Abstract
Tricuspid valve endocarditis frequently occurs in the setting of intravenous drug use. A case of tricuspid valve endocarditis in a 37-year-old woman with a history of intravenous cocaine use is described. Transthoracic echocardiography showed extension of the tricuspid valve mass through a patent foramen ovale and into the left atrium. One week after intravenous antibiotic treatment, the mass no longer traversed the patent foramen ovale, and only two smaller tricuspid valve vegetations remained. The present case demonstrates the value of performing a complete and thorough transthoracic echocardiography to visualize and evaluate both the right-and left-sided consequences of infective endocarditis in intravenous drug users. It also serves as a useful reminder to physicians caring for such patients that right-sided endocarditis can have important left-sided complications.
Keywords: Echocardiography, Infective endocarditis, Patent foramen ovale
Abstract
L’endocardite valvulaire tricuspidienne survient souvent dans le contexte des toxicomanies intraveineuses. On décrit ici un cas d’endocardite valvulaire tricuspidienne chez une femme de 37 ans ayant des antécédents de cocaïnomanie intraveineuse. L’échocardiographie transthoracique a révélé l’extension de la masse valvulaire tricuspidienne à travers un foramen ovale persistant et vers l’oreillette gauche. Une semaine après une antibiothérapie intraveineuse, la masse ne traversait plus le foramen ovale persistant et seules deux petites végétations valvulaires tricuspidiennes persistaient. Ce cas démontre l’utilité de l’échocardiographie transthoracique complète et approfondie pour visualiser et évaluer les complications gauches et droites de l’endocardite infectieuse chez les utilisateurs de drogues i.v. Il rappelle également fort à propos aux médecins qui soignent de tels patients atteints d’endocardite droite que des complications importantes peuvent affecter le cœur gauche.
Tricuspid valve endocarditis is a frequent complication of intravenous drug use (IVDU). An unusual case of tricuspid valve endocarditis with extension of a complicated mass into the left atrium through a patent foramen ovale (PFO) is reported. This complication was detected by transthoracic echocardiography and resolved within days of antibiotic treatment, leaving two residual tricuspid valve vegetations.
CASE PRESENTATION
A 37-year-old woman with a history of IVDU presented to the hospital with fever, hematuria and general malaise. She had been feeling unwell for one month before presentation and was experiencing persistent shortness of breath. The patient also described progressively increasing abdominal and neck pain. She had associated nausea, vomiting, fatigue and polyarthralgia, and had no known history of valve abnormality.
On examination, the patient was febrile and was found to have a supine blood pressure of 111/67 mmHg with a sitting blood pressure of 90/57 mmHg. She had a heart rate of 130 beats/min and a respiratory rate of 22 breaths/min. She was hypoxic, and had diffuse abdominal, flank and costophrenic tenderness. Her jugular venous pulsation was elevated and she was found to have a grade 2/6 systolic murmur, loudest at the right upper sternal border, which had not been previously documented. No other stigmata of infective endocarditis were elicited on physical examination.
Laboratory investigations revealed a white blood cell count of 16.5×109/L (normal range 4.0×109/L to 10.5×109/L) and a hemoglobin level of 75 g/L (normal range 120 g/L to 160 g/L). Her creatinine was elevated at 353 μmol/L (normal is 80 μmol/L or lower). Her erythrocyte sedimentation rate was 140 mm/h (normal range 0 mm/h to 27 mm/h). A qualitative urine drug screen was positive for opiates, cocaine metabolites, cannabinoids and benzodiazepines.
