Abstract
OBJECTIVE:
The delay between the availability of clinical evidence and its application to the care of patients with acute coronary syndrome (ACS) in the Kingdom of Saudi Arabia remains undefined. The Saudi Project for Assessment of Coronary Events (SPACE) registry provides a comprehensive view of the current diagnostic and treatment strategies for patients with ACS; thus, the registry may be used to identify opportunities to improve the care of these patients.
METHODS:
Eight hospitals in different regions of Saudi Arabia were involved in the pilot phase of the registry, from December 2005 to July 2006. The study patients included individuals with ST segment elevation myocardial infarction (STEMI), non-STEMI and unstable angina.
RESULTS:
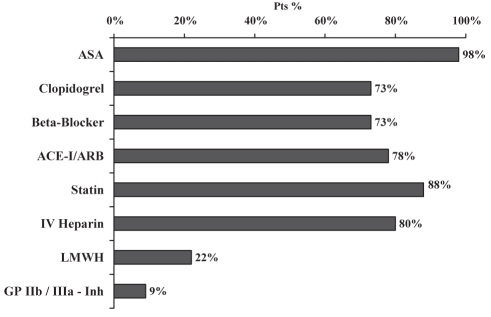
A total of 435 patients (77% men and 80% Saudis) with a mean age of 57.1 years were enrolled. Medical history included previously diagnosed ischemic heart disease (32%), percutaneous coronary intervention (12%), diabetes mellitus (53%), hypertension (48%), current smoking (39%), hyperlipidemia (31%) and family history of premature coronary artery disease (11%). The median door-to-needle time for fibrinolytic therapy received by patients with STEMIs was 90 min. Inhospital medications included acetylsalicylic acid (98%), clopidogrel (73%), angiotensin-converting enzyme inhibitors (74%), beta-blockers (73%), statins (88%), unfractionated heparin (80%), low-molecular weight heparin (22%) and glycoprotein IIb/IIIa inhibitors (9%). The inhospital mortality rate was 5%.
CONCLUSION:
The first nationwide registry of patients with ACS in the Kingdom of Saudi Arabia is presented. In contrast to registries from developed countries, our cohort is characterized by a younger age at presentation and a much higher prevalence of diabetes mellitus. Most patients with STEMIs did not receive fibrinolytic therapy within the time recommended in the American College of Cardiology/American Heart Association guidelines. The results of the present pilot study show potential targets for improvement in care.
Keywords: Acute coronary syndrome, Fibrinolytic therapy, Middle East, Registry, Saudi Arabia
Abstract
OBJECTIF :
On ne connaît pas le délai entre l’obtention des données cliniques et leur application aux soins des patients atteints d’un syndrome coronarien aigu (SCA) dans le Royaume d’Arabie saoudite. Le registre SPACE du projet saoudien pour évaluer les événements coronariens fournit un aperçu complet des stratégies diagnostiques et de traitement des patients ayant un SCA. Ainsi, il peut être utilisé pour repérer des occasions d’améliorer les soins de ces patients.
MÉTHODOLOGIE :
Huit hôpitaux de diverses régions d’Arabie saoudite ont participé à la phase pilote du registre entre décembre 2005 et juillet 2006. Les patients à l’étude incluaient des personnes ayant subi un infarctus du myocarde avec élévation du segment ST (IMÉST), un infarctus du myocarde sans élévation du segment ST (non IMÉST) ou ayant une angine instable.
RÉSULTATS :
Au total, 435 patients (77 % d’hommes et 80 % de Saoudiens), d’un âge moyen de 57,1 ans, ont participé. Leurs antécédents médicaux incluaient une maladie cardiaque ischémique déjà diagnostiquée (32 %), une intervention coronaire percutanée (12 %), le diabète (53 %), l’hypertension (48 %), le tabagisme courant (39 %), l’hyperlipidémie (31 %) et des antécédents familiaux de coronaropathie prématurée (11 %). Le temps médian entre l’arrivée et l’injection pour la thérapie fibrinolytique chez les patients ayant un IMÉST était de 90 minutes. Les médicaments administrés en milieu hospitalier incluaient l’acide acétylsalicylique (98 %), le clopidogrel (73 %), les inhibiteurs de l’enzyme de conversion de l’angiotensine (74 %), les bêta-bloquants (73 %), les statines (88 %), l’héparine non fractionnée (80 %), l’héparine à faible poids moléculaire (22 %) et les inhibiteurs de glycoprotéine IIb/IIIa. Le taux de mortalité en milieu hospitalier était de 5 %.
