Abstract
Th17 cells play an active role in autoimmune diseases. However, the nature of Th17 cells is poorly understood in cancer patients. We studied Th17 cells, the associated mechanisms, and clinical significance in 201 ovarian cancer patients. Tumor-infiltrating Th17 cells exhibit a polyfunctional effector T-cell phenotype, are positively associated with effector cells, and are negatively associated with tumor-infiltrating regulatory T cells. Tumor-associated macrophages promote Th17 cells through interleukin-1β (IL-1β), whereas tumor-infiltrating regulatory T cells inhibit Th17 cells through an adenosinergic pathway. Furthermore, through synergistic action between IL-17 and interferon-γ, Th17 cells stimulate CXCL9 and CXCL10 production to recruit effector T cells to the tumor microenvironment. The levels of CXCL9 and CXCL10 are associated with tumor-infiltrating effector T cells. The levels of tumor-infiltrating Th17 cells and the levels of ascites IL-17 are reduced in more advanced diseases and positively predict patient outcome. Altogether, Th17 cells may contribute to protective human tumor immunity through inducing Th1-type chemokines and recruiting effector cells to the tumor microenvironment. Inhibition of Th17 cells represents a novel immune evasion mechanism. This study thus provides scientific and clinical rationale for developing novel immune-boosting strategies based on promoting the Th17 cell population in cancer patients.
Introduction
Adaptive immunity plays a crucial role in tumor immunosurveillance.1–3 It has been shown that tumor-infiltrating effector T cells are associated with improved prognoses in multiple human cancers,4–6 whereas tumor-infiltrating regulatory T (Treg) cells are negatively associated with patient outcome.6,7 Th17 cells are newly identified effector CD4+ T cells. Th17 cells and interleukin-17 (IL-17) play an active role in inflammation and autoimmune diseases.8–15 Th17 cells are found in both mouse and human tumors.16,17 However, the biologic role of Th17 cells is poorly understood in the tumor microenvironment. In this report, we examined the phenotype, cytokine profile, generation, functional relevance, and immunologic and clinical predictive values of Th17 cells in 201 patients with ovarian cancers. We provide novel insight into the nature of Th17 cells in the tumor microenvironment in patients with cancer. This information may be useful for designing more effective cancer immunotherapies
Methods
Human subjects
We studied previously untreated patients with 201 ovarian carcinomas. Survival data were available for 85 patients (supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Patients gave written, informed consent in accordance with the Declaration of Helsinki. The study was approved by the University of Michigan Institutional Review Board.
Cells and tissues
Cells and tissues were obtained from ascites, blood, lymph nodes, and tumors as we described.16,18,19 Immune cells, including monocytes, macrophages, myeloid dendritic cells, plasmacytoid dendritic cells, and T-cell subsets, were enriched using paramagnetic beads (StemCell Technologies) and sorted with FACSAria (Becton Dickinson) as we described.16,18,19 Cell purity was more than 98% as confirmed by flow cytometry (LSR II; Becton Dickinson).
FACS
For cytokine detection, the cells were stimulated with phorbol myristate acetate (50 ng/mL; Sigma-Aldrich), ionomycin (1 μM; Sigma-Aldrich) for 4 hours before staining. Cells were first stained extracellularly with specific antibodies against human CD3, CD4, CD8, CD11b, CD11c, CD14, CD15, CD16, CD19, CD25, CD39, CD45, CD45RO, CD49a, CD49c, CD49d, CD49e, CD56, CD123, CD161, PD-1, CCR4, CCR6, CCR7, CXCR4, HLA-DR, and annexin V (BD Biosciences), CCR2, CXCR3, and CCR5 (R&D Systems), EpCam (StemCell Technologies), then were fixed and permeabilized with Perm/Fix solution (eBioscience), and finally were stained intracellularly with anti–IL-2, anti–IL-10, anti–IL-17, anti–tumor necrosis factor-α, anti–interferon-γ (IFN-γ), anti-Granzyme A, anti–Ki-67, and anti-FOXP3 (all from BD Biosciences, except anti–IL-17, eBioscience). Samples were acquired on a LSR II (BD Biosciences), and data were analyzed with DIVA software (BD Biosciences).
Th17 induction and suppression
Fresh peripheral blood and tumor-associated CD14+ macrophages were sorted19 and cocultured with T cells as indicated for 3 to 5 days in the presence of anti-CD3 (2.5-5 μg/mL) and anti-CD28 (1.2-2.5 μg/mL) monoclonal antibodies (BD Biosciences). Anti–IL-1 receptor (1 μg/mL) was used as indicated (R&D Systems). CD4+CD25high T cells were sorted from peripheral blood or ovarian cancer tissues.7 Different concentrations of tumor-associated Treg cells were added into the coculture. In some cases, ARL67156 (50 μM; Sigma-Aldrich) was added into the culture as described. T-cell phenotype and cytokine profile were determined by fluorescence-activated cell sorter (FACS) or enzyme-linked immunosorbent assay (ELISA; R&D Systems) as we described.16,18,19
siRNA knockdown of human IL-23 gene expression
HEK293 cells were transfected with a Flag-tagged IL-23 expression plasmid and either a nonfunctional scrambled control siRNA or IL-23–specific siRNA using Lipofectamine 2000 (Invitrogen). After the siRNA treatment, the hIL-23 silencing efficiency was measured by Western blot using anti-Flag tag (not shown). Blood or tumor-associated macrophages were transfected with the siRNA or pmaxGFP vector using Nucleofector technology (Macrophages Nucleofector Kit; Amaxa Biosystems) as we described.20 The transfection efficiency reached 60% to 80% as confirmed by pmaxGFP vector transfection.
Cytokine and chemokine detection
The mRNA levels of cytokines and chemokines were detected by real-time reverse-transcriptase polymerase chain reaction (PCR). All experiments were performed using gene-specific primer pairs and SYBR green I (Invitrogen) fluorescence detection in a Multiplex instrument (Eppendorf). Data analysis is based on the Ct method with normalization of the raw data to housekeeping gene.7,19 The protein levels of cytokines and chemokines were detected by either intracellular staining or ELISA kits (all from R&D Systems).