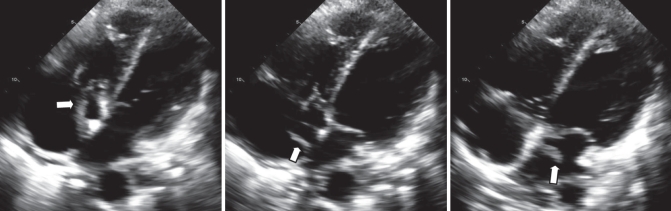
A transthoracic echocardiogram confirmed the suspicion of endocarditis (Figures 1 and 2). The patient had a large right atrial mass (40 mm in length) extending from the tricuspid valve to the atrial septum and then into the left atrium. The left atrial component of the mass was bulkier than the right atrial component and measured 20 mm × 6 mm. The mass appeared to cross the septum through a PFO. The mass was associated with severe tricuspid regurgitation, and there was moderate pulmonary hypertension with a pulmonary artery systolic pressure measured at 48 mmHg. Left and right ventricular size and systolic function were normal.
Figure 1).
Transthoracic echocardiography. Apical four-chamber view demonstrating the presence of a tricuspid valve mass (arrows) that extends to the atrial septum. In the middle frame, the mass appears to extend through the patent foramen ovale and into the left atrium. In the right frame, the mass appears to be bulkier in appearance within the left atrium
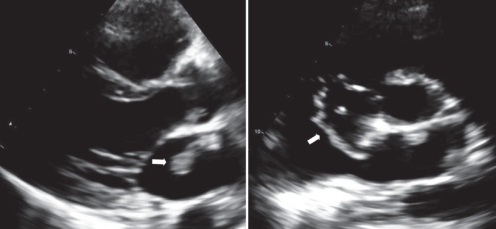
Figure 2).
Transthoracic echocardiography. Parasternal views showing the presence of a large mass (arrows) in the left atrium. In the right frame, the short-axis view shows that the mass appears to originate from the tricuspid valve and extends through a patent foramen ovale
Concurrently, the patient’s blood cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography (CT) of the chest revealed the presence of bilateral pleural effusions and multiple areas of airspace disease suggestive of septic emboli.
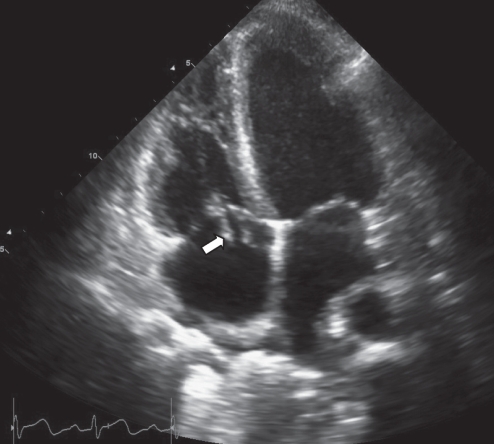
She was treated with fluid hydration, acetylsalicylic acid and intravenous cloxacillin 2 g every 4 h for eight weeks. Echocardiography conducted after seven days of treatment revealed significant improvement in the appearance of the tricuspid valve vegetation (Figure 3). The mass in the left atrium was no longer visible. Two masses on the tricuspid valve were seen, measuring 15 mm × 5 mm, and 13 mm × 4 mm. The severity of tricuspid regurgitation was reduced. Colour Doppler imaging did not indicate shunting across the septum following regression of the mass.
Figure 3).
Apical four-chamber view of an echocardiogram performed seven days after treatment, demonstrating significant improvement in the appearance of the vegetation since the previous echocardiogram. There is no longer any mass visible in the left atrium. There are two masses on the tricuspid valve, measuring 15 mm × 5 mm, and 13 mm × 4 mm (arrow indicates the larger mass)
DISCUSSION
Tricuspid valve endocarditis is frequently associated with IVDU (1). However, the present report is the first to describe a tricuspid valve vegetation extending through a patent foramen ovale into the left atrium. The organism involved in the present case was S aureus, which is known to be associated with aggressive presentations of infective endocarditis. Given the results of the urine drug screen, the patient was most likely administering intravenous drugs at that time. The rapid diminishment of the vegetation and the bulkier appearance within the left atrium suggest that the mass was complicated and likely associated with thrombi.