CONCLUSION :
Le premier registre national de patients ayant un SCA dans le Royaume d’Arabie saoudite est présenté. Contrairement aux registres des pays industrialisés, notre cohorte se caractérise par un plus jeune âge à la présentation et une prévalence beaucoup plus élevée de diabète. La plupart des patients ayant un IMÉST n’avaient pas reçu de thérapie aux fibrinolytiques dans les délais recommandés dans les lignes directrices de l’American College of Cardiology et l’American Heart Association. Les résultats de la présente étude pilote démontrent des cibles potentielles d’amélioration des soins.
According to the World Health Organization, cardiovascular disease will be the leading worldwide cause of morbidity and mortality by the year 2020, and developing countries will be a major contributor to this increase (1–4). It is well known that there is an unacceptable delay between the availability of conclusive clinical trial evidence and its application to patient care (5). This knowledge-practice gap is evidenced in several registries. Moreover, these registries have contributed tremendously to defining the characteristics of patients with coronary artery disease (CAD), assessing care and facilitating the implementation of clinical guidelines (6–8).
The overall prevalence of CAD in Saudi Arabia has been reported to be 5.5% (9). Data regarding the clinical presentation and management of Saudi patients with acute coronary syndrome (ACS) are still lacking. Therefore, we have established an ACS registry in several representative regions of Saudi Arabia. Our objectives are to study current practice patterns in the management of ACS, assess the gap between clinical practice and guidelines, and potentially improve the quality of cardiac care.
The registry consists of three phases over a three-year period from December 2005 to December 2008:
Phase I: Baseline measurement of process of care, outcomes and health care services
This phase extended over a 12-month period from December 2005 to December 2006.
Phase II: Hospital-based report cards
The objective of this phase was to assess the role of report cards in the improvement of patient care (10–13). The results for a 12-month period will be provided to the participating hospitals in the registry (not announced publicly) by the principal coordinating centre. Each hospital will be expected to review its own performance relative to the hospitals contributing to the registry.
Phase III: Critical pathways
The objective of this phase was to identify whether using critical pathways for the management of ACS over a 12-month period will result in improvement of the quality of patient care and application of evidence-based therapies.
The current study describes the overall design of the registry and reports data from its pilot phase.
METHODS
Study population
The Saudi Project for Assessment of Coronary Events (SPACE) is a prospective study of all consecutive patients with ACS admitted to the participating hospitals. Ethics approval was obtained in all participating centres. Involvement in the registry was based on voluntary participation of governmental hospitals and the registry did not include nongovernmental hospitals. Eight hospitals participated in the initial phase; three of the eight hospitals were nontertiary care hospitals with no cardiac catheterization or cardiac surgery facilities.
Definitions of ACS
The diagnosis of the different types of ACS was based on the definitions of the Joint Committee of the European Society of Cardiology/American College of Cardiology (ACC) (14). Serum cardiac bio-markers used to assist in the diagnosis of myocardial injury were measured in each hospital’s laboratory using its own assays and reference ranges.
Study organization
A two-page standardized case report form for each patient with suspected ACS was completed on admission by assigned physicians working in each hospital, and then was completed throughout the hospital stay. All forms were verified by a cardiologist and then sent to the principal coordinating centre, where the forms were further checked for incomplete data and mistakes before submission for final analysis. To avoid double-counting patients, each patient’s national identification number was used. Patients with ACS who presented directly to the emergency department were labelled as ‘SPACE-own’, while those who were transferred from a non-registry hospital were called ‘SPACE-referral’.
Case report form data variables
Data collected included the following variables: patient demographics, medical history, provisional diagnosis on admission and final discharge diagnosis, electrocardiogram (ECG) findings, laboratory investigations, medical therapy, use of cardiac procedures and interventions, inhospital outcomes and inhospital mortality. Major bleeding was defined as overt clinical bleeding associated with a drop in hemoglobin by more than 5 g/dL (0.5 g/L) or bleeding causing hemodynamic instability or requiring blood transfusion.