Induction of CXCL9 and CXCL10
Th17 cells were polarized from tumor-associated T cells (106/mL) for 3 days with tumor-associated macrophages (0.5 × 106/mL) in the presence of Th17-inducing cytokine cocktail as we described.20 The polarized cells were extensively washed with fresh medium and cultured for additional 40 hours. The polarized Th17 cell supernatants were collected. Primary ovarian cancer cells (OC8) or macrophages (105/mL)19 were cultured with IL-17 (10 ng/mL), IFN-γ (0-50 ng/mL), IL-17 plus IFN-γ, or 100% Th17 cell-polarized culture supernatants for 2 to 3 days. In some cases, the neutralizing anti–human IFN-γ (2 μg/mL, clone 25723, IgG2b) and anti–IL-17 receptor (2 μg/mL, clone 133617, IgG) were added into the culture. The culture supernatants were subjected to measuring CXCL9 and CXCL10 with ELISA kits (R&D Systems).
Migration assay
CD8+ T-cell migration was assessed as we described21 using ovarian cancer–associated CD8+ T cells (5-20 × 104). T cells were induced to migrate with tumor ascites. In some cases, mouse anti–human CXCR3 (57226.11, IgG2b, 500 ng/mL) were added 2 hours before migration assay. Experiments were performed in triplicate. Migration was expressed as a percentage of migrated cells after subtracting the spontaneous migration (Migration index).
Tissue immunofluorescence staining
Immunofluorescence analysis was performed as described.22 Tissues were stained with monoclonal mouse anti–human CD8 (1/40 dilution, clone HIT8a, IgG2b, BD Biosciences), and mouse anti–human EpCam (1/40 dilution, clone 5E11, IgG1; StemCell Technologies) followed by AlexaFluor 568–conjugated goat anti–mouse IgG2b and AlexaFluor 488–conjugated goat anti–mouse IgG1 (all 2 μg/mL; Invitrogen). Positive cells were quantified by ImagePro Plus software and expressed as the mean number of the positive cells per mm2 tissue section.
Statistical calculations
Pearson coefficient was computed to assess relationships between proteins and immune cell subsets in the tumor environments. Student t tests were used to compare IL-17 expressions across stage (II/III vs IV), grade (0-2 vs 3), histology type (serous, mucinous, endometroid vs clear cells and undifferentiated), and debulking (optimal residual disease vs suboptimal residual disease) categories, with P values less than .05 considered significant. Overall patient survival was defined as the interval between date of diagnosis and date of death or last follow-up, whichever occurred earlier. The known tumor-unrelated deaths (eg, intercurrent disease and accidental death) were excluded from the death record for this study. Data were censored at the last follow-up for patients who were disease-free or alive at the time of last follow-up. Univariate association between IL-17, other factors, and overall survival was assessed using log-rank test, and survival function estimates were computed using the Kaplan-Meier method. Cox proportional hazards model was used to assess the effect of IL-17 on survival, after adjusting for surgical debulking. All analyses were performed using SAS 9.1 (SAS Institute Inc) and STATISTIC (StatSoft Inc) software.
Results
Distribution, phenotype, and cytokine profile of Th17 cells
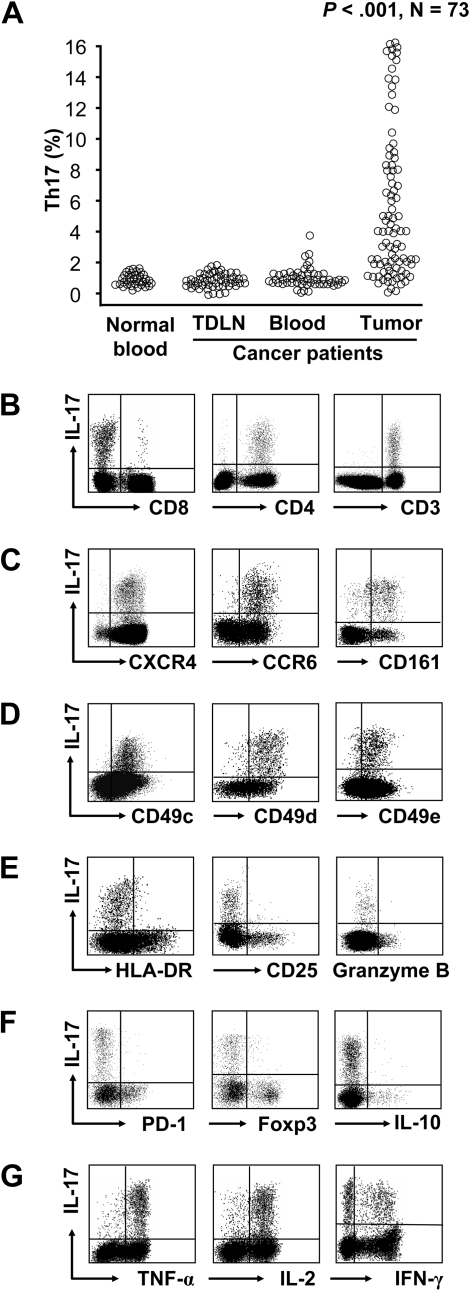
IL-17+CD4+ (Th17) cells are found in patients with cancer.16,17 However, the distribution, phenotype, and cytokine profile of Th17 cells remain poorly defined in human tumors. We first evaluated the tissue distribution of Th17 cells in ovarian cancer patients. The prevalence of Th17 cells was comparable in tumor-draining lymph nodes, cancer patient peripheral blood, and normal donor peripheral blood (Figure 1A). However, the proportion of Th17 cells was higher in tumors than these compartments (Figure 1A). This suggests that Th17 cells may be induced or/and migrate into the tumor microenvironment.16
Figure 1.