A previous report (2) described an atrial septal defect that was a complication of infective endocarditis of the mitral valve. In the present case, we could not exclude the possibility of an acquired interatrial communication resulting from bacterial seeding and perforation of the septum. However, this explanation seems unlikely because after one week of antibiotic treatment, there was a significant decrease in the size of the tricuspid valve mass, with no residual interatrial defect on echocardiography. Thus, bacterial seeding of the interatrial septum with perforation and subsequent healing of the septum within days seems less likely than a PFO. In another case (3), tricuspid valve endocarditis in conjunction with a PFO in an intravenous drug user was reported; however, no mass was seen. As in our patient, blood cultures were positive for S aureus and septic emboli were seen on a CT scan of the chest. In the case presented by Turek et al (3), it was believed that the patient’s PFO became apparent after tricuspid valve infection because the flail posterior leaflet directed a severe tricuspid regurgitation jet toward the septum in such a way that it pulled the flaplike structure away from the body of the septum. In the present case, we believe that the PFO opened because of increased right atrial pressures secondary to pulmonary hypertension (right ventricular systolic pressure 48 mmHg). The pulmonary hypertension was, in turn, due to septic pulmonary emboli diagnosed by CT and consistent with the patient’s initial presentation. Increased right atrial pressure was supported by bulging of the septum into the left atrium, which was observed on the initial transthoracic echocardiography. Although the extension of the mass through the PFO directly demonstrates right to left shunting on the initial echocardiogram, we were unable to conclusively demonstrate right to left shunting through a PFO on the second echocardiogram because the patient would not agree to a saline-enhanced (contrast) echocardiogram. However, colour Doppler imaging was performed and did not suggest shunting.
The etiology of the severe tricuspid regurgitation appeared to be due to two heavy vegetations that appeared to tether the septal and lateral valve leaflets, causing the tricuspid valve to visibly remain open throughout systole. Indeed, Figure 3 demonstrates that even as these two vegetations diminished in bulk with treatment, the tricuspid valve was disrupted. No prolapse, perforation or right ventricular dilation was seen to account for the tricuspid regurgitation. The possibility of a flail leaflet due to disruption cannot be excluded because of the extensive size of these vegetations.
Septic pulmonary emboli are eventually seen on chest x-ray in 77% of patients with tricuspid valve endocarditis related to IVDU (4). Thus, the finding of septic emboli to the lung in the present case was not unusual. It was believed that the patient’s severe acute renal failure may have been due to septic emboli to the kidneys. However, we were unable to confirm this because her renal function improved rapidly with hydration. Fortunately, there were no events suggesting systemic emboli as her left-sided vegetation resolved.
CONCLUSIONS
The present case illustrates the very rare occurrence of a tricuspid valve vegetation extending through a PFO into the left atrium. As a consequence of the tricuspid mass, the patient developed septic emboli to the lung and presumably to the kidney. The case demonstrates the value of performing a complete and thorough transthoracic echocardiogram to visualize and evaluate both the right- and left-sided consequences of infective endocarditis in intravenous drug users. The present case serves as a useful reminder to physicians that right-sided endocarditis can have important left-sided complications.
REFERENCES
- 1.Reisberg BE. Infective endocarditis in the narcotic addict. Prog Cardiovasc Dis. 1979;22:193–204. doi: 10.1016/0033-0620(79)90023-9. [DOI] [PubMed] [Google Scholar]
- 2.Prinz A, Akosah KO, Jackson P, et al. Acquired atrial septal defect as a complication of endocarditis. A case report. Angiology. 1998;49:865–9. doi: 10.1177/000331979804900912. [DOI] [PubMed] [Google Scholar]
- 3.Turek MA, Karovitch A, Aaron SD, Brais M. Persistent hypoxemia occurring as a complication of tricuspid valve endocarditis. J Am Soc Echocardiogr. 2000;13:412–4. doi: 10.1016/s0894-7317(00)70012-5. [DOI] [PubMed] [Google Scholar]
- 4.Levine DP, Crane LR, Zervos MJ. Bacteremia in narcotic addicts at the Detroit Medical Center. II Infectious endocarditis: A prospective comparative study. Rev Infect Dis. 1986;8:374–96. doi: 10.1093/clinids/8.3.374. [DOI] [PubMed] [Google Scholar]