Statistical analysis
Descriptive statistics are summarized as median or mean ± SD. Fisher’s exact tests or χ2 tests were used for categorical variables to assess group differences, and t tests were used to assess differences between continuous variables. All tests were two-sided, with a 5% level of significance. All analyses were performed using STATA version 9 (StataCorp LP, USA).
RESULTS
The SPACE registry enrolled 435 patients from December 2005 to July 2006. Patient demographics are presented in Table 1. The mean age was 57.1±13.6 years and 332 patients (77%) were men. Ischemic heart disease was previously diagnosed in 140 patients (32%). Diabetes mellitus (DM) was the most prevalent risk factor for CAD, present in 56% of patients, of which 3% were diagnosed after hospital admission. Hypertension was the second most prevalent risk factor, present in 208 patients (48%).
TABLE 1.
Patient demographics in the Saudi Project for Assessment of Coronary Events (SPACE) registry: Overall, SPACE-own* and SPACE-referral† cohorts
| SPACE overall (n=435) | SPACE-own (n=319) | SPACE-referral (n=116) | P | |
|---|---|---|---|---|
| Age, mean ± SD, years | 57.1±13.6 | 56.7±13.9 | 58.1±12.6 | 0.7 |
| Male sex | 332 (77) | 243 (77) | 89 (77) | 1.0 |
| Saudi nationality | 345 (80) | 240 (76) | 105 (91) | 0.005 |
| Medical history | ||||
| Known IHD | 140 (32) | 113 (35) | 27 (23) | 0.01 |
| PCI | 50 (12) | 40 (13) | 10 (9) | 0.25 |
| CABG | 24 (5) | 20 (6) | 4 (3) | 0.21 |
| CVA/TIA | 19 (4) | 13 (4) | 6 (5) | 0.64 |
| PAD | 8 (2) | 6 (2) | 2 (2) | 1.0 |
| Risk factors | ||||
| Current smoker within previous 1 year | 170 (39) | 131 (41) | 39 (34) | 0.18 |
| DM on insulin | 69 (16) | 44 (14) | 25 (22) | 0.044 |
| DM not on insulin | 161 (37) | 124 (39) | 37 (32) | 0.18 |
| Hypertension | 208 (48) | 158 (50) | 50 (43) | 0.19 |
| Hyperlipidemia | 135 (31) | 108 (34) | 27 (23) | 0.028 |
| Family history of premature CAD | 46 (11) | 31 (10) | 15 (13) | 0.37 |
| Discharge diagnosis | ||||
| STEMI | 198 (45) | 131 (41) | 67 (58) | 0.001 |
| NSTEMI | 120 (28) | 99 (31) | 21 (18) | 0.007 |
| Unstable angina | 117 (27) | 89 (28) | 28 (24) | 0.40 |
Data presented as n (%) unless otherwise specified.
Patient presented directly to the emergency department;
Transferred from a nonregistry hospital. CABG Coronary artery bypass graft; CAD Coronary artery disease; CVA Cerebrovascular accident; DM Diabetes mellitus; IHD Ischemic heart disease; NSTEMI Non-ST segment elevation myocardial infarction; PAD Peripheral arterial disease; PCI Percutaneous coronary intervention; STEMI ST segment elevation myocardial infarction; TIA Transient ischemic attack
Of the 435 patients, 198 (45%) were diagnosed with STEMI (41% in SPACE-own versus 58% in SPACE-referral cohorts; P=0.007), 120 (28%) with NSTEMI (31% in SPACE-own versus 18% in SPACE-referral cohorts; P=0.01), and 117 (27%) with unstable angina (28% in SPACE-own versus 24% in SPACE-referral cohorts; P=0.40).
There were 192 overall SPACE patients (own and referral) who presented with a provisional diagnosis of STEMI. Among these patients, 126 (66%) received fibrinolytic therapy. The most commonly used agent was streptokinase in 77 (61%), followed by retaplase in 43 patients (34%).