Distribution, phenotype, and cytokine profile of Th17 cells. Single-cell suspensions were made from fresh tumor specimens. The cells were subjected to membrane and intracellular staining and analyzed by FACS. One representative tumor specimen of 73 is shown in panels B to G. (A) The distribution of Th17 cells in patients with ovarian cancer. Results are expressed as the percentage of Th17 cells in CD4+ T cells in different tissues by gating on IL-17+CD4+CD3+ cells. Normal blood: n = 41. TDLN: Tumor-draining lymph nodes, n = 53. Cancer patient blood: n = 61. Ovarian cancer tissues: n = 73 (P < .001, compared with blood and TDLNs). (B) IL-17 expression in CD4+ and CD8+ T cells. IL-17 expression was analyzed in tumor-infiltrating CD45+ cells. (C) The expression of CXCR4, CCR6, and CD161 in tumor-infiltrating Th17 cells. (D) The expression of CD49C, CD49D, and CD49E in tumor-infiltrating Th17 cells. (E-F) The markers associated with T-cell activation/effector function and suppression. The expression of activation/effector molecules (CD25, HLA-DR, and granzyme B) in panel E and of suppression-associated molecules (PD-1, FOXP3, and IL-10) in panel F were analyzed in tumor-infiltrating Th17 cells. (G) The effector cytokine profile of Th17 cells. The cytokine profile was analyzed in tumor-infiltrating Th17 cells.
We next examined the phenotype of IL-17+ cells in the tumor microenvironment. We found that IL-17 was exclusively expressed by T cells. Less than 1% tumor-infiltrating CD8+ T cells expressed IL-17, whereas 99% of the tumor-infiltrating IL-17+ T cells were IL-17+CD4+ (Th17) cells (Figure 1B). Tumor-infiltrating Th17 cells expressed high levels of CXCR4, CCR6, and CD161 (Figure 1C) and multiple CD49 integrins (Figure 1D), but not CCR2, CCR5, and CCR7 (supplemental Figure 1). The expressed homing molecules may be associated with Th17 cell migration and retention within tumor.20
We also analyzed the markers for T-cell activation/effector function and immune suppression. Tumor-infiltrating Th17 cells expressed little HLA-DR, CD25, and granzyme B (Figure 1E). This suggests that Th17 cells may not be conventional effector T cells and may not mediate effector function through the granzyme B pathway. The B7-H1 receptor, PD-1, may be expressed in functionally exhausted T cells. The B7-H1/PD-1 pathway22 and FOXP3+ Treg cells7,23,24 contribute to immune suppression in the tumor microenvironment. We found that Th17 cells expressed minimal PD-1 and FOXP3 (Figure 1F). This indicates that Th17 cells are distinct from Treg cells and functionally exhausted PD-1+ T cells.
We further analyzed the cytokine profile of human tumor-infiltrating Th17 cells. IL-10+ and IL-10− Th17 cells have been observed in mice.25,26 We found that Th17 cells expressed minimal IL-10 (Figure 1F) and high levels of polyfunctional effector cytokines, including tumor necrosis factor-α, IL-2, and IFN-γ (Figure 1G). Tumor-infiltrating T cells, including Th17 cells, did not express IL-4 (not shown). Similar cytokine profiles were observed in 5 other human tumor types studied, including colon carcinomas, hepatocellular carcinomas, melanoma, pancreatic cancers, and renal cell carcinomas (not shown). These data indicate that Th17 cells exhibit an effector T-cell cytokine profile with polyfunctionality as described in infectious diseases.27,28
Th17 cells and their associations with immune cell subsets in the tumor microenvironment
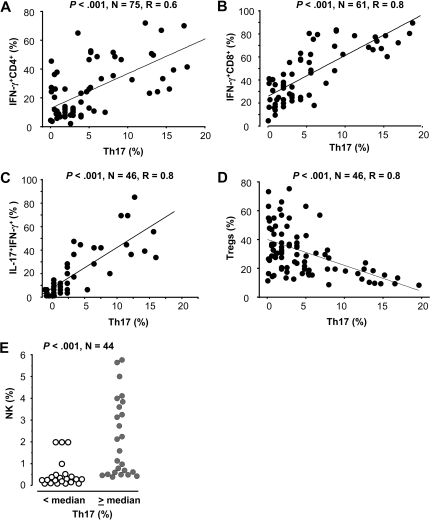
Multiple immune cell populations, including T-cell subsets and antigen-presenting cell (APC) subsets, infiltrated the tumor microenvironment. We evaluated the relationships between Th17 cells and immune cell subsets in the same ovarian cancer environment. We first analyzed the correlation between Th17 cells and T-cell subsets. We quantified Th17, IFN-γ+IL-17+ T cells, IFN-γ+CD8+, and IFN-γ+CD4+ T cells, and Treg cells in the same tumors. Flow cytometry analysis revealed that Th17 cells were positively correlated with IFN-γ–expressing T-cell subsets, including IFN-γ+CD4+ T cells (Figure 2A), IFN-γ+CD8+ (Figure 2B), and IFN-γ+IL-17+ T cells (Figure 2C) in the same tumor microenvironment. However, the proportion of Th17 and Treg cells was inversely correlated in the same tumors (Figure 2D).
Figure 2.
Th17 cells and their associations with immune cell subsets. (A-D) The correlation between Th17 cells and T-cell subsets in the same tumor environment. Multiple tumor-infiltrating T-cell subsets were defined with specific staining and analyzed by FACS. The percentages of Th17 cells in CD4+ T cells, IFN-γ+CD8+ T cells in CD8+ T cells, and IFN-γ+IL-17+CD4+ T cells in IL-17+CD4+ T cells (Th17 cells) were quantified in tumor tissues. The correlations between the percentages of Th17 cells and IFN-γ+CD4+ T cells (A), IFN-γ+CD8+ (B), IFN-γ+IL-17+ T cells (C), and FOXP3+CD4+ T cells (D) were evaluated. Correlation coefficients were computed to assess the relationship between Th17 cells and T-cell subsets in the same tumor environments. (E) The relationship between Th17 and NK cells in the same tumor environment. Th17 cells and NK cells were defined with specific staining and analyzed by FACS. Results are expressed as the percentage of NK cells in CD45+ cells. NK cells were quantified as the percentage of CD16+CD56+ cells in CD45+ cells in tumor ascites by gating on CD45+, non-T, B, and myeloid cells. The samples were divided into 2 groups based on median percentage of Th17 cells.
