
- 3.Mayer-Proschel M, Kalyani A J, Mujtaba T, Rao M S. Neuron. 1997;19:773–785. doi: 10.1016/s0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- 4.Johe K K, Hazel T G, Muller T, Dugich-Djordjevic M M, McKay R D. Genes Dev. 1996;10:3129–3140. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds B A, Weiss S. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 6.Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson A C, Reynolds B A. J Neurosci. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shihabuddin L S, Ray J, Gage F H. Exp Neurol. 1997;148:577–586. doi: 10.1006/exnr.1997.6697. [DOI] [PubMed] [Google Scholar]
- 8.Gage F H, Coates P W, Palmer T D, Kuhn H G, Fisher L J, Suhonen J O, Peterson D A, Suhr S T, Ray J. Proc Natl Acad Sci USA. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao M. Anat Rec. 1999;257:137–148. doi: 10.1002/(SICI)1097-0185(19990815)257:4<137::AID-AR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 10.Kalyani A J, Rao M S. Biochem Cell Biol. 1998;76:1051–1068. [PubMed] [Google Scholar]
- 11.Gensburger C, Labourdette G, Sensenbrenner M. FEBS Lett. 1987;217:1–5. doi: 10.1016/0014-5793(87)81230-9. [DOI] [PubMed] [Google Scholar]
- 12.Kalyani A J, Piper D, Mujtaba T, Lucero M T, Rao M S. J Neurosci. 1998;18:7856–7868. doi: 10.1523/JNEUROSCI.18-19-07856.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hulspas R, Tiarks C, Reilly J, Hsieh C C, Recht L, Quesenberry P J. Exp Neurol. 1997;148:147–156. doi: 10.1006/exnr.1997.6672. [DOI] [PubMed] [Google Scholar]
- 14.Ray J, Peterson D A, Schinstine M, Gage F H. Proc Natl Acad Sci USA. 1993;90:3602–3606. doi: 10.1073/pnas.90.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luskin M B, Zigova T, Soteres B J, Stewart R R. Mol Cell Neurosci. 1997;8:351–366. doi: 10.1006/mcne.1996.0592. [DOI] [PubMed] [Google Scholar]
- 16.Luskin M B. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 17.Lois C, Alvarez-Buylla A. Proc Natl Acad Sci USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lois C, Alvarez-Buylla A. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 19.Luskin M B, Boone M S. Chem Senses. 1994;19:695–714. doi: 10.1093/chemse/19.6.695. [DOI] [PubMed] [Google Scholar]
- 20.Zigova T, Betarbet R, Soteres B J, Brock S, Bakay R A, Luskin M B. Dev Biol. 1996;173:459–474. doi: 10.1006/dbio.1996.0040. [DOI] [PubMed] [Google Scholar]
- 21.Menezes J R, Luskin M B. J Neurosci. 1994;14:5399–5416. doi: 10.1523/JNEUROSCI.14-09-05399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menezes J R, Smith C M, Nelson K C, Luskin M B. Mol Cell Neurosci. 1995;6:496–508. doi: 10.1006/mcne.1995.0002. [DOI] [PubMed] [Google Scholar]
- 23.Betarbet R, Zigova T, Bakay R A, Luskin M B. Int J Dev Neurosci. 1996;14:921–930. doi: 10.1016/s0736-5748(96)00066-4. [DOI] [PubMed] [Google Scholar]
- 24.Roy N S, Wang S, Harrison-Restelli C, Benraiss A, Fraser R A R, Gravel M, Braun P E, Goldman S A. J Neurosci. 1999;19:9986–9995. doi: 10.1523/JNEUROSCI.19-22-09986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zigova T, Pencea V, Betarbet R, Wiegand S J, Alexander C, Bakay R A, Luskin M B. Cell Transplant. 1998;7:137–156. doi: 10.1177/096368979800700209. [DOI] [PubMed] [Google Scholar]
- 26.Lundberg C, Martinez-Serrano A, Cattaneo E, McKay R D, Bjorklund A. Exp Neurol. 1997;145:342–360. doi: 10.1006/exnr.1997.6503. [DOI] [PubMed] [Google Scholar]
- 27.Fricker R A, Carpenter M K, Winkler C, Greco C, Gates M A, Bjorklund A. J Neurosci. 1999;19:5990–6005. doi: 10.1523/JNEUROSCI.19-14-05990.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winkler C, Fricker R A, Gates M A, Olsson M, Hammang J P, Carpenter M K, Bjorklund A. Mol Cell Neurosci. 1998;11:99–116. doi: 10.1006/mcne.1998.0674. [DOI] [PubMed] [Google Scholar]
- 29.Svendsen C N, Clarke D J, Rosser A E, Dunnett S B. Exp Neurol. 1996;137:376–388. doi: 10.1006/exnr.1996.0039. [DOI] [PubMed] [Google Scholar]
- 30.Walsh C, Cepko C L. Experientia. 1990;46:940–947. doi: 10.1007/BF01939387. [DOI] [PubMed] [Google Scholar]
- 31.Williams B P. BioEssays. 1995;17:391–393. doi: 10.1002/bies.950170506. [DOI] [PubMed] [Google Scholar]
- 32.Peretto P, Merighi A, Fasolo A, Bonfanti L. Brain Res Bull. 1999;49:221–243. doi: 10.1016/s0361-9230(99)00037-4. [DOI] [PubMed] [Google Scholar]
- 33.Wichterle H, Garcia-Verdugo J M, Herrera D G, Alvarez-Buylla A. Nat Neurosci. 1999;2:461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- 34.Kukekov V G, Laywell E D, Suslov O, Davies K, Scheffler B, Thomas L B, O'Brien T F, Kusakabe M, Steindler D A. Exp Neurol. 1999;156:333–344. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- 35.Yoshino K, Yuasa S, Kawamura K. No To Shinkei. 1995;47:1149–1157. [PubMed] [Google Scholar]
- 36.Gage F H, Kempermann G, Palmer T D, Peterson D A, Ray J. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Quinn S M, Walters W M, Vescovi A L, Whittemore SR. J Neurosci Res. 1999;57:590–602. [PubMed] [Google Scholar]
- 38.Mujtaba T, Piper D R, Kalyani A, Groves A K, Lucero M T, Rao M S. Dev Biol. 1999;214:113–127. doi: 10.1006/dbio.1999.9418. [DOI] [PubMed] [Google Scholar]