Additional data regarding fibrinolytic therapy were available for only 124 SPACE-own patients with STEMI, 81% of whom presented within 12 h of symptom onset. Reasons for not receiving fibrinolytic therapy included primary percutaneous coronary intervention in 14 patients (23%), presence of symptoms for more than 12 h in 15 (25%), perceived contraindications in four (7%) and no reason recorded in 27 (45%). The vast majority of patients who received fibrinolytic therapy (76.5%) received it in the critical care unit or intensive care unit. The median door-to-needle time (DNT) was 90 min, with only five patients (4%) receiving therapy within 30 min. The median time between the first diagnostic ECG and commencement of therapy was 85 min.
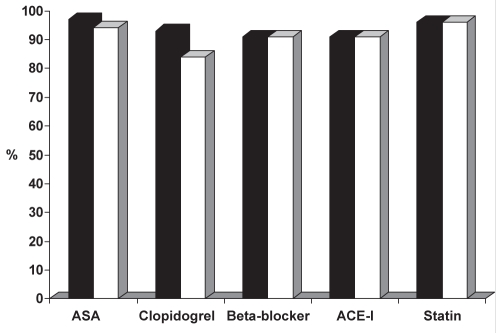
Figure 1 depicts medication usage during hospitalization in the total SPACE cohort. Figure 2 shows treatment of SPACE-own patients in the first 24 h of hospital admission and on discharge, with no significant difference between the two groups.
Figure 1).
Medical therapy for patients in the Saudi Project for Assessment of Coronary Events (SPACE) registry overall cohort. ACE-I Angiotensin-converting enzyme inhibitor; ARB Angiotensin receptor blocker; ASA Acetylsalicylic acid; GP IIb/IIIa-Inh Glycoprotein IIb/IIIa inhibitor; IV Intravenous; LMWH Low-molecular weight heparin
Figure 2).
Medical therapy for the Saudi Project for Assessment of Coronary Events (SPACE) registry SPACE-own patients in the first 24 h of hospitalization (black bars) and on hospital discharge (white bars). ACE-I Angiotensin-converting enzyme inhibitor; ASA Acetylsalicylic acid
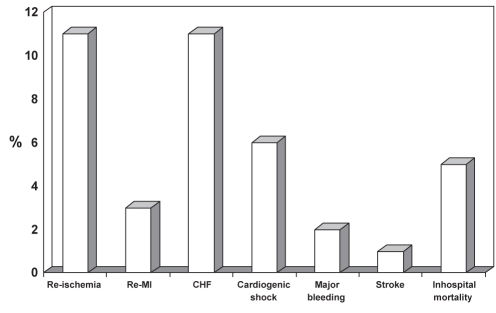
Coronary angiography was performed in 260 of the 435 patients in the entire cohort (60%); of these, 39% had percutaneous coronary intervention. Coronary artery bypass graft surgery was performed in 9% of patients. Figure 3 shows adverse inhospital outcomes for the total cohort. The incidence of stroke was 1% (two patients were ischemic, one was hemorrhagic and two were unknown). Major bleeding occurred in only eight patients (2%). The all-cause inhospital mortality rate for the entire study cohort was 5% (n=22). There were 65 patients (15%) transferred to non-SPACE hospitals for further management. Of these 65 patients, 40 (62%) were alive at discharge and two (3%) were deceased. Access to inhospital outcomes was not available for the remaining 23 (35%) patients.
Figure 3).
Adverse hospital outcomes for the Saudi Project for Assessment of Coronary Events (SPACE) overall cohort. CHF Congestive heart failure; MI Myocardial infarction; Re Recurrent
DISCUSSION
SPACE is the first ACS registry in the Kingdom of Saudi Arabia. The current report focuses on the overall design and results of its phase I pilot project. The first finding was that the mean age of presentation in the SPACE cohort (57.1 years) was eight to 11 years younger than in ACS registries in developed countries. In the Global Registry of Acute Coronary Events (GRACE) (15), the median age at ACS presentation was 65 years in patients with STEMI and 68 years in patients with NSTEMI. This difference in age at presentation is due in part to an overall younger population in developing countries than in developed countries (16) and may also be due to the high prevalence of poorly controlled CAD risk factors. Indeed, this is supported by the extremely high prevalence of DM (56%) in the present cohort. Data from other registries show that the prevalence of DM ranges from 21% to 33% (15,17,18). To our knowledge, the prevalence in our study is higher than what has been reported in any other ACS registry (15,17–19). The reasons for this finding are unclear; however, some plausible explanations are rising obesity rates, sedentary lifestyle, poor dietary habits and high rates of consanguinity (20,21). The fact that more than one-half of our patients had more than one CAD risk factor on hospital admission, yet most were probably not on optimal medical therapy, may explain the development of ACS at a younger age.