We further analyzed the relationship between Th17 cells and innate immune cells in the same ovarian cancer ascites. Eosinophils were rarely observed (supplemental Figure 2A). Moderate levels of mast cells (supplemental Figure 2B), neutrophils (supplemental Figure 2C), and NK cells (Figure 2E) were detected. However, Th17 cells had no correlation with eosinophils, mast cells, and neutrophils (supplemental Figure 2). We found that the levels of NK cells were higher in patients with high levels of Th17 cells than in patients with low levels of Th17 cells in the same tumor microenvironment (Figure 2E).
Finally, we analyzed the relationship between Th17 cells and APC subsets. Plasmacytoid dendritic cells,21 myeloid dendritic cells, and macrophages are the main APC populations in ovarian cancer19 (supplemental Figure 3A). These 3 APC subsets were found in the tumor ascites and tumor (supplemental Figure 3A). However, there were no quantitative correlations between Th17 cells, and myeloid DCs (supplemental Figure 3B), plasmacytoid DCs (supplemental Figure 3C), and macrophages (supplemental Figure 3D). We further investigated the functional association between Th17 cells and APC subsets in the subsequent studies.
Altogether, the data demonstrate that Th17 cells are quantitatively and positively correlated with NK cell–mediated innate immunity and adaptive T-cell immunity.
Induction and suppression of Th17 cell development in the tumor microenvironment
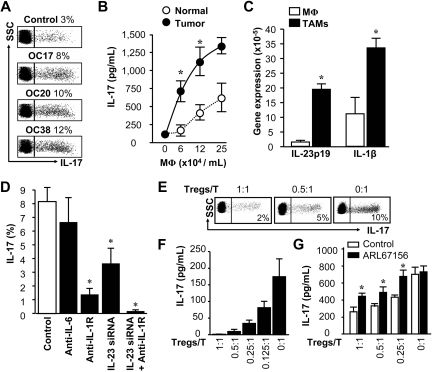
Th17 cells are basically found in the tumor microenvironment in patients with cancer.16 APCs contribute to T-cell polarization. We investigated the role of tumor-associated macrophages (TAMs), plasmacytoid DCs, and myeloid DCs in Th17 cell induction in ovarian cancer. We found that tumor-associated plasmacytoid DCs had minimal effects on Th17 cell induction (supplemental Figure 4). TAMs and myeloid DCs isolated from ovarian cancers stimulated Th17 cell induction from memory T cells, and not from naive T cells (supplemental Figure 4, Figure 3A). TAMs were more efficient than normal macrophages (Mφs) in eliciting T-cell IL-17 production, and the induction was dose dependent (Figure 3B). Macrophages outnumbered myeloid DCs in ovarian cancer19,21 (supplemental Figure 3) and were superior to inducing Th17 cells than myeloid DCs (supplemental Figure 4, Figure 3B).29 Our subsequent studies focused on tumor-associated macrophages.
Figure 3.
Induction and suppression of Th17 cell development. (A) TAMs induced Th17 cells. Normal blood T cells (5 × 105/mL) were stimulated with blood macrophages or TAMs (2.5 × 105/mL) from 3 ovarian cancer patients (OC17, OC20, and OC38). Th17 cells were analyzed by FACS. Results are expressed as the percentage of Th17 cells in CD4+ T cells. Similar results were observed in 8 ovarian cancer patients (P < .01, compared with control). (B) TAMs induced T-cell IL-17 production. Normal T cells (5 × 105/mL) were stimulated with different concentrations of normal macrophages (Mφ) or TAMs from donor OC20. IL-17 was detected by ELISA in the culture supernatants. Results are expressed as mean ± SEM; n = 5. P < .01. (C) The cytokine expression in TAMs. TAMs were isolated from ovarian cancer. Expression of IL-1β and IL-23p19 was detected by real-time PCR. Results are expressed as mean ± SEM; n = 5. P < .01. (D) The importance of IL-1β in TAM-mediated Th17 cell induction. T cells were stimulated for 5 days with TAMs with or without the indicated neutralizing antibodies. IL-23 was blocked by specific IL-23 siRNA as we reported.20 Th17 cells were detected by FACS. Results are expressed as the mean of Th17 cells in CD4+ T cells ± SEM; n = 5. *P < .05 compared with control. (E-F) Treg cells suppressed Th17 and T-cell IL-17 production induced by TAMs. T cells (5 × 105/mL) were stimulated with TAMs (2.5 × 105/mL) in the presence or absence different concentrations of tumor-associated Treg cells. Th17 cells were analyzed by FACS (E). Results are expressed as the percentage of Th17 cells in CD4+ T cells. IL-17 was detected by ELISA in the culture supernatants (F). n = 6. *P < .05 compared with control. (G) The relevance of the adenosinergic pathway in Treg cell-mediated Th17 suppression. In the culture system described (E-F), ARL67156 was added. IL-17 was detected by ELISA in the culture supernatants; n = 6. *P < .05 compared with control.
We investigated the mechanism by which TAMs induce Th17 cells. We found that TAMs expressed higher levels of IL-1β and IL-23p19 mRNA, compared with normal macrophages (Figure 3C). Blockade of IL-1, but not IL-6 and transforming growth factor-β (TGF-β), consistently and largely reduced TAM-mediated induction of Th17 cells (Figure 3D20; and data not shown). Blocking IL-23 with specific siRNA further helped reduce Th17 cell induction (Figure 3D). Our data suggest that IL-1β plays a predominant role in TAM-mediated Th17 cell induction in patients with ovarian cancer.
Because TAMs are potent Th17 cell inducers (Figure 3A-B,D), we examined why there were limited numbers of Th17 cells in the tumor microenvironment (Figure 1). We hypothesized that tumor-associated Treg cells might suppress Th17 cell development. To test this hypothesis, we first stimulated T cells with TAMs in the presence of tumor-associated Treg cells. Treg cells suppressed Th17 cells and T-cell IL-17 production in a dose-dependent manner (Figure 3E-F).
We further studied the mechanism by which Tregs suppressed Th17 induction. Tumor-associated Treg cells highly expressed CD39 (supplemental Figure 5A-B), an ectonucleotidase that converts ATP into adenosine. Mouse Treg cells may mediate T-cell suppression through adenosine induction.30,31 We found that ARL67156, a structural analog of ATP and an ectonucleotidase inhibitor, partially but significantly recovered T-cell IL-17 production suppressed by tumor-associated Treg cells (Figure 3G). These data indicate that Th17 cell development is partially suppressed by tumor-associated Treg cells through the adenosinergic pathway.
Th17, and Th1, Th2-type cytokines and chemokines
To further examine the relationships between Th17 cells and the types of immune responses in the ovarian cancer microenvironment, we quantified numerous representative cytokines and chemokines associated with Th17, Th1, and Th2-type responses in the ovarian cancer ascites.
Th17 cells were the only cell type expressing IL-17 in the ovarian cancer ascites. We detected variable levels of IL-17 in ascites fluid. Interestingly, the levels of IL-17 were positively correlated with IL-1β and IL-1α (supplemental Figure 6A-B), but not with TGF-β, IL-6 (supplemental Figure 6C; and data not shown), IL-21 (supplemental Figure 6), IL-23 (supplemental Figure 6E), and PGE2 (supplemental Figure 6F). IL-23 protein was barely detectable in most of the samples tested (supplemental Figure 6E). All these molecules have been reported to be associated with Th17 cell development.8–15,32 Given that the levels of IL-1α were less than 5 pg/mL (supplemental Figure 6B), the data further support that IL-1β plays a selective and crucial role in Th17 cell induction in the ovarian cancer microenvironment (Figure 3C-D).
Cytokines associated with Th1 and Th2-type responses, including IL-12, IL-2, and IL-4, were less than 10 pg/mL in ovarian cancer ascites. IL-17 has been reported to induce tumor angiogenesis.33,34 Consistent with previous reports, high levels of angiogeneic factors, including IL-8 and vascular endothelial growth factor, were detected in the ascites. However, IL-17 was not correlated with these angiogeneic molecules (supplemental Figure 7).
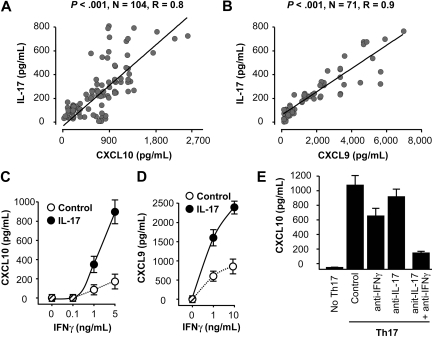
In addition to cytokines, we further evaluated the relationship between IL-17 and chemokines associated with Th1-type response, including CXCL9, CXCL10, and with Th2-type response, including CXCL12 and CCL22. Interestingly, we observed a significant positive correlation between IL-17, CXCL9, and CXCL10 (Figure 4A-B). Although we detected high levels of CXCL12 and CCL22, IL-17 had no association with these chemokines (supplemental Figure 8). The data indicate that, in addition to Th1-type effector T cells and NK cells (Figure 2), Th17 cells and IL-17 are correlated with Th1-type chemokines in the ovarian cancer microenvironment.
Figure 4.
Th17 cells induce Th1-type chemokines. (A-B) The correlations between IL-17 and Th1-type chemokines CXCL9 and CXCL10 in ovarian cancer ascites. IL-17, CXCL9, and CXCL10 were detected by ELISA in ovarian cancer ascites. The correlations between IL-17 and CXCL9 (A), and CXCL10 (B) were analyzed. (C-D) IL-17 and IFN-γ synergistically induced CXCL9 and CXCL10 production by primary ovarian tumor cells. Primary ovarian cancer cells (OC8) were cultured with IL-17 in the presence of variable concentrations of IFN-γ. CXCL9 and CXCL10 were detected in the cell supernatants by ELISA. Results are expressed as the mean values ± SEM (P < .05). (E) Th17 cells induced CXCL10 production by primary ovarian tumor cells through IL-17 and IFN-γ. Primary ovarian cancer cells were cultured with Th17-derived supernatants in the presence or absence of anti–IFN-γ and anti–IL-17. CXCL10 was detected in the cell supernatants by ELISA. Results are expressed as the mean values ± SEM (P < .05).
In addition, we examined the mechanistic relationship between Th17 cells and tumor immunity. Th17 cells or IL-17 had no direct effects on primary ovarian cancer cell proliferation and apoptosis (supplemental Figure 9). As Th17 cells are positively correlated with Th1-type chemokines and effector T cells, we hypothesized that Th17 cells induce Th1-type chemokines, and in turn recruit Th1-type effector T cells into tumor microenvironment. To test this hypothesis, we initially studied the effects of Th17 cells on Th1-type chemokine production. We found that IFN-γ and IL-17 synergistically induced the production of CXCL9 and CXCL10 by primary ovarian cancer cells and macrophages (Figure 4C-D; and data not shown). Consistent with this observation, real-time PCR revealed that the levels of IL-17 were positively correlated with that of CXCL9 and CXCL10 in the same tumor tissues (supplemental Figure 10). In further support, the supernatants derived from Th17 cells induced high levels of CXCL10 production. This production was blocked by neutralizing anti–human IFN-γ and anti–IL-17 (Figure 4E). These data indicate that Th17 cells induce Th1-type chemokine production.