The third major finding was the median DNT of 90 min, which is much longer than the benchmark DNT of less than 30 min recommended by the ACC/American Heart Association (ACC/AHA) guidelines. ECGs were performed in a timely fashion; however, the major component of delay occurred between the diagnosis of STEMI and the commencement of fibrinolytic therapy. Potential explanations are that fibrinolytic therapy was provided predominantly by the cardiology service, was administered mostly in the critical care unit or intensive care unit and was ordered from the hospital pharmacy in many hospitals (personal communication). Lambrew et al (22) demonstrated that improvement of the above factors could potentially result in reduction of the DNT.
The fourth critical observation was the relatively low rate of inhospital adverse outcomes and inhospital mortality. Possible explanations include the relatively young age at presentation, and the high rate of medical therapy and use of reperfusion strategies, which was likely because the study was conducted following the publication of updated clinical guidelines. Furthermore, data from the GRACE (15) and Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines (CRUSADE) (17) registries showed similar trends of improved management of ACS associated with reductions in the rates of heart failure and mortality. Conversely, it is possible that these low rates of inhospital complications may not reflect reality because of the small sample size recruited thus far, possible under-reporting of cardiac events and the absence of reported outcomes for many of the patients transferred to non-SPACE hospitals.
Limitations
The limitations of the present study include the possible selection bias inherent in any registry-based study. Furthermore, the limited number of participating hospitals in this pilot phase may limit the degree to which our findings can be generalized. We hope to reduce the effect of this limitation by recruiting more hospitals into the registry in the future.
CONCLUSIONS
The current study described the overall design of the SPACE registry and summarized the results of its pilot phase. Our patients were relatively young at the time of presentation with ACS and had a high prevalence of DM. Patients with STEMI had long DNTs, but otherwise, the overall medical therapy was similar to that recommended by the ACC/AHA guidelines (with the exception of underuse of glycoprotein IIb/IIIa inhibitors). There were low rates of adverse hospital outcomes and inhospital mortality. These results highlight several challenging areas and will hopefully form the basis for improving management practices and outcomes in patients with ACS in our country.
Acknowledgments
The SPACE registry is under the auspices of the Saudi Heart Association, and is financially sponsored by sanofi-aventis, which had no role in data extraction or analyses, the writing of the manuscript or the decision to submit the manuscript for publication. No authors have any conflicts of interest. The authors thank Zenaida Ramoso for secretarial assistance.
Footnotes
CASE REPORTS DATA COLLECTION BY: Ali Al-Shahrani, Nawaf Al-Majed, Rayan Al-Hazmi, Osama Al-Mogbel, Waleed Al-Harbi, Aws Al-Herbish, Ayman Al-Saleh, Eman Shisha, Adel Maria, Syed Asghar Hussain, Huda Abdullah Al-Mefarih, Ola Abdelmajeed, Asif Malik, Hassan Khalaf, Khalid Koumi, Hamad AlHabib, Ambreen Gul and Yahia Al Hossni.