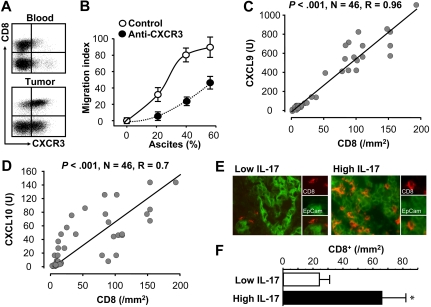
Th17, Th1-type chemokines, and effector T-cell trafficking
Tumor-associated effector CD8+ T cells highly expressed CXCR3, the receptor for CXCL9 and CXCL10 (Figure 5A). Tumor-associated effector CD8+ T cells efficiently migrated toward tumor ascites in a dose-dependent manner. The migration was reduced by neutralizing anti-CXCR3 (Figure 5B). We also quantified the number of tumor-infiltrating CD8+ T cells by immunofluorescence staining. The mRNA levels of CXCL9 and CXCL10 were positively correlated with tumor-infiltrating CD8+ T cells in the same tumor (Figure 5C-D). Furthermore, when we divided tumor tissues into 2 groups based on the median levels of IL-17, we observed that the levels of tumor ascites IL-17 were positively associated with tumor-infiltrating CD8+ T cells (Figure 5E-F). Altogether, the data support the notion that Th17 cells induce Th1-type chemokines through IL-17 and IFN-γ, and in turn recruit Th1-type effector T cells and NK cells into tumor microenvironment.
Figure 5.
Relationship between IL-17, Th1-type chemokines, and effector T-cell tumor trafficking. (A) Effector CD8+ T cells expressed CXCR3. Blood and tumor-associated T cells were stained for CXCR3; n = 8. P < .01. (B) Effector CD8+ T cells migrated toward tumor ascites through CXCR3. Tumor-associated CD8+ T cells were subject to migration to different concentrations of tumor ascites with or without anti-CXCR3. Results are expressed as the mean migration index ± SEM; n = 8. P < .01. (C-D) The correlation between the mRNA levels of CXCL9 (H), CXCL10 (I), and CD8+ T cells in the same tumors. The mRNA levels of CXCL9 and CXCL10 were quantified by real-time PCR. Tumor-infiltrating CD8+ T cells were defined by immunofluorescence staining and were quantified as described in “Tissue immunofluorescence staining.” (E-F) The correlation between the levels of IL-17 and CD8+ T cells in the same tumors. Tumor-infiltrating CD8+ T cells were defined by immunofluorescence staining and were quantified as described in “Tissue immunofluorescence staining.” Representative images showed CD8+ T-cell infiltration in low versus high levels of IL-17 (E). The numbers of tumor effector CD8+ T cells in patients with low versus high levels of tumor ascites IL-17 were compared (P = .009; F).
Increased tumor-associated IL-17 predicts improved patient survival
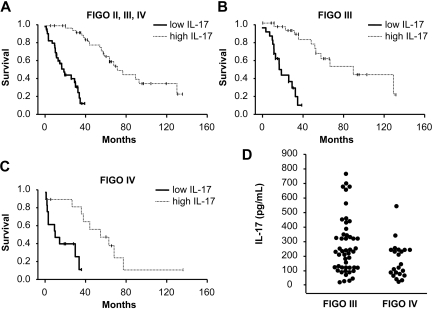
Our current data suggest that Th17 cells may contribute to protective tumor immunity in ovarian cancers. IL-17 is released into the tumor environment consisting of the abdominal cavity. IL-17 was detectable in all the ovarian cancer ascites we evaluated (Figure 4A-B). We analyzed the impact of IL-17 levels in the ascites on patient survival.
There was a significant association between ascites IL-17 levels and survival in the group as a whole (n = 85, P = .001), and also for patients in stage II/III (n = 57, P = .01) and stage IV (n = 28, P = .005). Tumor ascites IL-17 was a significant predictor of death hazard (95% confidence interval, P = .001) even after controlling for surgical debulking and other parameters using a Cox proportional hazards model (Figure 6; Table 1, supplemental Table 1).
Figure 6.
Increased tumor-associated IL-17 predicts improved patient survival. (A-C) Kaplan-Meier curve for overall survival by the levels of IL-17 in 85 patients in stage II-IV (A), stage III (B), and stage IV (C). Samples were divided into 2 groups based on the median levels of IL-17 in tumor ascites (“Statistical calculations”). Survival was significantly increased as a function of IL-17. (D) The levels of IL-17 in different stages were compared.
Table 1.
Relationship between IL-17 and clinical pathologic characteristics in ovarian cancer patients
| Variable | Unadjusted HR (95% CI) | Adjusted HR† (95% CI) |
|---|---|---|
| Age* | 1.003 (0.98-1.03) | NA |
| Stage, II/III vs IV | 1.84 (0.97-3.48) | NA |
| Grade, 0-2 vs 3 | 1.74 (0.80-3.78) | NA |
| Histology type, serous/mucinous/endometrial versus clear cells and undifferentiated | 1.36 (0.69-2.69) | NA |
| Debulking, optimal versus suboptimal residual disease | 0.17 (0.07-0.42) | 0.189 (0.078-0.458) |
| IL-17* | 0.994 (0.992-0.997) | 0.994 (0.991-0.997) |
NA indicates not applicable.
Age (in years) and IL-17 (in pg/mL) are continuous variables. Other variables are binary, as noted.
Adjusted HRs are based on a multivariable Cox proportional hazards model with debulking (binary: optimal vs suboptimal) and IL-17 (continuous) as covariates.
As an alternative analysis, patients were divided into 2 groups based on the median values of IL-17 (220 pg/mL). Survival functions were significantly different for the 2 groups (Figure 5A; P < .001). The median survival in the high IL-17 group was 78 months, compared with 27 months in the low IL-17 group. Tumor ascites IL-17 was a significant predictor of death, even after controlling for surgical debulking. Patients in the high IL-17 group had a significantly lower death hazard compared with those in the low IL-17 group (hazard ratio = 0.08; 95% confidence interval, 0.03-0.20, P < .001).
Furthermore, when the analyses were stratified by stage, we found significant association between ascites IL-17 and survival for patients in stage III (n = 52, P = .01; Figure 6B) as well as stage IV disease (n = 28, P = .005; Figure 5C). We additionally found that patients in stage IV had significantly reduced IL-17 in ascites compared with those in stage III (Figure 6D; P = .03).
Th17 cells are the IL-17 producers in the tumor. Therefore, decreased tumor ascites IL-17 or/and Th17 cells are a significant predictor of increased risk for reduced survival in ovarian cancer.
Discussion
In this study, we have applied multiple complementary strategies to map out the phenotype, mechanisms of induction, biologic function, and clinical relevance of Th17 cells in the tumor microenvironment of patients with ovarian cancer.
We have shown that tumor-infiltrating Th17 cells highly express effector cytokines, but little in the way of molecules associated with immune suppression. This cytokine profile reveals a phenotype for polyfunctional effector T cells similar to that observed in patients with infectious diseases.27,28 This phenotype was universally found in 6 different human cancer types that we examined. It suggests that tumor-associated Th17 cells may be functional effector T cells. In line with this possibility, we found that Th17 cells are negatively associated with the presence of Treg cells7 and are positively associated with effector immune cells, including IFN-γ+ effector T cells, CD8+ T cells, and NK cells in the same tumor microenvironment. The data are consistent with several lines of evidence. (1) Transgenic T cells polarized with TGF-β and IL-6 can induce tumor eradication in mice.35 (2) Forced expression of IL-17 ectopically in tumor cells can suppress tumor progression through enhanced antitumor immunity in immune-competent mice.36,37 (3) IL-17–deficient mice exhibit accelerated tumor growth and lung metastasis.38 (4) Both blocking indoleamine 2,3-dioxygenase39 and adjuvant IL-7 treatment40 result in improved antitumor immunity, which is associated with marked CD8+ T-cell activation and Th17 cell enhancement. (5) In patients with prostate cancer, a significant inverse correlation is found between Th17 skewing and tumor grade.17
Along this line, we have detected IL-17 in tumor-associated ascites, and the levels of IL-17 positively predict patient survival. Th17 cells are the sole cellular source for IL-17 in the human tumor microenvironment. Hence, the data provide evidence that Th17 cells may contribute to protective tumor immunity in humans with advanced tumors. In addition to CD8+ effector T cells,4–6 our data indicate that Th17 cells are an important immune component in tumor immunosurveillance.1,2
The next question is how Th17 cells mediate antitumor immunity in patients with cancer. Th17 cells do not express granzyme B and perforin, and have no direct effects on primary ovarian cancer cell proliferation and apoptosis. Th17 cells may not mediate a direct tumor cytotoxic activity against tumor cells. Recent compelling evidence demonstrates that trafficking properties and location of effector T cells play a central role in the control of tumor growth and recurrence.4–6 In line with this notion, we found that IL-17 was positively associated with tumor-infiltrating IFN-γ+ effector T cells and with Th1-type chemokines CXCL9 and CXCL10, but not with Th2-type chemokines CXCL12 and CCL22. Mechanistically, Th17 cell–derived IL-17 and IFN-γ synergistically induced the production of CXCL9 and CXCL10, and in turn promoted effector T-cell migration toward tumor. The levels of CXCL9 and CXCL10 were directly correlated with tumor-infiltrating CD8+ T cells and NK cells. The data suggest that Th17 cells may play a role in promoting effector T-cell and NK-cell tumor trafficking and retainment, and the polyfuctional cytokine profile (IFN-γ+IL-17+) of Th17 cells is essential for synergistically inducing Th1-type chemokines. In support of this notion, human psoriatic environmental Th17 cells express both IL-17 and IFN-γ and synergistically induce β-defensin, a functional marker for human psoriasis.20 Furthermore, polarized Th17 cells mediate tumor regression in an IFN-γ–dependent manner in mice.35
Notably, the role of IL-17 and IL-23 in tumor is controversial in the murine system. Earlier studies have shown that exogenous IL-17 either enhances antitumor immunity36,37 or promotes tumor growth by inducing tumor vascularization in tumor-bearing mice.33,34 Recent studies have also revealed opposite roles of IL-23 in mouse tumors.41,42 It is worthwhile to point out that the potential role of endogenous IL-17 (or Th17 cells) has not been examined in tumor initiation in spontaneous mouse tumor models, including those induced by infectious pathogens and chemical carcinogens or in humans with preclinical diseases. It is possible that endogenous IL-17 (or Th17 cells) may play distinct roles in tumor initiation versus established tumor growth. In addition to IL-17, Th17 cells express a polyfunctional cytokine profile in human tumors. This polyfunctional cytokine profile may not be observed in a specific mouse system. The collaborative effects among these cytokines, including IL-17 and IFN-γ, may be decisive in determining the biologic activities of Th17 cells in human tumors as demonstrated in this and other human studies.20 Further, the roles of exogenous and endogenous IL-17 may potentially be distinct resulting from local biologic levels of IL-17 and environment. In patients with ovarian cancer, IL-17 is quantitatively and mechanistically associated with CXCL9 and CXCL10 but not with the well-defined angiogeneic factors IL-8 and vascular endothelial growth factor in ovarian cancer.43 In addition to attracting effector T cells, CXCL9 and CXCL10 are 2 potent antiangiogeneic cytokines.44 IL-17 is also not associated with IL-23, and IL-23 plays a minor role, if any, in Th17 cell development in human ovarian cancer. The data do not rule out the potential angiogeneic and proinflammatory roles of IL-17 derived from Th17 cells in human tumors. However, these potential effects may possibly be overweighed by the antitumor immunity and antiangiogeneic activities mediated by Th17 cell–induced CXCL9 and CXCL10 in patients with cancer.