REFERENCES
- 1.WHO . The World Health Report 1998. Geneva: World Health Organization; 1998. pp. 148–85. [Google Scholar]
- 2.Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med. 1998;4:1241–3. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 3.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–60. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 4.Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation. 1998;97:596–601. doi: 10.1161/01.cir.97.6.596. [DOI] [PubMed] [Google Scholar]
- 5.European Secondary Prevention Study Group Translation of clinical trials into practice: A European population-based study of the use of thrombolysis for acute myocardial infarction. Lancet. 1996;347:1203–7. [PubMed] [Google Scholar]
- 6.Steg PG, Goldberg RJ, Gore JM, et al. GRACE Investigators Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE) Am J Cardiol. 2002;90:358–63. doi: 10.1016/s0002-9149(02)02489-x. [DOI] [PubMed] [Google Scholar]
- 7.Rosengren A, Wallentin L, Simoons M, et al. Age, clinical presentation, and outcome of acute coronary syndromes in the Euroheart acute coronary syndrome survey. Eur Heart J. 2006;27:789–95. doi: 10.1093/eurheartj/ehi774. [DOI] [PubMed] [Google Scholar]
- 8.Wiviott SD, Morrow DA, Frederick PD, Antman EM, Braunwald E. Application of the Thrombolysis In Myocardial Infarction risk index in non-ST-segment elevation myocardial infarction: Evaluation of patients in the National Registry of Myocardial Infarction. J Am Coll Cardiol. 2006;47:1553–8. doi: 10.1016/j.jacc.2005.11.075. [DOI] [PubMed] [Google Scholar]
- 9.Al-Nozha MM, Arafah MR, Al-Mazrou YY, et al. Coronary artery disease in Saudi Arabia. Saudi Med J. 2004;25:1165–71. [PubMed] [Google Scholar]
- 10.Tu JV, Donovan LR, Austin PC, et al. Quality of Cardiac Care in Ontario – Phase I. Report 2. Toronto: Institute for Clinical Evaluative Sciences; 2005. [Google Scholar]
- 11.Lindenauer PK, Remus D, Roman S, et al. Public reporting and pay for performance in hospital quality improvement. N Engl J Med. 2007;356:486–96. doi: 10.1056/NEJMsa064964. [DOI] [PubMed] [Google Scholar]
- 12.Hibbard JH, Stockard J, Tusler M. Hospital performance reports: Impact on quality, market share, and reputation. Health Aff (Millwood) 2005;24:1150–60. doi: 10.1377/hlthaff.24.4.1150. [DOI] [PubMed] [Google Scholar]
- 13.Marshall MN, Shekelle PG, Leatherman S, Brook RH. The public release of performance data: What do we expect to gain? A review of the evidence. JAMA. 2000;283:1866–74. doi: 10.1001/jama.283.14.1866. [DOI] [PubMed] [Google Scholar]
- 14.Cannon CP, Battler A, Brindis RG, et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) J Am Coll Cardiol. 2001;38:2114–30. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 15.Fox KAA, Steg PG, Eagle KA, GRACE investigators Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA. 2007;297:1892–900. doi: 10.1001/jama.297.17.1892. [DOI] [PubMed] [Google Scholar]
- 16.The International Bank for Reconstruction and Development/The World Bank. World Development Report. 2007:288–9. [Google Scholar]
- 17.Mehta RH, Roe MT, Chen AY, CRUSADE investigators Recent trends in the care of patients with non-ST segment elevation acute coronary syndromes: Insights from the CRUSADE initiative. Arch Intern Med. 2006;166:2027–34. doi: 10.1001/archinte.166.18.2027. [DOI] [PubMed] [Google Scholar]
- 18.Yan AT, Yan RT, Tan M, Canadian ACS Registry Investigators Management patterns in relation to risk stratification among patients with non-ST elevation acute coronary syndromes. Arch Intern Med. 2007;167:1009–16. doi: 10.1001/archinte.167.10.1009. [DOI] [PubMed] [Google Scholar]
- 19.Abdallah MH, Arnaout S, Karrowni W, Dakik HA. The management of acute myocardial infarction in developing countries. Int J Cardiol. 2006;111:189–94. doi: 10.1016/j.ijcard.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf S, Hawken S, Ounpuu S, INTERHEART investigators Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 21.El-Hazmi MA, Al-Swailem AR, Warsy SA, et al. Consanguinity among Saudi Arabian population. J Med Genetics. 1995;32:623. doi: 10.1136/jmg.32.8.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambrew CT, Bowlby LJ, Rogers WJ, et al. Factors influencing the time to thrombolysis in acute myocardial infarction. Time to Thrombolysis Substudy of the National Registry of Myocardial Infarction-1. Arch Intern Med. 1997;157:2577–82. [PubMed] [Google Scholar]