We have further demonstrated that tumor-associated macrophages are capable of inducing Th17 cell development in vitro. IL-1β, but not IL-1α, IL-6, TGF-β, and IL-23, is crucial for Th17 cell induction and is positively associated with IL-17 in ovarian cancer ascites. Consistent with this observation, the levels of IL-1α and IL-23 are negligible in ovarian cancer ascites. It suggests that IL-1α and IL-23 play a minor role in Th17 cell development in human ovarian cancer. However, IL-1α, IL-1β, and IL-23 are involved in memory Th17 cell expansion in patients with psoriasis.20,29 It is possible that the molecular mechanisms are distinct in inducing Th17 cells in patients with tumors versus autoimmune diseases. The role of IL-6 and TGF-β in Th17 cell development remains controversial in humans.45–48 High levels of IL-6 and TGF-β are often detected in the tumor microenvironment.49 If IL-6 and TGF-β haveplayed potent roles in promoting Th17 cells, one may expect substantial numbers of Th17 cells in human tumors. However, it is evident that the numbers of Th17 cells are limited, compared with Treg cells and other T-cell subsets in the tumor microenvironment.16 Blockade of IL-1, rather than IL-6 and TGF-β, reduced Th17 cell induction. Furthermore, IL-17 and Th17 cells are not quantitatively associated with IL-6 and TGF-β. Therefore, at least these 2 cytokines are not crucial for Th17 cell development in the ovarian cancer microenvironment. The role of IL-1β is relatively selective in Th17 cell development in the human tumor microenvironment.
We have also investigated the underlying mechanisms by which Th17 cells are limited in the tumor microenvironment. Interestingly, the levels of Treg cells and Th17 cells are inversely associated in the same tumors. Tumor-associated Treg cells highly express CD39, an ectonucleotidase that converts ATP into adenosine, and suppress Th17 cell development through the adenosinergic pathway. Although it has been reported that mouse Treg cells may apply this pathway to suppress T-cell activation,30,31 we demonstrated, for the first time, that human tumor-associated Treg cells inhibit Th17 cells with a similar molecular mechanism. In addition to multiple modes of immune-suppressive mechanisms demonstrated in the tumor microenvironment,49–53 as human Th17 cells probably mediate protective tumor immunity, inhibition of Th17 cell development may be a novel immunoediting mechanism for tumor to escape tumor immunity.
In conclusion, we have extensively defined the nature of Th17 in the human tumor microenvironment. Our data provide immunologic and clinical evidence linking Th17 cells to immune protection in human cancer and suggest that inhibition of Th17 cell development is a novel immune evasion mechanism. This study thus provides the rationale for developing novel immune-boosting strategies based on promoting the Th17 cell population in cancer patients.
Supplementary Material
Acknowledgments
This work was supported in part by the National Cancer Institute (CA123088, CA099985; W.Z.) and the Marsha Rivkin Center for Ovarian Cancer Research (I.K.).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: I.K., R.L., and W.Z. designed research, analyzed data, and wrote the paper; M.B. analyzed data; P.C., E.H., E.F., D.S., T.H.W., A.C., G.C., and R.L. provided specimen and clinical information and reviewed the paper; and I.K., L.V., W.S., and S.W. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Weiping Zou, MSRB II C560B, 1150 W Medical Center Dr, University of Michigan School of Medicine, Ann Arbor, MI 48109-0669; e-mail: wzou@med.umich.edu; or Ilona Kryczek, MSRB II C560B, 1150 W Medical Center Dr, University of Michigan School of Medicine, Ann Arbor, MI 48109-0669; e-mail: ilonak@med.umich.edu.
References
- 1.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 3.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 4.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 6.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 8.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 10.Wynn TA. T(H)-17: a giant step from T(H)1 and T(H)2. Nat Immunol. 2005;6:1069–1070. doi: 10.1038/ni1105-1069. [DOI] [PubMed] [Google Scholar]
- 11.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 12.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 14.Tato CM, O'Shea JJ. Immunology: what does it mean to be just 17? Nature. 2006;441:166–168. doi: 10.1038/441166a. [DOI] [PubMed] [Google Scholar]
- 15.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kryczek I, Wei S, Zou L, et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 17.Sfanos KS, Bruno TC, Maris CH, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kryczek I, Wei S, Vatan L, et al. Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J Immunol. 2007;179:1423–1426. doi: 10.4049/jimmunol.179.3.1423. [DOI] [PubMed] [Google Scholar]
- 19.Kryczek I, Zou L, Rodriguez P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kryczek I, Bruce AT, Gudjonsson JE, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou W, Machelon V, Coulomb-L'Hermin A, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 22.Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;21:21. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 23.Yu P, Lee Y, Liu W, et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628–636. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGeachy MJ, Bak-Jensen KS, Chen Y, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 26.Fitzgerald DC, Zhang GX, El-Behi M, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 27.Precopio ML, Betts MR, Parrino J, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almeida JR, Price DA, Papagno L, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kryczek I, Wei S, Gong W, et al. Cutting edge: IFN-gamma enables APC to promote memory Th17 and abate Th1 cell development. J Immunol. 2008;181:5842–5846. doi: 10.4049/jimmunol.181.9.5842. [DOI] [PubMed] [Google Scholar]
- 30.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borsellino G, Kleinewietfeld M, Di Mitri D, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 32.Chizzolini C, Chicheportiche R, Alvarez M, et al. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood. 2008;112:3696–3703. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Numasaki M, Watanabe M, Suzuki T, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 34.Numasaki M, Fukushi J, Ono M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 35.Muranski P, Boni A, Antony PA, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benchetrit F, Ciree A, Vives V, et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 37.Hirahara N, Nio Y, Sasaki S, et al. Inoculation of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumor-specific immunity in mice. Oncology. 2001;61:79–89. doi: 10.1159/000055357. [DOI] [PubMed] [Google Scholar]
- 38.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma MD, Hou DY, Liu Y, et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113:6102–6111. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pellegrini M, Calzascia T, Shahinian A, et al. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat Med. 2009;15:528–536. doi: 10.1038/nm.1953. [DOI] [PubMed] [Google Scholar]
- 41.Langowski JL, Zhang X, Wu L, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 42.Kaiga T, Sato M, Kaneda H, Iwakura Y, Takayama T, Tahara H. Systemic administration of IL-23 induces potent antitumor immunity primarily mediated through Th1-type response in association with the endogenously expressed IL-12. J Immunol. 2007;178:7571–7580. doi: 10.4049/jimmunol.178.12.7571. [DOI] [PubMed] [Google Scholar]
- 43.Kryczek I, Lange A, Mottram P, et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005;65:465–472. [PubMed] [Google Scholar]
- 44.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 45.Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 46.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 47.Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 50.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 51.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 52.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 53.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.